Abstract
Extracorporeal photopheresis (ECP) has been considered an efficient dendritic cell (DC) therapy, used for treating both T cell malignancy, as well as T cell-mediated diseases. During the ECP procedure leucocytes are exposed to photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A radiation (PUVA) prior to reinfusion. Despite its clinical efficacy the mechanism of action remains elusive. As it has been reported that ECP might promote the differentiation of monocytes into immature DCs, we investigated the effects of UVA light (2 J/cm2) and 8-MOP (100 ng/ml) on in vitro monocyte-to-DC differentiation from normal donors. DCs were generated from human purified CD14+ cells. Because monocytes are killed by PUVA and taking into account that only 5–10% of circulating mononuclear cells are exposed to PUVA during the ECP procedure, we developed an assay in which 10% of PUVA-treated monocytes were co-cultured with untreated monocytes. We first demonstrate that the presence of 10% apoptotic cells and monocyte activation were not enough to induce monocyte differentiation into DCs. Adding cytokines to our culture system, we obtained immature DCs characterized by significantly higher phagocytic activity and human leucocyte antigen D-related (HLA-DR) expression. These DCs preserved the capacity to be activated by lipopolysaccharide, but showed a reduced capacity to induce allogeneic T cell proliferation when first co-cultured with 10% of PUVA-treated cells. Our experimental design provides a novel insight into the real action of 8-MOP and UVA light on dendritic cell biology, suggesting an additional mechanism by which 8-MOP and UVA light exposure may influence immune responses.
Keywords: dendritic cells, photopheresis, psoralen, UVA light
Introduction
Dendritic cells (DCs) represent a rare and heterogeneous population of professional antigen-presenting cells (APCs) able to initiate and regulate immune responses [1]. Immunogenic DCs play a key role in host defences inducing T cell activation and polarization, whereas in normal physiology they also have a central role in the induction of peripheral tolerance [2–4]. Therefore, as DCs represent an ideal therapeutic target for pharmacological manipulation, identification of the checkpoints of DC biology (differentiation, antigen uptake, migration, maturation and survival) is crucial in order to select the potential pharmacological targets [4]. In their immature state, DCs are localized in the peripheral tissues, express low levels of co-stimulatory molecules and major histocompatibility complex (MHC) molecules and have high endocytic activity. Following their encounter with antigens and/or ‘danger’ signals, such as proinflammatory cytokines, DCs initiate their maturation process and migrate to regional lymph nodes where they activate and mobilize effector cells. This process leads to increased expression of co-stimulatory molecules and MHC molecules, as well as the production of immunomodulatory cytokines [5–8]. Even in an immature state, small numbers of DCs migrate continuously to the lymph node and present antigens. Immature or incompletely mature DCs, however, are not well equipped for the induction of effector T cells, and either interacting antigen-specific T lymphocytes are anergized or regulatory T cells are induced [5–8]. Thus the presence or absence of maturation signals for immature DCs may act as inductors to either an adaptive immune response or tolerance [5,6].
Several clinical approaches are currently under way for the development of DC immunotherapy for cancer vaccines or graft survival [4,9,10]. The extracorporeal photopheresis (ECP), performed using UVAR apparatus (Therakos Inc., Exton, PA, USA), is currently considered to be a simple and efficient dendritic cell therapy [8,11–13]. During ECP, leukopheresed concentrated white blood cells are exposed to photoactivable DNA-intercalating agent 8-methoxypsolaren (8-MOP) (administered directly into the concentrated white blood cell bag) and ultraviolet A (UVA) radiation (320–400 nm) and then returned to the patient [14,15]. This method is used currently both for treating T cell malignancy (e.g. cutaneous T cell lymphoma, CTCL) [13–18], and for reporting benefits in T cell-mediated diseases [e.g. graft-versus-host-disease (GVHD) and organ transplant rejection] [14,15,19–24]. Nevertheless, the phenotypic and functional mechanisms in the 8-MOP photo-treated immunocompetent cells, including DC, have not been elucidated fully. It remains puzzling as to how a single treatment modality could both activate the immune system in protection against cancer and suppress T cell activity in autoreactive disorders. The clinical efficacy of ECP in the cutaneous T cell lymphoma setting might be explained by two simultaneous and synergistic UVA light-induced phenomena: apoptosis of exposed T and B lymphocytes by 8-MOP activation, activation of passaged monocytes and their differentiation into DCs [11–13,15,17,18,21,22]. The mechanisms to explain the beneficial effect of ECP in T cell-mediated diseases are less well understood. Recently, evidence has been found to indicate that ECP induces tolerance to alloantigens correlated with the shift in peripheral blood T cell populations from T helper 1 (Th1) to Th2 responses [19].
Therefore, in this study we investigated the in vitro effects of UVA light and 8-MOP (using the same therapeutic doses as in ECP) on monocyte-to-DC differentiation. In a previous study we demonstrated that monocytes treated in vitro with PUVA at the beginning of culture underwent apoptosis within 48 h [25]. Therefore, as it is known that only 5–10% of the patient's mononuclear cell population is affected in a single photopheresis treatment [15], we developed an assay in which 10% of PUVA-treated monocytes were co-cultured with PUVA-untreated monocytes of the same healthy donor. For this performance, purified CD14+ cells were divided into two aliquots: one was treated with 8-MOP and UVA light in the same conditions as above, and the other was incubated at 37°C without any pretreatment. Then, PUVA-exposed CD14+ cells were co-cultured with the remaining untreated cells at a ratio of 1 : 9. These cultures were induced to generate DCs. Using this model we have been able to add new insight into understanding of the mechanism of action of ECP on DC functions.
Materials and methods
Generation of dendritic cells
Human peripheral blood mononuclear cells (PBMC) from normal donors (Centro Trasfusionale, Ospedale Cisanello, Pisa, Italy) were isolated from buffy coats over a Ficoll density gradient centrifugation (Ficoll-Hypaque, Pharmacia Biotech, Uppsala, Sweden). CD14+ cells were purified from PBMC by high-gradient magnetic sorting using the MIDIMACS Technique (MACS Miltenyi Biotec, Bergish Gladbach, Germany), according to the manufacturer's instructions. Briefly, PBMC were incubated with microbeads conjugated with monoclonal mouse anti-human CD14 antibodies for 15 min on ice, washed in phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin and 2 mM ethylenediamine tetraacetic acid (EDTA). Labelled and positively enriched cells were eluted from magnetic columns by the removal of columns from the magnetic device. Purified CD14+ cells were resuspended at a concentration of 1 × 106 cells/ml and cultured for 6 days in six-well multi-well tissue culture plates in RPMI-1640 (Gibco laboratories, Milan, Italy) supplemented with 10% fetal calf serum (FCS; Eurobio Biotechnology, France), 2 mM l-glutamine, 100 µg/ml streptomycin, 100 IU/ml penicillin, in the presence of 50 ng/ml recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF; Novartis, Basel, Switzerland) and 20 ng/ml recombinant human interleukin 4 (IL-4; PeproTech EC Ltd, London, UK). Fresh cytokines were replaced every 3 days. Some cultures received 10–100 ng/ml of bacterial lipopolysaccharide (LPS; Sigma-Aldrich, Poole, UK), known to stimulate DC maturation during the last 24 h. Cell morphology and cell-surface markers were analysed on days 3, 6 and 7.
Light source
Cell suspensions were irradiated with a UVA light box (Therakos, West Chester, PA, USA) containing a series of 12 linear fluorescent tubes. We evaluated the radiation emission of the lamp and the transmission characteristics of the plastic plates used in our tissue cell cultures in order to obtain the appropriate doses of UVA. Tubes emitted UVA light ranging from 320 to 400 nm, with a peak of emission at 365 nm. UVA emission power was measured by a photodiode, removing the visible light (> 400 nm) with a low-pass filter. In this condition, the irradiated power was measured at 42 W/m2. The filter absorption in the UVA region was approximately 10%, very close to the absorption of the plastic plate top (8%). Therefore, the actual power delivered to the culture was 42 W/m2, for an exposure of 8 min, providing a total applied energy of 2 J/cm2.
In vitro treatment of cells with 8-MOP/UVA
An 8-MOP stock solution was prepared in 80% ethanol and stored in aliquots at −80°C. CD14+ cells (1 × 106 cells/ml) in complete medium were incubated with 8-MOP (100 ng/ml; Sigma) for 20 min at 37°C and then exposed at room temperature to 2 J/cm2 dose of UVA light. These doses of 8-MOP and UVA radiation were chosen on the basis of the ones used in clinical settings (in each photopheresis cycle). In a previous study we demonstrated that monocytes treated in vitro with PUVA at the beginning of culture underwent apoptosis within 48 h [25]. Therefore, as it is known that only 5–10% of the patient's mononuclear cell population is affected in a single photopheresis treatment [15], we developed an assay in which 10% of PUVA-treated monocytes were co-cultured with PUVA-untreated monocytes. For this performance, purified CD14+ cells were divided into two aliquots: one was treated with 8-MOP and UVA light in the same conditions as above, and the other was incubated at 37°C without any pretreatment. Then, PUVA-exposed CD14+ cells were co-cultured with the remaining untreated cells at a ratio of 1 : 9. These cultures were induced to generate DCs in the above-described assay. This is the culture system used in this paper, and will be referred to as ‘PUVA-mixed culture’ throughout.
It has been reported that photopheresis might contribute to monocyte-to-DCs differentiation and an adaption of ECP termed transimmunization [11–13] enhances its efficacy for more phagocytosis by the addition of an overnight incubation period.
Therefore, to investigate the potential role of the presence of 10% PUVA-treated monocytes and plastic adherence on DC differentiation, we performed experiments containing cells cultured in the absence of GM-CSF and IL-4.
Morphological examination
To assess cultured cell morphology, cytospin slides were prepared by cytocentrifuging (800 g, 10 min) 5 × 104 cells/ml (cytospin-2 centrifuge; Shandon, Astmoor, UK) onto glass microscope slides. These were air-dried, fixed with methanol, stained using May–Grünwald–Giemsa stain and then examined by light microscopy (Leitz Laborlux S, ×100).
Evaluation of apoptosis
Viability of mature DC (after LPS, 100 ng/ml) was monitored in both PUVA-mixed cultures and PUVA-untreated systems (24 h after treatment) by light microscopy and flow cytometry. Apoptosis and secondary necrosis were determined using annexin-V–propidium iodide (PI) (Bender MedSystem GmbH, Vienna, Austria), according to the manufacturer's recommendations. Cells were harvested, washed, labelled with fluorescein isothiocyanate (FITC)–annexin V, the mixture was incubated for 10 min in the dark at room temperature, and subsequently 1 mg/ml propidium iodide (PI) was added. AnnexinV–PI staining was analysed on a fluorescence activated cell sorter (FACScan; Becton Dickinson, San Jose, CA, USA) using CellQuest software. Data are expressed as percentage of annexin-V+ cells in the PI population.
Flow cytometric analysis
Antibodies used for cell surface staining included CD14, CD1a, human leucocyte antigen D-related (HLA-DR) (Caltag Laboratories, Burlingame, CA, USA – Valter Occhiena, Torino, Italy), CD40, CD80, CD83 (Immunotech, Marseille, France). All monoclonal antibodies were conjugated to FITC or phycoerythrin (PE). Data acquisition and analysis were performed on both Epics-XL (Beckman Coulter, Inc., Miami, FL, USA) with Expo32 software and FACScan (Becton Dickinson, San Jose, CA, USA) with CellQuest software. DCs were gated according to their light-scattering properties, and dead cells were excluded from the analysis. Five thousand events were acquired for each experiment. Results were expressed as a percentage of positive cells or as the mean fluorescence intensity (MFI) of positive cells. Modulation was calculated as (MFI of untreated group − MFI of PUVA-treated group)/MFI of the untreated group.
Uptake of FITC–dextran
Mannose receptor mediated endocytosis was measured as cellular uptake of FITC–dextran. Approximately 1 × 106 of immature, 6-day-cultured DCs for the sample were incubated in media containing FITC–dextran (2 mg/ml; Sigma) for 60 min at 37°C or on ice (the latter for the assessment of background staining due to unspecific external binding). After incubation, cells were washed twice with PBS to remove excess dextran and fixed in cold 1% formalin. The cells were then analysed by flow cytometry. At least 5000 gated events were evaluated for each condition. The level of antigen uptake by DCs was expressed as the difference in MFI (ΔMFI) between the test (37°C) and control (0°C) tubes for each sample.
Mixed leucocyte culture reaction (MLR)
The primary T cell stimulatory capacity of DCs was tested in MLR. Allogenic purified T cells were used as responder cells in 96-well round-bottomed cell culture plates in a culture volume of 200 µl. All experiments were performed in triplicate. Graded numbers of stimulator cells were added to 2 × 105 responder cells while control cultures, used to evaluate the background proliferation, contained responder or stimulator cells only.
After 6 days of culture in 5% CO2 at 37°C the cells were harvested, counted and diluted to about 1 × 106/ml and fixed in 70% ethanol. Proliferation was assessed by flow cytometry for the S phase of the cell cycle measure using DNA staining with PI (5 µg/ml). Data acquisition and analysis were performed on a Epics-XL (Beckman Coulter) using MultiCycle software.
Statistical analysis
Data were expressed as mean ± standard deviation (s.d.). Comparisons were performed by Student's t-test. A P-value < 0·05 was considered statistically significant.
Results
PUVA treatment and plastic adherence did not induce monocyte to DC differentiation
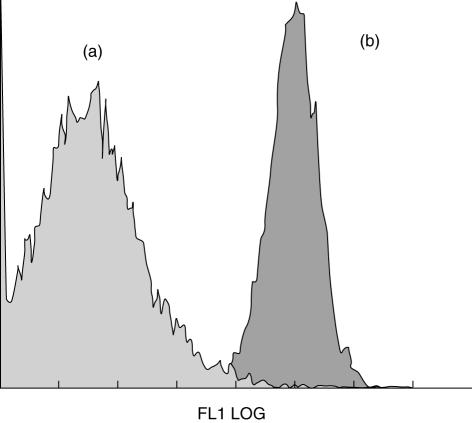
It has been reported that photopheresis might contribute to monocyte-to-DCs differentiation and its efficacy may be improved by incubating treated cells overnight before reinfusion [11–13]. Because we have reported that PUVA-treated monocytes undergo apoptosis [25], we sought to determine whether the presence of 10% PUVA-treated monocytes in co-cultures and plastic adherence were able to induce DC differentiation. Cells were co-cultured for 6 days in the absence of IL-4 and GM-CSF. Cells retrieved from this culture system displayed a monocyte/macrophage morphology (data not shown) and retained high CD14 expression (Fig. 1). This indicates that neither plastic adherence nor incubation with PUVA cells can induce monocyte-to-DC differentiation in our system.
Fig. 1.
Photoactivable agent 8-methoxypsoralen (8-MOP) and ultraviolet (UV) A (UVA) light (PUVA) treatment and plastic adherence did not induce monocyte differentiation towards dendritic cells (DCs). Photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A (PUVA)-exposed CD14+ cells were cultured with IL-4 and GM-CSF or without cytokines. After 6 days of culture, cells were stained with fluorescein isothiocyanate (FITC)–CD14 and analysed by flow cytometry. Plot of CD14 expression in cells generated by culturing PUVA-treated monocytes with (a) or without (b) GM-CSF/IL-4. Cells lose CD14 when cultured with IL-4 and GM-CSF (a) but they remained CD14+ in the absence of cytokines (b). Data from one representative experiment are provided.
Cytokines are requisite to induce DC differentiation from monocytes
Analysis of cultured cell morphology
Following culture with GM-CSF and IL-4, purified CD14+ cells in both control and PUVA-mixed cultures differentiated into non-adherent, floating and clustering cells.
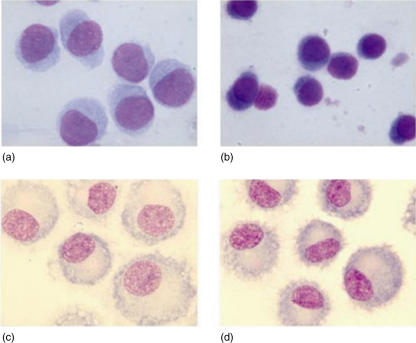
In three experiments we investigated the morphological profile of cells harvested on day 3 of culture. Cells derived from PUVA-mixed cultures were larger in size, with more cytoplasm displaying weak basophilia, than control cells (Fig. 2a,b).
Fig. 2.
The morphology of monocyte-derived dendritic cells (DCs). (a,b) Morphological profile of cells harvested during the pre-DC stage of development. Each DC preparation was examined by light microscopy (May–Grunwald–Giemsa staining). At early time-point (day 3) of culture with interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) (as described in Material and methods), cells derived from photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A (PUVA)-treated cultures (a) were larger in size with more cytoplasm, displaying weak basophilia, than control DCs (b). (c,d) DCs were differentiated from CD14+ cells (control Mo-DCs) cultured for 7 days with GM-CSF and IL-4. Cultures received 10 ng/ml of lipopolysaccharide (LPS) to stimulate DC maturation during the last 24 h. Cells derived from both cultures of PUVA-treated (c) and untreated monocytes (d) showed typical shapes of mature DCs such as cytoplasmic dendritic projections in both samples: the first were larger, giant-sized cells (c) in contrast to control DCs (d).
On day 7, after LPS addition, more than 80% of cells displayed features of mature myeloid DCs, including a homogeneous smooth intensely stained cytoplasm, irregular membrane ruffling, dendrites and a laterally positioned nucleus. Cells derived from PUVA-mixed cultures showed dendritic morphology but were larger, giant-sized cells in comparison to control DCs (Fig. 2c,d).
Immunophenotype of cultured cells
In two experiments we examined the effect of 10% PUVA-treated monocytes on the immunophenotype during the pre-DC stage of development in IL-4/G-CSF cultured monocytes. At an early time-point (day 3) of culture with IL-4 and GM-CSF, PUVA-mixed derived cells showed lower CD1a expression when compared to control cells (range of reduction: 21–23%). No differences in CD14 and CD40 expression were observed between PUVA-mixed and control cells. Pre-DCs have a low expression of CD80 and no CD83, therefore no significant differences in the expression of these markers could be detected at this early time-point. However, cells derived from PUVA-mixed cultures showed a higher HLA-DR expression than control cells (range of increase 8–10%) (data not shown).
As expected, monocytes cultured for 6 days in IL-4 and GM-CSF developed into DCs characterized by the acquisition of CD1a (84% ± 10·6%) and the loss of CD14 (2·7% ± 1·4%) antigens at the cell surface. They expressed the pattern of molecules characteristic of immature DCs: low levels of co-stimulatory molecules (CD40 and CD80), low expression MHC class II molecules (25·6% ± 5%, MFI: 345·4 ± 57·1) and negativity of the maturation-specific marker CD83 (10·6% ± 2·1%). These antigens were up-regulated when DCs matured in response to LPS (Table 1).
Table 1.
Phenotype of immature dendritic cells (DCs) and lipopolysaccharide (LPS)-activated DCs. DCs were exposed to photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A (PUVA) treatment as described in Materials and methods. Immature DCs were activated to mature DCs with addition of 10 ng/ml LPS on day 6. Results are presented as mean of mean fluorescence intensity/or percentage ± s.d. of seven independent experiments.
| CD1a | CD14 | CD40 | CD80 | CD83 | HLA-DR | |
|---|---|---|---|---|---|---|
| Immature DCs | ||||||
| Control | 84 ± 10·6% | 2·7 ± 1·4% | 10·6 ± 2·1% | 345·4 ± 57·1 | ||
| PUVA treatment | 81·2 ± 3% | 3·8 ± 2·9% | 9·9 ± 1·9% | 380·8 ± 45* | ||
| Mature DCs | ||||||
| Control | 816·7 ± 52 | 474·7 ± 38·6 | 429·3 ± 44 | 476·5 ± 36·8 | ||
| PUVA treatment | 807 ± 55 | 485·8 ± 35·3 | 421·1 ± 41·4 | 526·2 ± 17·1 | ||
Statistical significance (P < 0·01) compared to untreated control DCs. HLA-DR: human leucocyte antigen D-related.
Incubation with 10% PUVA-treated monocytes did not substantially alter MFI and percentage values of CD1a and CD14 (81·2% ± 3% and 3·8% ± 2·9%, respectively), CD40, CD80 antigens, except HLA-DR (Table 1). Indeed, immature DCs derived from PUVA-mixed cultures expressed significantly higher levels of HLA-DR (MFI ± s.d.: 380·8 ± 44·9) than control DCs (P < 0·01). They preserved the capacity to be activated by LPS, as shown by the increase in the expression of HLA-DR, co-stimulatory (CD80, CD40) and CD83 molecules compared to control DCs (Table 1).
The presence of 10% PUVA-treated monocytes during DC differentiation from purified CD14+ cells did not influence cell viability, as indicated by trypan blue exclusion and annexin-V values on flow cytometry (data not shown).
Effects of PUVA treatment on antigen capture activity
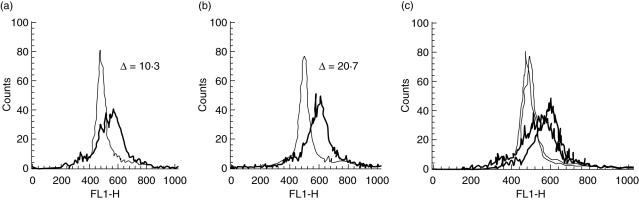
As endocytic activity is regarded as a property of myeloid DCs at immature states, the decrease in antigen uptake has been used to monitor DC maturation. We analysed the phagocytic activity of immature DCs by means of fluorescein-conjugated dextran uptake. PUVA-treated DCs showed a vigorous endocytosis of FITC–dextran, higher than control DCs (ΔMFI, 40·8 ± 28 and 22·6 ± 10, mean ± s.d. of PUVA-treated and control DCs, respectively, P < 0·05) (Fig. 3).
Fig. 3.
Photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A (PUVA) treatment increases the antigen uptake by dendritic cells (DCs). Cells were incubated for 30 min (at 0°C and 37°C) with fluorescein isothiocyanate (FITC)-labelled dextran, washed and analysed by flow cytometry. Representative analysis of antigen uptake by monocyte-derived (control) DCs (a) and by DCs generated from co-cultures containing PUVA-treated and untreated monocytes in the ratio 10 : 90 (b). The difference in uptake [difference in mean fluorescence intensity (ΔMFI)] between FITC–dextran uptake at 37°C (thick lines) and the control uptake at 0°C (thin lines) on day 6 is shown. Overlay of FITC–dextran uptake (c). The result is representative of eight independent experiments.
Effect of PUVA treatment on allostimulatory capacity of DCs
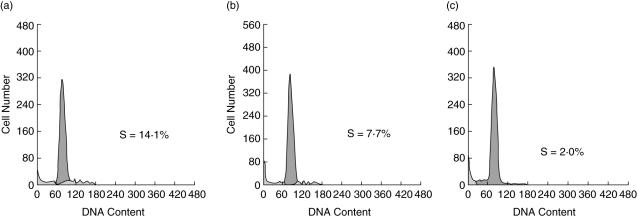
The ability of DCs to stimulate T cells is a function that increases with their maturation and may be observed in allo-mixed lymphocyte reactions (MLR). We compared the ability of mature PUVA-treated DCs to induce allogeneic T cell proliferation to that of mature untreated DCs in a MLR test. As shown in Fig. 4, both treated and untreated DCs were effective stimulators compared with control cultures of unstimulated responder T cells (P < 0·05). The activity of control DC stimulator cells in each of three independent experiments was slightly higher than that of PUVA-treated DCs derived from the same donors (S phase: 15·2% ± 1·05% versus 11·5% ± 0·9% in control and PUVA cultures, respectively; P = 0·05).
Fig. 4.
The mixed leucocyte reaction (MLR) stimulatory capacity of control dendritic cells (DCs) versus photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A radiation (PUVA)-treated DCs. Mature DCs were washed extensively and added in graded doses to allogeneic responder T cells (2 × 105 cells/well) in 96-well round-bottomed microtest plates. Each group was performed in triplicate. On day 6, cells were fixed in 70% ethanol, stained with propidium iodide (10 mg/l) and processed in a flow cytometer (Epics-XL, Coulter), followed by estimating the S-phase distribution of cell cycle. Distribution of S-phase of each group: control DC stimulator cells (a), DC stimulator cells derived from co-cultures of PUVA-exposed CD14+ cells with untreated monocyte (ratio 10 : 90; see Material and methods) (b) and responder T cells only (c). The percentage of T cells in S phase is shown. Representative data are shown through one of four independent experiments with different donors.
With both stimulators the maximal response was observed at a higher DC/T ratio (1 : 10).
Discussion
Photopheresis is a cell therapy used in pathological conditions in which immune mechanisms are considered contributory to their pathogenesis [14–16,18,19,26–28]. Despite the fact that this cell therapy is now used widely, the mechanisms of its action remain characterized inadequately. The enigma of ECP therapy is how the damage to a small proportion of the total circulating leucocytes induces a distant response in untreated cells; cutaneous and haematological responses could be induced by monthly exposure of less than 10% of peripheral blood leucocytes to 8-MOP and UVA [13,29]. Several mechanisms have been proposed to explain the action of ECP, the most widely explored being the induction of apoptosis within the lymphocyte population, including malignant CTCL cells [22,25–32]. However, the effect of ECP is probably not based solely on malignant or pathogenic apoptosis, as only a fraction of these cells are treated. It has been suggested that monocytes are resistant to apoptotic effects induced by ECP [29,33,34] and are activated during leukopheresis. It has been suggested that during the leukapheresis step of the ECP procedure, cell environment changes (such as transient plastic-surface adherence, temperature in ECP device, reinfusion, cell-to-cell interaction, etc.) could increase monocyte activation, as demonstrated by increased cytokine release [IL-1β, IL-6, IL-10 and especially tumour necrosis factor (TNF)-α] [19,22,35–37], and possibly induce differentiation into dendritic cells [38].
Recent works have been focused on the effects of photopheresis on DC biology, due to their crucial role in initiating specific immune responses [8,11–13,17,18,22,39,40].
It has been suggested that ECP-induced monocyte-to-DC differentiation produces cells with the capacity to stimulate an anti-tumour T cell response (as in CTCL), yet leads to down-regulation of the activity of the T cell clones (as in the treatment of organ transplant rejection, GVHD and autoimmune diseases). The immunomodulatory effects of ECP have been explored more extensively in the context of CTCL. Berger et al. [12] demonstrated that ECP-induced DCs can phagocytose apoptotic T cells actively and present tumour antigen loaded onto MHC class I molecules capable of stimulating a potent anti-tumour cytolitic CD8+ T cell response.
The immunological mechanisms of ECP in GVHD and in autoimmune diseases are still debated. In addition to lymphocyte apoptosis and DC induction, the clinical response to ECP in patients with chronic GVHD has been associated with an increase in natural killer cells, induction of the regulatory T cell subset and a change in the cytokine profile [8,22,39–42]. Furthermore, Plumas et al. [13] have discussed these experimental results, suggesting that additional studies must be carried out in order to know whether DCs could be involved in the control of GVHD after ECP.
The seemingly opposite effects of ECP (stimulatory and tolerogenic) have not been explained fully. It is known that DC functional properties depend on different elements such as maturational state, stimulating modalities (co-stimulatory molecules, cytokine environment) and therapy used in the different clinical conditions. All these factors must be taken into account when considering the type of immune response to ECP in any single disease and to explain the possible switching between tolerance and immunity. The fact that different clinical response times of ECP treatment resulting in CTCL after 2–3 months and in allograft rejection after only a few days [21] suggests that multiple mechanisms of action may be applicable.
In contrast to literature reports, in our culture system monocytes were sensitive to PUVA apoptotic effects. Thus, we were unable to examine the in vitro effects of 8-MOP and UVA light treatment directly on monocyte-to-DC differentiation [25].
Taking account of the fact that the total number of circulating leucocytes treated ex vivo per cycle of ECP has been estimated as between 5% and 10%, we designed a culture system in which 10% of PUVA-treated monocytes were co-cultured with untreated monocytes.
As reported previously, ECP might promote differentiation of monocytes into immature DCs [11–13]. We used the above-described experimental design to investigate whether 8-MOP and UVA light exposure, in the absence of differentiating cytokines, might induce monocyte-to-DC differentiation. We have demonstrated that the presence of 10% apoptotic cells and the monocyte activation by plastic adherence were not sufficient to induce monocyte differentiation to DCs.
To investigate whether IL-4 and GM-CSF were a requisite for DC generation, we added these cytokines to our experimental system. Under these conditions, we showed that PUVA treatment did not influence monocyte-to-DC differentiation. Interestingly, immature DCs generated in the presence of 10% PUVA-treated cells were characterized by a significantly higher phagocytic activity with respect to untreated DCs. This finding is consistent with the recent data from Spisek et al. [8], showing the presence of an immature state of DCs after ECP.
Immature PUVA-treated DCs also displayed a higher expression of MHC class II molecules. Despite the increase in endocytic activity DCs retained their capacity to maturate following LPS stimulation, as shown by the increase in co-stimulatory molecules and CD83 expression. However, mature PUVA-treated DCs showed a lower ability to induce T cell proliferation with respect to control DCs. As reported by Piemonti et al., the higher MHC expression on membrane before maturation may result in a lower disposability of MHC molecules and a lower capacity to process and form peptide–MHC complexes in the intracellular space [43]. An increase in antigen uptake activity may result in higher antigen presentation if DCs process antigen effectively and present peptide in the context of MHC molecules [43]. The simultaneous increase in antigen uptake activity and MHC molecules expression in our system may induce a reduction in the capacity to induce T cell proliferation.
In summary, we have found that while PUVA treatment kills even APCs, the presence of 10% PUVA-treated cells does not inhibit DC generation from monocytes but does induce DCs with a tolerogenic phenotype and function.
Our report, designed in the absence of any concomitant therapy, contributes to greater knowledge of the mode of action of 8-MOP and UVA light on dendritic cell biology. This mechanism can be useful for the generation of immature tolerogenic DCs in vitro. Further studies are needed to elucidate the role of 8-MOP and UVA light on DCs in the context of an assay performed in the presence of immunosuppressive agents in order to dissect the effects of ECP from those of immunosuppressive drugs.
Our work might also provide a rational basis for the selection of UVA light-based therapies in different clinical settings.
Acknowledgments
This study was supported by a grant from Interdepartmental Projects, University of Pisa, Italy, 2003. We would like to thank the Blood Transfusion Center and in particular Dr Cecilia Pardi at Cisanello Hospital (Pisa, Italy) for organizing blood volunteers, and the volunteers who provided blood specimens. We would also like to thank Mr Bruno Fiori for his assistance with the immunohistochemistry and optic microscopy. We are grateful to Dr Gino Malvaldi (Department of Experimental Pathology, University of Pisa) for his critical comments on this manuscript.
References
- 1.Ardavin C, Amigorena S, Reis e Sousa C. Dendritic cells: immunobiology and cancer immunotherapy. Immunity. 2004;20:17–23. doi: 10.1016/s1074-7613(03)00352-2. [DOI] [PubMed] [Google Scholar]
- 2.Usharauli D. Dendritic cells and the immunity/tolerance decision. Med Hypoth. 2005;64:112–13. doi: 10.1016/j.mehy.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 3.Xiao BG, Huang YM, Link H. Dendritic cell vaccine design: strategies for eliciting peripheral tolerance as therapy of autoimmune diseases. Biodrugs. 2003;17:103–11. doi: 10.2165/00063030-200317020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Schlichting CL, Schareck WD, Nickel T, Weis M. Dendritic cells as pharmacological targets for the generation of regulatory immunosuppressive effectors. New implications for allo-transplantation. Curr Med Chem. 2005;12:1921–30. doi: 10.2174/0929867054546627. [DOI] [PubMed] [Google Scholar]
- 5.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of T-cells. J Immunol. 1999;163:1817–26. [PubMed] [Google Scholar]
- 7.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 8.Spisek R, Gasova Z, Bartunkova J. Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease. Transfusion. 2006;46:55–65. doi: 10.1111/j.1537-2995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Kagamu H, Yoshizawa H, et al. The duration of signaling through CD40 directs biological ability of dendritic cells to induce antitumor immunity. J Immunol. 2003;171:5828–36. doi: 10.4049/jimmunol.171.11.5828. [DOI] [PubMed] [Google Scholar]
- 10.Coates PT, Colvin BL, Hackstein H, Thomson AW. Manipulation of dendritic cells as an approach to improved outcomes in transplantation. Exp Rev Mol Med. 2002;18:1–21. doi: 10.1017/S1462399402004283. [DOI] [PubMed] [Google Scholar]
- 11.Edelson RL. Cutaneous T cell lymphoma: the helping hand of dendritic cells. Ann NY Acad Sci. 2001;941:1–11. [PubMed] [Google Scholar]
- 12.Girardi M, Berger CL, Wilson LD, et al. Transimmunization for cutaneous T cell lymphoma: a phase I study. Leuk Lymph. 2006;47:1495–503. doi: 10.1080/10428190600581419. [DOI] [PubMed] [Google Scholar]
- 13.Plumas J, Manches O, Chaperot L. Mechanisms of action of extracorporeal photochemotherapy in the control of GVHD. involvement of dendritic cells. Leukemia. 2003;17:2061–2. doi: 10.1038/sj.leu.2403114. [DOI] [PubMed] [Google Scholar]
- 14.McKenna KE, Whittaker S, Rhodes LE, et al. British Photodermatology Group & UK Skin Lymphoma Group. Evidence-based practice of photopheresis 1987–2001: a report of a workshop of the British Photodermatology Group and the UK Skin Lymphoma Group. Br J Dermatol. 2006;154:7–20. doi: 10.1111/j.1365-2133.2005.06857.x. [DOI] [PubMed] [Google Scholar]
- 15.Heshmati F, Andreu G. for the French Group of Extracorporeal Photochemotherapy. Extracorporeal photochemotherapy: a historical perspective. Tranfus Apher Sci. 2003;28:25–34. doi: 10.1016/S1473-0502(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 16.Duvic M, Chiao N, Talpur R. Extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma. J Cutan Med Surg. 2003;7:3–7. doi: 10.1007/s10227-003-5001-1. [DOI] [PubMed] [Google Scholar]
- 17.Duvic M. Photopheresis versus transimmunization? Leuk Lymph. 2006;47:1449. doi: 10.1080/10428190600604872. [DOI] [PubMed] [Google Scholar]
- 18.Edelson RL. Transimmunization: the science catches up to the clinical success. Transfus Apher Sci. 2002;26:177–80. doi: 10.1016/s1473-0502(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 19.Greinix HT, Socie G, Bacigalupo A, et al. Assessing the potential role of photopheresis in hematopoietic stem cell transplant. Bone Marrow Transplant. 2006;38:265–73. doi: 10.1038/sj.bmt.1705440. [DOI] [PubMed] [Google Scholar]
- 20.Rubegni P, Cuccia A, Sbano P, et al. Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol. 2005;130:271–5. doi: 10.1111/j.1365-2141.2005.05586.x. [DOI] [PubMed] [Google Scholar]
- 21.Dall'Amico R, Murer L. Extracorporeal photochemotherapy: a new therapeutic approach for allograft rejection. Tranfus Apher Sci. 2002;26:197–204. doi: 10.1016/s1473-0502(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 22.Marshall SR. Technology insight: ECP for the treatment of GVHD − can we offer selective immune control without generalized immunosuppression? Nat Clin Pract Oncol. 2006;3:302–14. doi: 10.1038/ncponc0511. [DOI] [PubMed] [Google Scholar]
- 23.Urbani L, Mazzoni A, Catalano G, et al. The use of extracorporeal photopheresis for allograft rejection in liver transplant patients. Transplant Proc. 2004;36:3068–70. doi: 10.1016/j.transproceed.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 24.Kumlien G, Genberg H, Shanwell A, Tyden G. Photopheresis for the treatment of refractory renal graft rejection. Transplantation. 2005;79:123–5. doi: 10.1097/01.tp.0000147197.24050.61. [DOI] [PubMed] [Google Scholar]
- 25.Legitimo A, Consolini R, Di Stefano R, Bencivelli W, Mosca F. Psoralen and UVA light: an in vitro investigation of multiple immunological mechanisms underlying the immunosuppression induction in allograft rejection. Blood Cells Mol Dis. 2002;29:24–34. doi: 10.1006/bcmd.2002.0533. [DOI] [PubMed] [Google Scholar]
- 26.Knobler RM, French LE, Kim Y, et al. A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol. 2006;54:793–9. doi: 10.1016/j.jaad.2005.11.1091. [DOI] [PubMed] [Google Scholar]
- 27.Martelli AM, Cappellini A, Tazzari PL, et al. Caspase-9 is the upstream caspase activated by 8-methoxypsoralen and ultraviolet-A radiation treatment of Jurkat T leukemia cells and normal T lymphocytes. Haematologica. 2004;89:471–9. [PubMed] [Google Scholar]
- 28.Bladon J, Taylor PC. Extracorporeal photochemotherapy induces apoptosis in the lymphocytes of cutaneous T-cell lymphoma and graft-versus-host disease patients. Br J Haematol. 1999;107:707–11. doi: 10.1046/j.1365-2141.1999.01773.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolnicka-Glubisz A, Rijnkels JM, Sarna T, Beijersbergen vanHenegouwen GM. Apoptosis in leukocytes induced by UVA in the presence of 8-methoxypsoralen, chlorpromazine or 4,6,4′-trimethylangelicin. J Photochem Photobiol B. 2002;68:65–72. doi: 10.1016/s1011-1344(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 30.Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction by ultraviolet light A and photochemotherapy in cutaneous T-cell lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107:235–42. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- 31.Legitimo A, Consolini R, Di Stefano R, Bencivelli W, Calleri A, Mosca F. Evaluation of the immunomodulatory effects of photochemotherapy in transplantation. Transplant Proc. 2001;33:2266–8. doi: 10.1016/s0041-1345(01)01985-6. [DOI] [PubMed] [Google Scholar]
- 32.Bladon J, Taylor PC. Extracorporeal photopheresis in cutaneous T-cell lymphoma and graft-versus-host disease induces both immediate and progressive apoptotic processes. Br J Dermatol. 2002;146:59–68. doi: 10.1046/j.1365-2133.2002.04560.x. [DOI] [PubMed] [Google Scholar]
- 33.Bladon J, Taylor PC. Treatment of cutaneous T cell lymphoma with extracorporeal photopheresis induces Fas-ligand expression on treated T cells, but does not suppress the expression of co-stimulatory molecules on monocytes. J Photochem Photobiol B. 2003;69:129–38. doi: 10.1016/s1011-1344(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 34.Bladon J, Taylor PC. Extracorporeal photopheresis differentially regulates the expression of phosphorylated STAT-1 and STAT-5 in treated monocytes and T cells, respectively. J Cutan Med Surg. 2004;8:148–56. doi: 10.1007/s10227-004-0102-z. [DOI] [PubMed] [Google Scholar]
- 35.Craciun LI, Stordeur P, Schandene L, et al. Increased production of interleukin-10 and interleukin-1 receptor antagonist after extracorporeal photochemotherapy in chronic graft-versus-host disease. Transplantation. 2002;74:995–1000. doi: 10.1097/00007890-200210150-00017. [DOI] [PubMed] [Google Scholar]
- 36.Bladon J, Taylor PC. Lymphocytes treated by extracorporeal photopheresis can down-regulate cytokine production in untreated monocytes. Photodermatol Photoimmunol Photomed. 2005;21:293–302. doi: 10.1111/j.1600-0781.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 37.Vowels BR, Cassin M, Boufal MH, Walsh LJ, Rook AH. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992;98:686–92. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- 38.Heshmati F. Mechanisms of action of extracorporeal photochemotherapy. Tranfus Apher Sci. 2003;29:61–70. doi: 10.1016/S1473-0502(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 39.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus host disease. Blood. 2002;100:941–7. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 40.Maeda A, Shwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–76. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 41.Peritt D. Potential mechanisms of photopheresis in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:7–12. doi: 10.1016/j.bbmt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Sabelin K, Shulzki A, Kloetzel PM, Dörken B, Pezzuto A, Subklewe M. Impairment of circulating myeloid dendritic cells in immunosuppressed renal/pancreas transplant recipients. Transplantation. 2006;82:779–87. doi: 10.1097/01.tp.0000235741.96013.08. [DOI] [PubMed] [Google Scholar]
- 43.Piemonti L, Monti P, Allavena P, Leone BE, Caputo A, Di Carlo V. Glucocorticoids increase the endocytic activity of human dendritic cells. Int Immunol. 1999;11:1519–26. doi: 10.1093/intimm/11.9.1519. [DOI] [PubMed] [Google Scholar]