Abstract
Most of the studies on 60-kDa and 10-kDa chlamydial heat shock proteins (HSPs) to date have been carried out with blood lymphocytes or serum antibody responses, which do not provide a clear picture of the actual pathogenesis as they do not differentiate primary infection from recurrent infection. Thus, in the present study induction of the immune response was evaluated by studying lymphoproliferation of both cervical and peripheral lymphocytes to synthetic peptides of cHSP60, cHSP10 and major outer membrane protein (MOMP) antigen. In addition, cervical antibody prevalence to MOMP antigen, cHSP60 and cHSP10 and cytokine levels in cervical washes was also determined. Positive proliferative responses of cervical lymphocytes to cHSP10 peptide were significantly higher (P < 0·05) in women with recurrent infections and that to MOMP antigen were significantly higher in primary infection. On proliferation of PBMCs with the above antigens, no significant difference was observed between primary and recurrent infection. Prevalence of cervical IgG and IgA antibodies to Chlamydia trachomatis was significantly higher (P < 0·05) during primary infection than recurrent infections. In contrast, prevalence of IgG and IgA antibodies to cHSP10 and IgG antibodies to cHSP60 was higher during recurrent infections than primary infections. Interferon (IFN)-γ levels were significantly higher in cervical washes of women with recurrent infection and correlated strongly with cHSP60 antibody titres. Our data thus suggest that mucosal responses are more appropriate in understanding the pathogenesis of chlamydial infection and IFN-γ could be involved in the modulation of immune responses towards chlamydial infection directly, by causing acute inflammation, or indirectly through modulation of HSP expression.
Keywords: Chlamydia trachomatis, chlamydial heat shock proteins, interferon-gamma, mucosal immune response, recurrent infection
Introduction
Chlamydia trachomatis infections are the most prevalent sexually transmitted bacterial infections recognized throughout the world, and 90 million new chlamydial infections are detected annually worldwide [1]. In India alone, a high chlamydial prevalence rate (up to 30%) has been reported among symptomatic women [2]. Chlamydial infection of the lower genital tract infection usually spreads to the upper genital tract and is then responsible for more serious consequences of chlamydial infection, such as infertility, ectopic pregnancy, pelvic pain and pelvic inflammatory disease (PID) [3]. In addition, infection with C. trachomatis facilitates the transmission of HIV [4] and might be a co-factor in human papilloma virus (HPV)-induced cervical neoplasia [5,6]. Chlamydial infections are often asymptomatic, mild infections that are usually self-limiting, but repeated or persistent infections can cause severe damage to the inflamed tissue [7].
Heat shock proteins (HSPs) are highly conserved proteins present in almost all prokaryotic and eukaryotic organisms. They are members of a family of stress response proteins, which protects the cells from a variety of insults [8]. A number of infectious diseases have been associated with activated humoral and cellular responses to microbial HSPs [9]. The chlamydial 60-kDa and 10-kDa HSPs (cHSP60 and cHSP10) are thought to be major target antigens which stimulate a strong pathogenic inflammatory response [10] in both animal models and among patients with chlamydial genital tract infections.
A strong association of serum cHSP60 antibodies with tubal factor infertility has been demonstrated [11]. Women with a history of multiple episodes of salpingitis have been found to exhibit lymphocyte proliferation in response to cHSP60 more frequently than healthy women or women with a history of a single episode of salpingitis [12]. A specific role for cHSP60 in the pathogenesis of salpingitis has also been suggested by an experimental monkey model of infection [13]. Recently, a study in Cameroon demonstrated a significant correlation between anti-cHSP10 and anti-cHSP60 antibodies with secondary infertility [14].
The above data support the idea that cHSPs are important in deciding the immune response of the host towards chlamydial infection, and to date most studies demonstrating the role of cHSPs in pathogenesis of chlamydial infection have been performed primarily with serum antibodies or blood lymphocyte responses. Thus, our first objective was to characterize and compare mucosal and peripheral immune responses to cHSPs in women with either primary chlamydial infection or recurrent infections. The second aim was to determine the role of cytokines in modulation of immune responses towards cHSPs. The study was also aimed at defining local mucosal immune markers, which could help in identifying women with increased risk for development of sequelae to infection.
Materials and methods
Study population
After obtaining informed written consent, 362 patients attending the gynaecology outpatient department of Safdarjung Hospital, New Delhi, India were enrolled into the study. All women underwent careful pelvic examination. Forty-five healthy age-matched controls attending the family planning department for birth control measures and with no previous history of any sexually transmitted disease (STD) were also enrolled. Patients with positive urine pregnancy test, recent antibiotic therapy and history of previously treated STD infection were excluded from the study. Because variations in sex hormones are known to influence cytokine concentrations and immune cell populations, cervical samples were collected during mid-cycle (median: 13 days, range: days 9–15 of the menstrual cycle). None of the patients had had sexual intercourse 3 days or more prior to collection of sample. The study received approval from the hospital's ethics review committee.
Collection of samples
The vulva was examined for lesions and the cervix for warts, ulcers, ectopy, erythema and discharge, if any. After cleaning the endocervix with a sterile cotton swab, three endocervical swabs (HiMedia, Mumbai, India) were collected from patients and controls for diagnosis of C. trachomatis and other sexually transmitted pathogens. An additional vaginal cotton swab was collected for screening of pathogens such as Candida spp., bacterial vaginosis and Trichomonas vaginalis.
For collection of cervical cells, a cytobrush was placed within the endocervical canal so that the cells from the endocervical region and the zone between the endocervical and ectocervical region (transformation zone) could be obtained. The cytobrush was then transferred to a sterile centrifuge tube containing sterile phosphate-buffered saline (PBS) (pH 7·2) supplemented with 100 U penicillin/ml, 100 µg streptomycin/ml and 100 µg glutamine/ml. All cytobrush samples had negative results for blood contamination. Cervical washes were collected in 5 ml of sterile saline administered through a sterile Pasteur pipette and recovered after washing the cervix thoroughly and were then stored at 4°C until they were transported to the laboratory within 1 h. Two ml non-heparinized blood for separating serum and 10 ml venous blood was collected into heparinized vials for isolation and culture of lymphocytes. At the laboratory serum and cervical wash samples were aliquoted and stored at − 80°C until assay and the cells were processed within 1 h.
Microbiology
Five-mm spots were made on clean glass slides using endocervical swabs. These were stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies to C. trachomatis major outer membrane protein (MOMP) using the direct specimen test kit (Microtrak, Palo Alto, CA, USA), according to the manufacturer's instructions. A sample was considered to be positive when at least 10 elementary bodies (EBs) were detected. Samples with greater than one and less than 10 EBs were confirmed for positivity by polymerase chain reaction (PCR) analysis using a primer specific for 517 base pairs (bp) plasmid of C. trachomatis [15].
Gram-stained cervical and vaginal smears were examined for the presence of yeast cells (Candidiasis) and clue cells for diagnosis of bacterial vaginosis. A Gram stain showing the predominance of Lactobacillus morphotype was interpreted as normal. When showing the Gardnerella morphotype or mixed flora they were interpreted as consistent with bacterial vaginosis. Wet mount microscopy was performed for diagnosis of T. vaginalis. For detection of Neisseria gonorrhoeae cervical specimens were incubated at 35°C in a humidified CO2 incubator for 48 h on Thayer Martin medium. Colony growth was noted and N. gonorrhoeae was identified on basis of Gram-stained smears. pleuropneumonia-like organism (PPLO) broth was used for the identification of Mycoplasma hominis and Ureaplasma urealyticum by diluting the cervical samples in arginine-containing and urea-containing liquid media, respectively, thereafter incubating the media at 37°C until there was a colour-change after 5–7 days for M. hominis and 2 days for U. urealyticum.
Lymphoproliferative assay
The tube containing the cytobrush was vortexed before removing the brush and the cells were filtered through a sterile 70-µm nylon cell strainer (Becton Dickinson, San Diego, CA, USA) to make a homogeneous preparation of cells. These were then centrifuged at 200 g for 10 min, the resulting pellet yielding endocervical cells. The population of epithelial cells and lymphocytes in the cytobrush sample was counted with a haemocytometer and samples containing less than 1 million lymphocytes/ml were excluded.
Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque density gradient centrifugation. The endocervical cells and PBMCs were then washed three times with Hank's balanced salt solution (Sigma, St Louis, MO, USA) and suspended in RPMI-1640 medium (Sigma) supplemented with 10% heat-inactivated human AB serum for lymphoproliferation assay. Briefly, the cells were cultured in triplicate in round-bottomed 96-well plates (5 × 104 cells/well) with or without antigen in a total volume of 200 µl. Cultures were incubated in humidified 5% CO2 at 37°C for 6 days. Synthetic biotinylated peptides corresponding to epitopes of MOMP antigen (VLGTSMAEFISTNVIS) and C. trachomatis HSP60 and HSP10 (Techno Concept, New Delhi, India) were used as antigens at a protein concentration of 2·5 µg/ml. The peptides corresponded to amino acids 151–162 (SANNDAEIGNLI) for cHSP60 and 79–87 (SGQELTVEG) for cHSP10 [16,17]. Phytohaemaglutinin (Sigma, 1 µg/ml) was used as a positive control mitogen in each experiment. Optimum concentrations of antigens and mitogen were determined in preliminary experiments as minimum concentrations giving maximum proliferations. [3H]Tritiated thymidine (Bhabha Atomic Research Centre, Mumbai, India) was added to the cultures before the last 18 h of incubation. Proliferative responses were measured as counts per minute (cpm) of incorporated radioactivity using a liquid scintillation counter (Packard Biosciences, Downers Grove, NE, USA). Results were expressed as stimulation indices (SI = mean cpm in the presence of antigen divided by mean cpm in its absence). A SI value > 2 was considered a positive response. After 6 days supernatants were collected after pelleting down the cells and stored for detection of antibodies against cHSP60 and cHSP10.
Flow cytometry
Quantification of different T cell subsets and B cells in the samples was performed by standard flow cytometric technology. Endocervical cells obtained were stained with FITC-conjugated anti-CD4 and CD19 antibodies, and phycoerythrin (PE)-conjugated CD8 (Becton Dickinson, San Jose, CA, USA) for 25 min on ice. Preparations were then washed with buffer [PBS supplemented with 0·1% NaN3 and 2% fetal bovine serum (FBS)] and acquired using a fluorescence activated cell sorter (FACS) (FACSCaliber; BD Biosciences, San Jose, CA, USA). A total of 10 000 events were acquired. Appropriate isotype-matched control antibodies were used to rule out non-specific fluorescence.
Antibody assays
Sera of patients and controls was assayed for IgG antibodies to C. trachomatis surface components by commercially available enzyme-linked immunosorbent assay (ELISA) kit (Ridascreen, AG, Darmstadt, Germany), according to the manufacturer's instructions. Results were obtained as mean absorbance of duplicated samples at 450 nm. An optical density (OD) > 1·1 was considered positive.
Cervical washes of patients and controls were assayed for presence of IgG and IgA antibodies to synthetic peptides for cHSP60, cHSP10 and MOMP antigen. Briefly, the peptides were bound to the wells of a microtitre plate (2·5 µg/well) in carbonate buffer (14·2 mM Na2CO3, 34·9 mM NaHCO3, 3·1 mM NaN3, pH 9·5) and were incubated overnight at 4°C. After washing the unbound peptides the non-specific binding sites were blocked with phosphate-buffered saline (PBS)−0·5% bovine serum albumin (BSA) at 37°C for 60 min; 100 µl of cervical wash was then added, and after incubation at 37°C for 120 min 100 µl of 1 : 10 000 dilution of peroxidase-conjugated goat antibody to human IgA and IgG (Jackson Immunoresearch, Baltimore, MD, USA) was added to each well. After further incubation of 60 min at 37°C, the peroxidase substrate tetramethylene benzidine was added. The reaction was stopped with 0·5 M H2SO4 and the plates were read at 450 nm. Known positive and negative controls (cervical washes of known C. trachomatis-positive and -negative women) were always assayed in parallel to test samples. A positive sample was defined as one yielding an OD value that was at least 2 standard deviations (SD) above the mean value of known negative samples.
Quantification of cytokines
Quantification of interleukin (IL)-1β, IL-6, interferon (IFN)-γ and IL-10 in cervical washes was performed by commercially available ELISA kits (Ebiosciences, San Diego, CA, USA), in accordance with the manufacturer's instructions. The absorbances were read at 450 nm, log–log standard curves were generated and unknowns were interpolated.
Statistical analysis
The Kruskal–Wallis non-parametric test was used to compare continuous variables among multiple groups. The Mann–Whitney U-test was used for comparing two groups. Categorical variables were compared using the χ2 test. Correlation was tested with Spearman's correlation coefficient.
Results
Study population
Cervical C. trachomatis infection was diagnosed by direct fluorescent assay/PCR in 197 patients. Twenty of these patients were found to be co-infected with either Candida sp., bacterial vaginosis, T. vaginalis, M. hominis, U. urealyticum or N. gonorrhoeae in the cervix and were thus excluded from the study. Six Chlamydia-positive patients were excluded, as the lymphocyte population in the cervical cells was less than 1 million/ml. Based on the clinical history and diagnosis the patients were categorized into two groups. Group I (n = 44) comprised patients without any previous history of chlamydial infections, as confirmed by the absence of IgG antibodies against C. trachomatis in serum of infected women. Group II (n = 81) comprised women having recurrent chlamydial infections and being tested positive for infection on ≥ 2 consecutive visits separated by 6 months. All women positive for chlamydial infection received antibiotic therapy after their each visit. The clinical profile of these patients is summarized in Table 1. The median ages of women with primary or recurrent infections and controls were comparable (27, 29 and 28 years, respectively). Women with recurrent infections have a higher prevalence of multiple spontaneous abortions than controls or women with primary infections, but the difference was non-significant (Table 1).
Table 1.
Characteristics of study population.
| Positive LP response to no (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cervical lymphocytes | PBMCs | |||||||
| Groups | Age, median years (range) | Multiple sp. abortions (%) | MOMP | cHSP10 | cHSP60 | MOMP | cHSP10 | cHSP60 |
| Control (n = 45) | 28 (20–45) | 1 (2) | 4 (9) | 3 (7) | 3 (7) | 5 (11) | 4 (9) | 4 (9) |
| Primary infection (n = 44) | 27 (22–43) | 0 (0) | 13 (30) | 4 (9) | 6 (14) | 17 (38)c | 7 (16) | 6 (14) |
| Recurrent infection (n = 81) | 29 (19–48) | 13 (16)a | 9 (11)d | 21 (26)b | 18 (22) | 20 (25) | 11 (14) | 17 (21) |
Figures in parentheses denote percentage. Major outer membrane protein (MOMP); cHSP: chlamydial heat shock proteins; LP = lymphoproliferative; PBMC: peripheral blood mononuclear cells.
P < 0·05 compared with all others
P = 0·01 compared with all others
P < 0·05 compared to controls
P < 0·05 compared to primary infection group.
T lymphocyte subsets in the cervix of women
The median range of CD4 and CD8 lymphocytes among endocervical leucocytes in women which were included in the study was between 59% and 86% and that of B lymphocytes was between 1% and 4%. The median CD4 percentage in controls was 75% and that of CD8 was 60% among endocervical leucocytes (ratio CD4 : CD8, 1·25 : 1). In women with primary chlamydial infections the median CD4 percentage was 84% and that of CD8 cells was 65% (ratio CD4 : CD8, 1·29 : 1). In women with recurrent infection the median CD4 percentage was 80%, and that of CD8 was 63% (ratio CD4 : CD8, 1·26 : 1). No significant difference was observed in the median percentages of B lymphocytes between the groups.
Lymphocyte proliferative responses to synthetic peptides for MOMP antigen
Cell-mediated immune response was assessed by stimulating local endocervical lymphocytes and PBMCs with synthetic peptides for MOMP antigen. Positive responses (SI > 2) to MOMP were more common in both cervical lymphocytes (CL) and PBMCs of patients with primary infections (Table 1), but was not statistically significant from other groups. No significant difference was observed in median SI of CL or PBMCs to MOMP (CL: controls SI 1·14, range 0·5–1·9; primary infection SI 1·71, range 0·72–6·22; recurrent infection SI 1·4, range 0·7–4·83) and (PBMCs: controls SI 1·21, range 0·71–2·14; primary infection SI 1·89, range 0·73–5·46; recurrent infections SI 1·25, range 0·62–3·96) but the difference was not statistically significant.
Lymphocyte proliferative responses to synthetic peptides of cHSP10
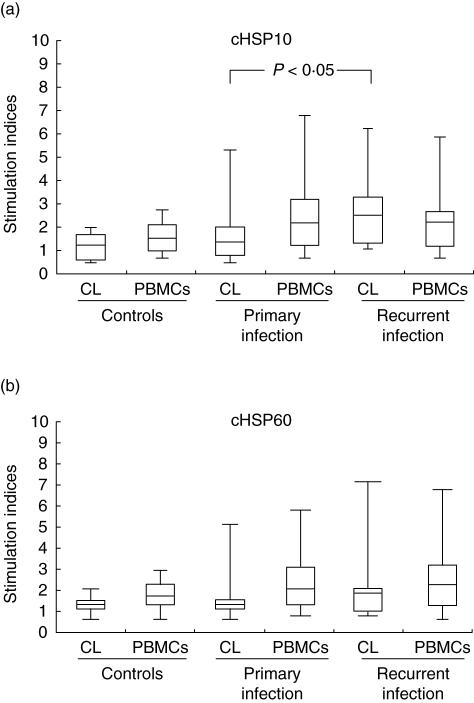
Positive responses (SI > 2) to cHSP10 were more common in patients with recurrent infections (Table 1) and was statistically significant (P < 0·05) from other groups in the case of CLs. No significant difference was observed in median SI of CLs or PBMCs to cHSP10 (CLs: controls SI 1·2, range 0·2–3·9; primary infections SI 1·1, range 0·6–4·4; recurrent infection SI 1·78, range 0·7–8·2) and (PBMCs: controls SI 1·52, range 0·62–2·71; primary infections SI 2·05, range 0·7–6·4; recurrent infections SI 1·9, range 0·79–5·42) (Fig. 1a). No correlation was found between local IgA or IgG antibodies to cHSP10 and proliferative responses of CLs or PBMCs to cHSP10 in any group.
Fig. 1.
Proliferative responses (stimulation index) of cervical lymphocytes and peripheral blood mononuclear cells (PBMCs) to (a) chlamydial heat shock proteins (cHSP)10 and (b) cHSP60 in women with primary or recurrent Chlamydia trachomatis infection and controls. The horizontal line in the middle of the box is the median value of the responses and the lower (upper) 25th (75th) percentile.
Lymphocyte proliferative responses to synthetic peptides of cHSP60
Cell-mediated immune response to synthetic peptides of cHSP60 did not differ significantly between groups in either CLs or PBMCs (CLs: controls median SI 1·4, range 0·8–1·9; primary infections SI 1·4, range 0·6–5·4; recurrent infections SI 1·85, range 0·8–8·43) and (PBMCs: 1·65, range 0·52–2·91; primary infection SI 1·94, range 0·73–5·77; recurrent infections SI 1·98, range 0·51–6·71) (Fig. 1b). Positive responses (SI > 2) to cHSP60 were more numerous in patients with recurrent infections in both CLs and PBMCs (Table 1) but was not statistically different from women with primary infections. A significant positive correlation (r= 0·54; P < 0·01) was found between cervical IgA antibodies to cHSP60 peptide and proliferative responses of CLs of patients with recurrent infections to peptides of cHSP60.
Cervical antibodies to C. trachomatis and heat shock proteins
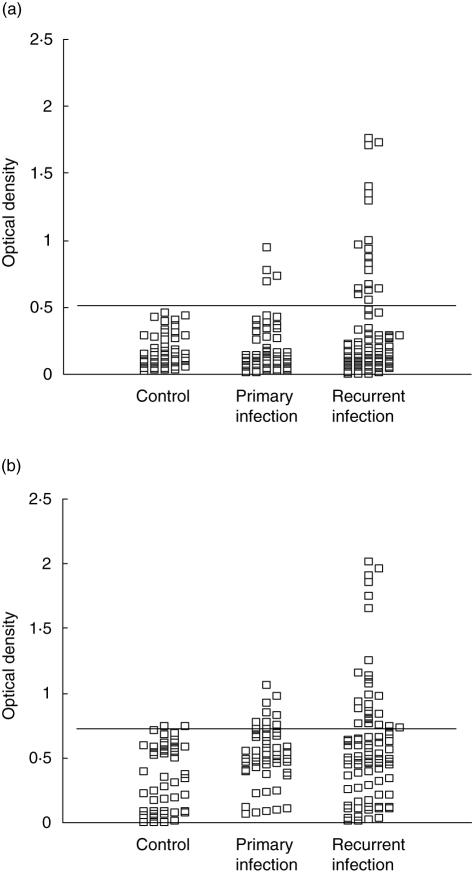
The prevalence of antibodies in cervical washes is shown in Table 2. The prevalence of IgG and IgA antibodies to C. trachomatis MOMP antigen was significantly higher in patients with primary infections than in patients with recurrent infections (P < 0·0001 and P < 0·05). In contrast, the prevalence of IgG and IgA antibodies against peptides of cHSP10 and IgG antibodies to cHSP60 was significantly higher (P < 0·01) in patients with recurrent infections compared to other groups. Individual results for testing for local IgA antibodies to peptides of cHSP60 and cHSP10 are shown in Fig. 2a,b.
Table 2.
Prevalence of antibodies in cervical washes of women.
| Control (n = 45), no (%) | Primary infection (n = 44), no (%) | Recurrent infection (n = 81), no (%) | |
|---|---|---|---|
| Ctr-IgG+ | 3 (7) | 35 (78)a | 19 (23) |
| Ctr-IgA+ | 3 (7) | 23 (52)b | 30 (37) |
| cHSP10 IgG+ | 5 (11) | 9 (20) | 35 (43)c |
| cHSP10 IgA+ | 0 (0) | 4 (9) | 20 (25)d |
| cHSP60 IgG+ | 4 (9) | 11 (25) | 36 (44)e |
| cHSP60 IgA+ | 4 (9) | 12 (27) | 27 (33) |
Ctr = Chlamydia trachomatis
P < 0·0001
P = 0·04
P = 0·0008
P = 0·007
P = 0·004.
P-values are in comparison with both controls and patient groups. cHSP: chlamydial heat shock protein.
Fig. 2.
Immunoglobulin A (IgA) antibodies to synthetic peptides corresponding to epitopes of (a) chlamydial heat shock proteins (cHSP)10 and (b) cHSP60 in cervical washes of women with primary or recurrent infection and controls. The horizontal line indicates the lower boundary of a positive antibody response.
We also evaluated in vitro secretion of antibodies by cervical lymphocytes upon stimulation with peptides for cHSP10 and cHSP60. Only the endocervical lymphocytes obtained from patients with recurrent infections were able to proliferate and secrete IgG antibodies significantly (P < 0·01) over cells obtained from patients with primary infections (data not shown).
Concentration of IL-1β and IFN-γ in cervical washes
The concentrations of IL-1β, IL-6, IFN-γ and IL-10 in cervical washes are given in Table 3. IL-1β levels were higher in recurrent infections than primary infections, but the increase was not significant. IFN-γ levels were significantly higher in recurrent infections than in primary infections (P < 0·01). There was a significant correlation of IFN-γ levels with antibodies to cHSP60 during recurrent infections (r= 0·67; P < 0·0001). No significant difference was observed in the levels of IL-6 and IL-10 in all patient groups.
Table 3.
Cytokine levels in cervical washes of women.
| Control (n = 45) | Primary infection (n = 44) | Recurrent infection (n = 81) | P | |
|---|---|---|---|---|
| IFN-γ median (range; pg/ml) | 109 (10·6–597) | 64 (7·3–324) | 207 (0–500) | < 0·01 |
| IL-6 median (range; pg/ml) | 11·9 (0–127) | 14·6 (0–158) | 8·6 (0–117) | n.s. |
| IL-1β median (range; pg/ml) | 35 (0–350) | 23 (0–164) | 77 (7·6–568) | n.s. |
| IL-10 median (range; pg/ml) | 6·8 (0–16·8) | 8·8 (0–30·9) | 6·9 (0–22·8) | n.s. |
IFN: interferon; IL: interleukin; n.s.: not significant.
Discussion
In women, chlamydial infections are often asymptomatic, and subsequent reinfections lead to inflammatory responses with pathological sequelae [18]. Because all the studies on cHSPs to date have been performed with PBMCs, we also looked for differences in cytokine production between cervical lymphocytes and PBMCs. Cervical cells are the actual cells encountering the pathogen, and their responses would help towards a much better understanding of the immunopathogenesis of chlamydial disease; thus, we evaluated the mucosal immune responses to cHSPs and compared them with peripheral responses during primary and recurrent chlamydial infection. We enrolled healthy controls and C. trachomatis-positive women, as the cells obtained from controls act as naive cells mimicking the primary immune response; cells from C. trachomatis-positive women with primary infections will mimic the secondary immune response, as they are already exposed to the pathogen; and cells obtained from women with recurrent infection will mimic cells which have encountered the antigen a few times previously.
We found that women with recurrent infections have significant humoral and cell-mediated immune responses towards cHSP10 and cHSP60. Our results correlated with previous studies, suggesting that immune sensitization to HSPs probably requires prolonged exposure of them at elevated concentrations [19]. Our results demonstrate that cHSP10 induces proliferation of cervical lymphocytes obtained from women with recurrent infections more significantly than cHSP60, thus showing that its role can be more important than cHSP60 in the pathogenesis of chlamydial infections. cHSP10 is associated with chronic genital tract infection and is homologous to human chaperonin (Cpn10) and early pregnancy factor (EPF), a form of Cpn10 that is secreted especially at the start of pregnancy. It has been reported previously that infertility was associated with the presence of anti-cHSP10 and anti-EPF antibodies in serum [20]. A study by La Verda et al. [17] demonstrated that women with acute infection and tubal factor infertility (TFI) recognized cHSP10 more frequently, with infertile women having greater seroreactivity to cHSP10 than acutely infected women. They also demonstrated that among women with similar exposure to chlamydiae, serological responses were greater to HSP10 in the TFI group than HSP60 or MOMP. As for cHSP60, there have been reports that during repeated and severe C. trachomatis infection there is enhanced recognition of cHSP60 by circulating lymphocytes [12,21] and it has been shown that PBMCs from women with tubal factor infertility responded more frequently to cHSP60 antigen [22]. However, our results show that, although not significant, there was lesser recognition of cHSP10 and more of cHSP60 by PBMCs obtained from women with recurrent infection compared to CLs from the same women, which recognize cHSP10 very strongly. These results suggest that PBMCs and CLs differ in their responses towards cHSPs and CLs begin to recognize cHSPs only after infection has taken place more than once. Thus, studies involving PBMCs may, in fact, not present the true picture of chlamydial pathogenesis.
We also found a higher prevalence of antibodies to cHSP60 and cHSP10 peptides in cervical washes of women with recurrent infection in contrast to antibodies against C. trachomatis surface antigens, which were significantly higher during primary infections. As postulated previously [23], cells infected chronically with C. trachomatis synthesize only low levels of structural components but continue to produce cHSP60 at higher levels. Because bacterial and human HSP share ∼50% amino acid sequence homology [24], it has been proposed that prolonged exposure of the immune system to cHSP60 may lead to autoantibody formation [25]. A high prevalence of cHSP60 IgA has been shown in 20·7% of the patients undergoing in-vitro fertilization (IVF) and none of these women had ever had a recognized chlamydial infection. The presence of this antibody has thus been shown to be considered as reflecting an acute immune response [26]. Our study also reported a higher prevalence of multiple spontaneous abortions among women with recurrent infections. As postulated previously [27], the presence of cervical IgA antibodies to the immunodominant epitope of cHSP60 corresponding to 260–271 amino acids correlated with embryo loss after transient implantation of embryos in the uterus of women undergoing IVF. Another study by Witkin et al. [27] implied that cervical IgA antibody to conserved HSP60 epitopes and the failure of successful implantation after embryo transfer is interrelated. We also found a significant prevalence of IgA cSHP10 antibodies along with cHSP60 antibodies in women with recurrent chlamydial infections, and these results are in concordance with previous studies that demonstrated that cHSP10 is co-expressed with cHSP60 [28].
Earlier studies in our laboratory have also reported increased levels of IFN-γ in infertile women infected with C. trachomatis[29]. It has been reported previously that under conditions of IFN-γ-mediated deprivation of tryptophan, a decrease in MOMP and 60-kDa OMP expression was observed, but the synthesis of cHSP60 is maintained as it contains no tryptophan residues, as deduced from the nucleotide sequence [30]. Another possibility is that under conditions of stress, mRNA for stress response proteins continues to be transcribed, whereas that for structural proteins is down-regulated. Our results showed higher levels of IFN-γ during recurrent infections and the levels have a significant correlation with cHSP60 antibody titres. As a balance of pro- and anti-inflammatory cytokines is important for immune protection during chlamydial infection, IL-10 levels were studied in cervical washes, but no significant difference was found in the levels between primary and secondary infections. In addition, IL-1β and IL-6, both proinflammatory cytokines, did not show any correlation with the clinical conditions or with the presence of cHSP antibodies. None of these three cytokines was found to have any correlation with either cHSP10 or cHSP60 antibody titres. Thus, it can be suggested that IL-1β, IL-6 and IL-10 may not play a significant role in the clinical outcome of chlamydial infection.
In conclusion, our study reveals that mucosal immune responses towards chlamydial infection are different from those of PBMCs and knowledge of the exact mechanism involved in the pathogenesis can be gained through studies involving cervical lymphocytes and not PBMCs. Among the four cytokines studied, IFN-γ was found to be the only cytokine which could be involved in modulation of the immune responses towards chlamydial infection directly, by causing acute inflammation, or indirectly through modulation of HSPs expression. We also propose that levels of IFN-γ, along with IgA antibody responses to C. trachomatis, cHSP10 and cHSP60 in cervical washes, can be used to screen and identify women who are at increased risk of the development of sequelae, thus helping in their timely treatment.
Acknowledgments
We thank Mrs Madhu Badhwar, Mrs Asha Rani and Mrs Rosamma Thomas for providing technical assistance. This study was supported by a grant from the Defense Research Developmental Organization (DRDO), Government of India (DLS/RD-81/48222/LSRB-51/ID/2003). We also acknowledge the University Grants Commission for providing financial assistance to Tanvi Agrawal in the form of a research fellowship.
References
- 1.Gerbase AC, Rowley JT, Mertens TE. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351(Suppl. 3):S2–4. doi: 10.1016/s0140-6736(98)90001-0. [DOI] [PubMed] [Google Scholar]
- 2.Singh V, Salhan S, Das BC, Mittal A. Predominance of Chlamydia trachomatis serovars associated with urogenital infections in females in New Delhi, India. J Clin Microbiol. 2003;41:2700–2. doi: 10.1128/JCM.41.6.2700-2702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schachter J. Chlamydial infections. N Engl J Med. 1978;298:428–35. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 4.Plummer FA. Cofactors in male–female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–9. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 5.Anttila T. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Gopalkrishna V, Aggarwal N, Malhotra VL, et al. Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions. Clin Microbiol Infect. 2000;6:88–93. doi: 10.1046/j.1469-0691.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- 7.Golden M, Schillinger J, Markowitz L, St Louis M. Duration of untreated genital infections with Chlamydia trachomatis: a review of the literature. Sex Transm Dis. 2000;27:329–7. doi: 10.1097/00007435-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 9.Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerrone MC, Ma JJ, Stephens RS. Cloning and sequence of gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of protein. Infect Immun. 1991;59:79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartog JE, Land JA, Stassen FRM, Kessels AGH, Bruggeman CA. Serological markers of persistent C. trachomatis infections in women with tubal factor subfertility. Hum Reprod. 2005;20:986–90. doi: 10.1093/humrep/deh710. [DOI] [PubMed] [Google Scholar]
- 12.Witkin SS, Jeremias J, Toth M, Ledger WJ. Proliferative response to conserved epitopes of Chlamydia trachomatis and human 60-kilodalton heat-shock proteins by lymphocytes from women with salpingitis. Am J Obstet Gynecol. 1994;171:455–60. doi: 10.1016/0002-9378(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 13.Patton DL, Sweeney YT, Kuo CC. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathologic mechanism of tubal damage. J Infect Dis. 1994;169:680–3. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 14.Dadamessi I, Eb F, Betsou F. Combined detection of Chlamydia trachomatis specific antibodies against the 10 and 60-kDa heat shock proteins as a diagnostic tool for tubal factor infertility: results from a case–control study in Cameroon. FEMS Immunol Med Microbiol. 2005;45:31–5. doi: 10.1016/j.femsim.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Singh V, Rastogi S, Garg S, et al. Polymerase chain reaction for detection of endocervical Chlamydia trachomatis infection in Indian women attending gynaecology outpatient department. Acta Cytol. 2002;46:540–4. doi: 10.1159/000326874. [DOI] [PubMed] [Google Scholar]
- 16.Yi Y, Zhong G, Brunham RC. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect Immun. 1993;61:1117–20. doi: 10.1128/iai.61.3.1117-1120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Verda D, Byrne GI. Use of monoclonal antibodies to facilitates identification, cloning and purification of Chlamydia trachomatis hsp10. J Clin Microbiol. 1997;35:1209–15. doi: 10.1128/jcm.35.5.1209-1215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RL, Holand MJ, Whittle HC, Mabey DC. Subject recovering from human ocular chlamydial infection have enhance lymphoproliferative responses to chlamydial antigens compare with those of persistently diseased controls. Infect Immun. 1995;63:389–92. doi: 10.1128/iai.63.2.389-392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkin SS, Askienazy-Elbhar M, Henry-Suchet J, Belaisch-Allart J, Tort-Grumbach J, Sarjdine K. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60 kDa heat shock protein (hsp60) in infertile couples and its relationship to antibodies to C. trachomatis surface antigens and the Escherichia coli and human HSP60. Hum Repod. 1998;13:1175–9. doi: 10.1093/humrep/13.5.1175. [DOI] [PubMed] [Google Scholar]
- 20.Betsou F, Borrego MJ, Guillaume N, et al. Cross reactivity between Chlamydia trachomatis heat shock protein 10 and early pregnancy factor. Clin Diagn Lab Immunol. 2003;3:446–50. doi: 10.1128/CDLI.10.3.446-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnunen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis heat shock protein 60 induced interferon-gamma and interleukin 10 production in infertile women. Clin Exp Immunol. 2003;131:299–303. doi: 10.1046/j.1365-2249.2003.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witkin SS, Jeremias J, Toth M, Ledger WJ. Cell mediated immune response to the recombinant 57-kDa heat shock protein of Chlamydia trachomatis in women with salpingitis. J Infect Dis. 1993;167:1379–83. doi: 10.1093/infdis/167.6.1379. [DOI] [PubMed] [Google Scholar]
- 23.Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon-γ mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinnik TM. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Topics Microbiol Immunol. 1991;167:145–60. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- 25.Witkin SS, Neuer A, Giraldo P, et al. Chlamydia trachomatis infection, immunity and pregnancy outcome. Infect Dis Obstet Gynecol. 1997;5:128–32. doi: 10.1155/S1064744997000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witkin SS, Sultan KM, Neal GS, Jeremias J, Grifo JA, Rosenwaks Z. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol. 1994;171:1208–14. doi: 10.1016/0002-9378(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 27.Witkin SS, Jeremias J, Neuer A, et al. Immune recognition of the 60kD heat shock protein: implications for subsequent fertility. Infect Dis Obstet Gynecol. 1996;4:152–8. doi: 10.1155/S1064744996000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spandorfer SD, Neuer A, LaVerda D, et al. Previously undetected Chlamydia trachomatis infection, immunity to heat shock proteins and tubal occlusion in women undergoing in vitro fertilization. Hum Reprod. 1999;14:62–4. doi: 10.1093/humrep/14.1.60. [DOI] [PubMed] [Google Scholar]
- 29.Reddy BS, Rastogi S, Verma S, Das B, Salhan S, Mittal A. Cytokine expression pattern in the genital tract of C. trachomatis women − implication for T cell responses. Clin Exp Immunol. 2004;37:552–8. doi: 10.1111/j.1365-2249.2004.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison RP, Su H, Lyng K, Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groEL stress response of Escherichia coli. Infect Immun. 1990;58:2701–5. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]