Abstract
CD1d-restricted invariant natural killer T (iNK T) cells activated by their experimental ligand α-galactosylceramide (α-GC) can produce both T helper 1 (Th1) and Th2 cytokines and display regulatory functions. Recent studies identified CD4+ and CD4– CD8– double-negative (DN) iNK T cells as the two major components of the human population and suggest that they display a Th2 and a Th1 profile, respectively. We compared the Th2-promoting activity of freshly isolated human CD4+ and DN iNK T cells in terms of their capacity to induce Ig production by autologous B cells. Secretion of IgG and IgE but not IgM was enhanced by the CD4+ T cell subset (including iNK T cells) but not by its DN counterpart. iNK T cells were directly responsible for this pro-Th2 effect, as demonstrated by the requirement for both α-GC stimulation and CD1d presentation, as well as by its disappearance upon iNK T cell depletion. Interaction with iNK T cells led to progressive accumulation of isotype-switched and activated B cells. Myeloid dendritic cells (DC) completely block the induction of Ig production in co-culture. This dominant inhibitory effect of myeloid DC was concomitant with a specific loss of interleukin (IL)-4 production by CD4+ iNK T but not by conventional T cells. These data support the conclusion that, conversely to the interferon (IFN)-γ-producing DN human iNK T cell population, interleukin (IL)-4-producing CD4+ iNK T cells can activate and help B cells to produce both IgG and IgE through a CD1d-dependent mechanism, in keeping with a functional Th1/Th2 dichotomy between these subsets.
Keywords: B cells, dendritic cells, human studies, immunoglobulins, iNK T cells
Introduction
Invariant natural killer T (iNK T) cells are a distinct subpopulation of α/β T cells, which co-express activated/memory and NK markers and appear to be involved in several disease conditions [1–3]. They express a conserved canonical T cell receptor (TCR) (Vα14Jα18-Vβ8 in the mouse and Vα24Jα18-Vβ11 in the human), and are restricted by the major histocompatibility complex (MHC) class I-like antigen-presenting molecule CD1d [4,5]. Most mouse Vα14+ and human Vα24+ CD1d-restricted T cells have been found to respond strongly to CD1d-mediated presentation of α-galactosylceramide (α-GC), an unusual glycosphingolipid derived from a marine sponge [6,7]. Notwithstanding the recent identification of natural antigens recognized by iNK T cells [8–10], α-GC is used usually to achieve in vitro expansion of iNK T cells from peripheral blood mononuclear cells (PBMC). In contrast to conventional T helper 1 (Th1) and Th2 cells, iNK T cells show no restricted cytokine production profile and generate both Th1 and Th2 cytokines after stimulation by anti-TCR αβ or α-GC [6,11].
It is generally believed that the capacity to produce Th1 and Th2 cytokines promptly in large amounts upon TCR engagement endows iNK T cells with regulatory functions during the immune response. The implication of iNK T cells in the outcome of various disease conditions such as autoimmune diabetes [12,13], infections [14,15] and tumour growth [16–18] is consistent with this idea. However, it is unclear whether effector functions of iNK T cells influence adaptive immune responses towards a type 1 or type 2 differentiation. Early investigations have suggested that iNK T cells come into play early during the onset of the immune response to produce the interleukin (IL)-4 needed for the development of Th2 response. Three studies have shown that repeated α-GC injections allowed increased IgE production in the context of a global Th2 switch of the immune response [19–21]. Nevertheless, other investigators reported normal levels of antigen-specific IgE in iNK T-deficient mice [22]. Cui et al. [23] reported that iNK T cells activated by their cognate ligand α-GC could exert a potent inhibitory effect on Th2 cell differentiation and subsequent IgE production by producing large amounts of interferon (IFN)-γ. In the latter case, the authors assigned a major role to myeloid dendritic cells (DC), which are able to polarize iNK T cells towards a Th1 profile [24]. Indeed, this cross-talk between myeloid DC and iNK T cells promoted a Th1 differentiation of conventional T cells [23].
Because most mouse and human B cells express CD1d [25], a relevant question is whether iNK T cells can directly activate B cell functions. Attempts to demonstrate the CD1d-dependent antibody responses have produced conflicting results in mice [19,20,23]. By addressing this question in humans, Galli et al. [26] demonstrated recently that iNK T cells promote proliferation of memory and naive B lymphocytes together with IgG and IgM production while, surprisingly, IgE production is not affected. Whether this finding was due to the use of a panel of iNK T cell clones instead of freshly isolated iNK T cells is still unresolved. In the same line of evidence, it should be stressed that the CD4+ and DN iNK T cell clones generated by these authors did not differ in terms of cytokine production profile. This latter finding contrasts with those of Gumperz et al. [27] and Lee et al. [28], who showed that freshly isolated human CD4+ iNK T cells produce Th2 cytokines, whereas their DN counterpart displays a strict Th1 profile.
In the present study, starting with freshly isolated iNK T cells, we were capable to confirm the Th1/Th2 dichotomy in term of cytokine production between CD4+ and DN iNK T cells. Based on these data, we hypothesized that these phenotypic differences might reflect distinct functional capacities in terms of B cell activation, including the amount and the isotype of the antibodies produced. We thus co-cultured T cell preparations containing either CD4+or DN iNK T cells with B cells loaded with α-GC and evaluated the effect on Ig production. We found that the CD4+ and not the DN iNK T cell subset induced both IgG and IgE production upon exposure to α-GC and further examined whether myeloid DC could control this helper function.
Materials and methods
Preparation of primary human lymphocytes and myeloid DC
Human venous blood samples were drawn from healthy volunteer donors. PBMC were isolated by Ficoll (Biochrom KG, Berlin, Germany) centrifugation and depleted in monocytes prior to further cell purification using anti-CD14-coated magnetic microbeads and VarioMACS® separation columns according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). CD19+ B cells were purified by magnetic cell separation using CD19 microbeads (Miltenyi Biotech) and CD4+ cells were selected positively using CD4-conjugated magnetic microbeads. CD8+ cells were depleted magnetically from remaining cells by CD8 microbeads to obtain DN cells. Cell fractions were > 95% pure by flow cytometry analysis (see below). In some cases, Vα24+ cells were removed from CD4+ cells after staining with anti-Vα24 fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MoAb) followed by anti-FITC microbeads.
Myeloid DC were derived from purified CD14+ monocytes cultured for 72 h in RPMI-1640 Glutamax medium (Gibco, Paisley, Scotland, UK) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Sigma-Aldrich, St Louis, MO, USA), 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco) containing 50 ng/ml granulocyte–macrophage-colony-stimulating factor (GM-CSF) (Schering-Plough, Levallois-Peret, France) and 10 ng/ml IL-4 (Sanofi, Toulouse, France). Maturation of myeloid DC was assessed by cytometric analysis of CD80, CD86, CD11c, CD1c, CD14 and human leucocyte antigen-D related (HLA-DR) expression. Mature myeloid DC were generated by further exposure to 1 µg/ml lipopolysaccharide (LPS) (from Escherichia coli 026:B6, Sigma-Aldrich) or 2·5 µg/ml anti-CD40 MoAb (clone mAb89) for 18 h.
Cell cultures
Co-cultures were performed in humidified 5% CO2 at 37°C in 96-well round-bottomed microplates (Nunc, Roskilde, Denmark), generally at a ratio of 15 × 104 CD4+ or DN cells for 5 × 104 B cells and/or 5 × 104 myeloid DC per well (200 µl final volume), in quadruplicate to sextuplicate, in the presence of IL-2 (10 ng/ml, Sanofi) or IL-7 (35 ng/ml, Sanofi). B cells and myeloid DC were pulsed or not with α-GC (Kirin Brewery, Gunma, Japan, 100 ng/ml) for 18 h and washed by centrifugation with culture medium prior to co-culture. In control experiments, the blocking anti-CD1d MoAb (clone 20H2, 20 µg/ml) was added simultaneously to B cells and/or myeloid DC. In some experiments, co-cultures were performed with purified B cells that were preincubated for 18 h with the superantigen TSST1 (Sigma-Aldrich, 10 ng/ml). Expansion of iNK T and Vβ2+ T cells were measured on day 7. Supernatants were harvested on day 12 and stored at −20°C until assayed for Ig levels.
B cell proliferation was measured using 5(6)-carboxyfluoresceine diacetate succinimidyl ester (CFSE) staining. Briefly, magnetically purified CD19+ cells were incubated for 5 min at 37°C in phosphate-buffered saline (PBS) (Gibco) containing 0·2% heat-inactivated FCS and 0·5 µM CFSE (Molecular Probes, Leiden, the Netherlands) and then washed four times in culture medium. Cells were loaded with α-GC and co-cultured as described previously. After co-culture, B cells were stained and analysed by flow cytometry.
Intracytoplasmic amounts of cytokines in CD4 and DN lymphocytes were determined ex vivo or in vitro after co-culture at a concentration of 5 × 106 cells/ml or 1 × 106 cells/ml, respectively. Cells were cultured for 5 h in the presence of 1 ng/ml phorbol myristate acetate (PMA), 0·5 µg/ml calcium ionophore (all from Sigma-Aldrich) and GolgistopTM (BD Biosciences, San Diego, CA, USA) and analysed by flow cytometry, as described below.
Ig secretion assay
IgE and IgM concentrations in culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA), as described previously [29]. For IgG measurement, goat anti-human γ chain antibody (1 µg/ml, Southern Biotechnology, Birmingham, AL, USA) and peroxidase-conjugated mouse Ig adsorbed goat anti-human γ chain (Caltag, Burlingame, CA, USA) were used as capture and detection antibodies, respectively.
Flow cytometry staining and analysis
Cell surface staining was performed as described elsewhere [30]. Anti-CD4-phycoerythrin (PE), anti-CD4-allophycocyanin, anti-IL-4-PE, anti-IFN-γ-FITC, anti-CD80-PE, anti- CD86-PE, anti-CD11c-PE, anti-CD14-allophycocyanin, anti-HLA-DR-PE, anti-CD19-Cy, anti-CD19-allophycocyanin and anti-CD27-PE were purchased from BD Biosciences, anti-Vα24-FITC, anti-Vβ11-PE and anti-TCRαβ-PE from Beckman Coulter (Marseille, France) and anti-CD1c-FITC from Miltenyi Biotec. For ex vivo experiments, stained cells were fixed in PBS containing 2·5% paraformaldehyde. For in vitro experiments, dead cells were excluded by propidium iodide staining. For intracytoplasmic cytokine determination cells were membrane-labelled, then permeabilized with Cytofix/CytopermTM (BD Biosciences) and washed with Perm/WashTM (BD Biosciences). After staining with the appropriate anti-cytokine MoAb, cells were again washed with Perm/WashTM. Samples were gated on lymphocytes using forward- and side-scatter parameters. Positive staining for each marker was determined by comparison with appropriate isotype-matched negative controls. A fluorescence activated cell sorter (FACS)caliburTM flow cytometer (Becton Dickinson, Mountain View, CA, USA) was used and a minimum of 5 × 104 events gated from viable cells were acquired with CellQuestTM software (Becton Dickinson).
Statistical analysis
Statistical analyses were performed using Student's paired t-test and Wilcoxon's rank tests as appropriate. P-value of less than 0·05 were considered statistically significant.
Results
CD4+ T lymphocytes, but not their DN counterpart, enhance IgE and IgG production by α-GC-loaded B cells
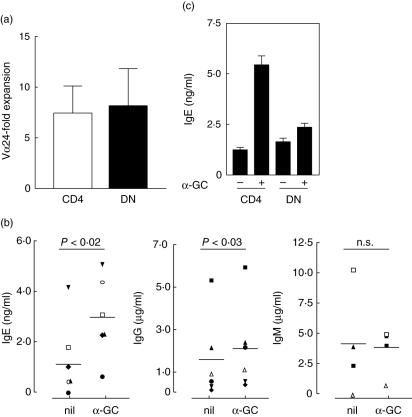
In a first series of experiments, purified B cells were co-cultured with autologous CD4+ or DN cell fractions. Because of the low frequency of iNK T cells in these fractions (0·02–0·8%, as assessed using an association of anti-TCR Vα24 and Vβ11 MoAb), co-cultures were performed in the presence of IL-2 or IL-7, two cytokines that enhance preferentially the expansion of α-GC-stimulated iNK T cells among peripheral blood lymphocytes (PBL) [25,31]. Because human B cells express CD1d [25], we expected that their cross-talk with iNK T cells could be initiated by loading B cells with the cognate ligand of iNK T cells, α-GC. Incubation of CD4+ or DN cells with IL-2 in the presence of autologous α-GC-pulsed B cells resulted within 7 days in a dramatic (approximately 10-fold) increase in the proportion of both CD4+ Vα24+ and DN Vα24+ cells detected (Fig. 1a). In order to avoid interpretation problems because of possible non-iNK T cell proliferation, iNK T cell expansion was calculated using the absolute number of Vα24-positive T cells per well after culture instead of their frequency. A similar expansion occurred upon exposure to IL-7 (data not shown).
Fig. 1.
IgE and IgG production by α-galactosylceramide (α-GC)-loaded B cells is enhanced by CD4+ T lymphocytes but not by their double-negative (DN) counterpart. Purified CD4+ or DN lymphocyte fractions (15 × 104 cells) were co-cultured in medium containing interleukin (IL)-2 with 5 × 104 B cells either non-loaded or loaded with α-GC. (a) Similar fold expansion of DN invariant natural killer T (iNK T) cells and CD4+ iNK T cells in response to α-GC-loaded B cells. Expansion was calculated after a 7-day culture by dividing the number of Vα24-positive T cells in co-cultures with α-GC-loaded B cells by their number in co-cultures with non-loaded B cells. The total number of iNK T cells recovered ranged from 0·75 × 103−25·7 × 103 cells/well for CD4+ fraction (n = 4) and from 0·8 × 103 to 17·5 × 103 cells/well for their DN counterpart (n = 4), depending on their frequency in fresh peripheral blood lymphocytes (which varied considerably between individuals). Data are mean values ± s.e.m. of experiments performed with PBL obtained from four healthy donors. (b) CD4+ lymphocytes promote Ig production by α-GC-loaded B cells. Concentrations of IgM, IgG and IgE in supernatants were determined after a 12-day culture. Data are from experiments performed with cells from four to six healthy donors, each symbol corresponding to one donor; significant differences (P < 0·02 and P < 0·03, respectively, according to Wilcoxon's test) were found for IgE and IgG only. (c) Comparison of IgE levels in supernatants of B cells co-cultured with CD4+ or DN cells in the presence or absence of α-GC. Data are means ± s.e.m. from one typical experiment of three.
We next examined Ig secretion by α-GC-pulsed or not-pulsed B cells after 12 days of co-culture with CD4+ or DN lymphocyte fractions in the presence of IL-2. As shown in Fig. 1b, the mean IgE level in culture supernatants was significantly higher when CD4+ cells were co-cultured with α-GC-pulsed B cells [means ± standard error of the mean (s.e.m.): 2·9 ± 0·6 ng/ml] than in co-culture with non-loaded B cells (1·3 ± 0·6 ng/ml). Similar effects, but to a lesser extent, were observed on IgG production (respectively, 2·1 ± 0·8 µg/ml versus 1·5 ± 0·8 µg/ml when B cells were α-GC-loaded or not), with significant differences within each donor. The important variations in Ig levels within each condition in response to α-GC-pulsed B/iNK T cell mixtures probably reflect the marked differences in iNK T cell frequency between individuals. Secretion of IgM was not modified significantly under these conditions (Fig. 1b). In comparative experiments with the CD4+ fraction, DN cells, which showed a similar expansion of iNK T cells, failed to enhance IgE and IgG production when co-cultured with α-GC-loaded B cells (Fig. 1c, and data not shown).
Promotion of IgE and IgG production is mediated by CD4+ iNK T cells
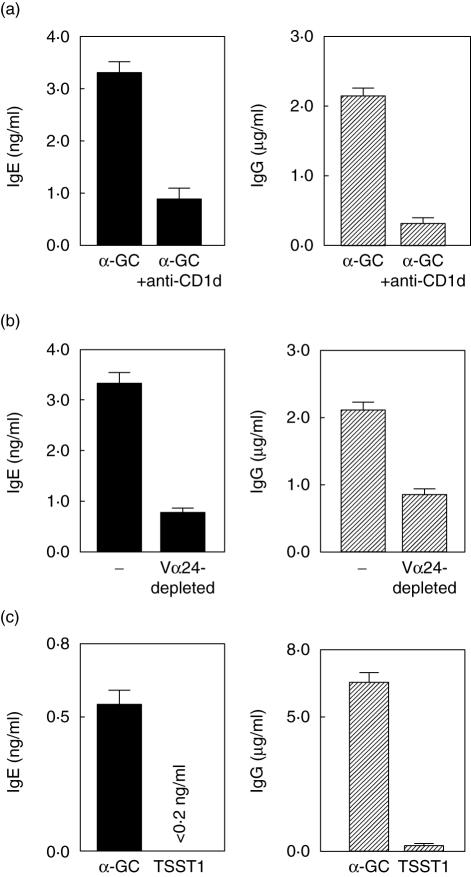
To investigate the role of CD1d in iNK T cell activation, we used a specific anti-CD1d MoAb [32]. Specificity of the neutralizing activity of this anti-CD1d MoAb was controlled by its capacity to block the expansion of human iNK T cells cultured with α-GC but not that of conventional T cells stimulated by IL-2. As shown in Fig. 2a, CD1d blockage resulted in a dramatic reduction of IgE (3·7 ± 0·1-fold; mean ± s.e.m.) and IgG (15·0 ± 11·0-fold, mean ± s.e.m.) levels in supernatants of α-GC-pulsed B cells co-cultured with autologous CD4+ cells. We then depleted CD4+ T lymphocytes in Vα24 cells, which strongly reduced Ig production by α-GC-loaded B cells by 3·6-fold and 2·5-fold for IgE and IgG, respectively (Fig. 2b). Actually, in the absence of the Vα24 cell fraction, IgG and IgE levels in culture supernatants did not differ from baseline values measured in response to non-loaded B/total CD4+ cell mixtures. Additionally, co-cultures were performed in the presence of the superantigen TSST1, which stimulates selectively the MHC-class II-restricted CD4+ T cells that express a Vβ2 chain-containing TCR [33]. Although TSST1 induced Vβ2+ cell expansion (1·5–2·9-fold increase), it failed to induce any detectable IgE and IgG production by B cells (Fig. 2c).
Fig. 2.
Promotion of IgE and IgG production by CD4+ T lymphocytes in response to α-galactosylceramide (α-GC)-loaded B cells is mediated by invariant natural killer T (iNK T) cells. Purified CD4+ lymphocyte fractions (15 × 104 cells) were co-cultured in interleukin (IL)-2-containing medium with 5 × 104 α-GC-loaded B cells (a,b) or with 5 × 104 B cells and the TSST1 superantigen (c). Supernatant concentrations of IgG and IgE were determined as above. Data are means ± s.e.m. from four to six wells (one typical experiment of three). (a) CD1d-dependence of the CD4+/α-GC-loaded B cell cooperation in Ig production. Blocking anti-CD1d monoclonal antibody (20 µg/ml) was added or not to α-GC-loaded B cells before co-culture. (b) Vα24-depleted CD4+ lymphocytes fail to help B cells to produce Ig. Depletion of Vα24+ cells from CD4+ cells was achieved by magnetic cell sorting. (c) CD4+ lymphocytes activated by the TSST1 superantigen do not help B cells to produce Ig.
CD4+ iNK T lymphocytes enhance the proliferation of the memory B cell compartment
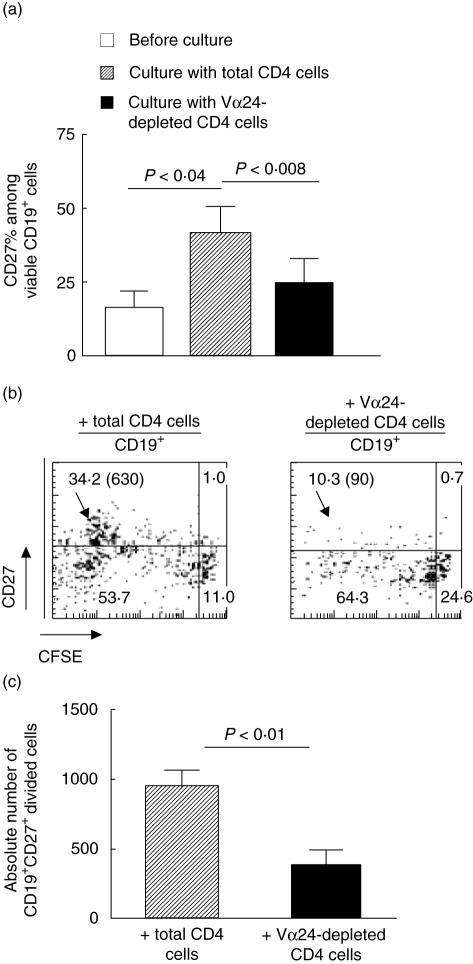
With regard to the identity of the responder B cells, we speculated that CD4+ iNK T cells interacted at least in part with isotype-switched B cells or induced their differentiation. As shown in Fig. 3a, the proportion of α-GC-pulsed CD19+ cells that express CD27, a marker used commonly to identify memory B cells, was significantly higher after a 10-day co-culture with CD4+ cells than prior to culture (41·7 ± 8·6% versus 17·0 ± 4·5%, respectively; mean ± s.e.m., P < 0·04). Moreover, these CD19+ CD27+ cells were all activated, as evidenced by their stage of cell division (Fig. 3b). When co-cultures were performed with Vα24-depleted CD4+ T lymphocytes, a decrease in both percentages (Fig. 3a,b) and absolute number (Fig. 3c) of CD19+ CD27+ B cells occurred. Altogether, these data indicate that CD4+ but not DN iNK T cells are crucial for the accumulation of isotype-switched B cells through a CD1d-dependent CD4+ iNK T/B cell interaction in our model.
Fig. 3.
CD4+ invariant natural killer T (iNK T) lymphocytes enhance the proliferation of the memory B cell compartment. Purified 5(6)-carboxyfluoresceine diacetate succinimidyl ester (CFSE)-labelled CD19+ B cells were preincubated with α-galactosylceramide (α-GC) and co-cultured with CD4+ or iNK T-depleted CD4+ cells for 8–10 days. Data are means ± s.e.m. from four experiments. (a) Frequency of CD27+ cells among CD19+ lymphocytes before and after culture. The increase of CD27+ cell frequency along culture depends on CD4+ iNK T cells. (b, c) Proliferation of memory B cells depends on CD4+ iNK T cells. Proliferation of gated CD19+ B cells was analysed on day 10 according to CD27 expression and to dilution of CFSE staining. Dot plots show percentages of cells in each quadrant, and absolute number of dividing memory (CD27+) cells (CFSE dilution ≥ 1 division) are shown between brackets.
Functional dichotomy in term of cytokine production between freshly isolated CD4+ and DN iNK T cells
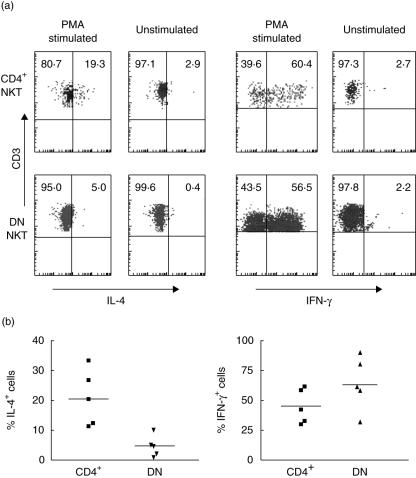
We then verified whether the functional dichotomy in promoting Ig production by B cells between CD4+ and DN iNK T cells was associated with differences in cytokine production profiles. Freshly drawn PBL were treated or not for 5 h with PMA and calcium ionophore in culture medium containing GolgiStopTM to avoid exocytosis and then analysed for cytokine production by intracytoplasmic staining. The CD4+ subset produced both IL-4 and IFN-γ, in contrast to DN iNK T cells, which generated predominantly IFN-γ (Fig. 4a,b).
Fig. 4.
Comparison of interleukin (IL)-4 and interferon (IFN)-γ expression by CD4+ and double-negative (DN) freshly drawn invariant natural killer T (iNK T) cells. Cells were prepared and stimulated as described in the Materials and methods section and analysed by flow cytometry. (a) A representative experiment; (b) data from five experiments. Differences for IL-4 expression between CD4+ and DN iNK T cells were significant by the paired t-test. (P < 0·006). There was no significant difference in terms of IFN-γ production. No cytokines were detected in unstimulated cells.
The pro-Th2 effect of CD4+ iNK T cells on B cells is controlled by myeloid DC
Finally, we addressed the question of a possible interference of myeloid DC with the induction of Ig production in our human in vitro model. Indeed, α-GC-pulsed myeloid DC completely blocked IgE and IgG production mediated by CD4+ iNK T/α-GC-pulsed B cell interactions (Fig. 5a). Because the capacity of myeloid DC to polarize iNK T cells towards a Th1 profile was reported in the mouse [24], we searched for a similar effect in our human in vitro co-culture model. Co-cultured cells were treated further for 5 h with PMA and calcium ionophore and then analysed by intracytoplasmic staining with anti-cytokine MoAb. As shown in Fig. 5b,c, co-culture with myeloid DC enhanced the expression of IFN-γ and dramatically decreased that of IL-4 in CD4+ iNK T cells. Importantly, the major inhibitory effect of myeloid DC on IL-4 expression was selective for the iNK T cell subset. Indeed, conventional CD4+ T cells retained their capacity to express IL-4 although their proportion to express IFN-γ was increased (Fig. 5b,d). Contrasting with myeloid DC, B cells did not modify the pattern of IL-4 and IFN-γ expression by CD4+ iNK T cells (see Fig. 4a,b, compared to Fig. 5b,c).
Fig. 5.
Myeloid dendritic cells (DC) block Ig production promoted by CD4+ invariant natural killer T (iNK T) cells. CD4+ T lymphocyte fractions (15 × 104 cells) were co-cultured in medium containing interleukin (IL)-2 with 5 × 104 α-galactosylceramide (α-GC)-loaded B cells in the presence or not of 5 × 104 α-GC-loaded myeloid DC. (a) Myeloid DC block the CD4+ cell-induced Ig production of α-GC-loaded B cells. Supernatant concentrations of IgG and IgE were determined on day 12 of co-culture. Data are arithmetic means from three separate experiments. (b,c,d) Myeloid DC modify the pattern of interferon (IFN)-γ and IL-4 production by CD4+ iNK T and conventional CD4+ T cells (Vα24–) co-cultured with α-GC-loaded B cells. After 10–13 days of culture, iNK T cells and conventional T cells were analysed for intracytoplasmic IL-4 and IFN-γ expression as described in the Materials and methods section. Dot plots show percentages of cells of either population that produced the relevant cytokine. Data are from experiments performed with cells from six healthy donors; significant differences were found for IL-4 and IFN-γ expression by CD4+ iNK T cells (P < 0·02 and P < 0·03, respectively) and IFN-γ expression by conventional T cells (P < 0·002), according to paired t-tests.
Discussion
In view of the recent reports suggesting that CD4+ and DN iNK T cells in humans display Th2 and Th1 profiles, respectively, it could be expected that these two cell subsets differ in terms of their capacity to induce Ig production by autologous B cells. Here, by addressing this question, we demonstrated that freshly isolated CD4+ iNK T cells can activate and help B cells to produce IgG, whereas DN iNK T cells do not. Moreover, by showing that CD4+ iNK T cells are also capable of promoting IgE production, a characteristic feature of Th2 response, we afforded a definitive evidence for a Th2-promoting activity of this cell subset.
In a previous study by Galli et al. [26], improvement of IgE production by CD4+ iNK T cells could not be demonstrated. This discrepancy with our data could be explained by the fact that these investigators used iNK T cell clones instead of freshly isolated cells, which are clearly distinct in their cytokine production pattern. Indeed, as reported recently by others [27,28], we observed a dichotomy concerning the cytokine profile generated by human CD4+ and DN iNK T cells; these data contrasted with the results of Galli et al., who observed that CD4+ and DN iNK T cell clones displayed the same cytokine profile. The discrepancies between the two studies also probably reflect differences in the experimental system. Galli et al. used autologous iNK T cell clones with a ratio of one B for one T cell. In our study, B cells were co-cultured with unpurified sources of iNK T cells, in order to avoid them being in a much greater proportion than under physiological conditions. For this reason, it was necessary to prove that iNK T cells were responsible for the observed effects. Altogether, the requirement for α-GC stimulation and CD1d presentation, the inhibitory effect of iNK T cell depletion and their cytokine secretion profile provide strong evidence for the role of iNK T cells in the induction of IgG and IgE synthesis. The undetectable IgE and IgG production by TSST1-loaded B cells co-cultured with CD4+ cells suggests that conventional CD4+ T cells play no role under these experimental conditions. Finally, we have confirmed our results with CD4+ iNK T cell lines. We have purified and raised CD4+ T lymphocytes with autologous B lymphocytes presenting α-GC. After 18 days, iNK T cells were purified by magnetic sorting with the MoAb anti-Vα24 or the Tet-CD1d. These iNK T cell lines were raised with autologous feeder cells, and they induce IgE and IgG production by autologous B cells in co-culture (data not shown and manuscript in preparation).
Evidence suggesting that iNK T cells can promote Ig production has already been provided in mice [19,20,34]. However, in some experimental designs iNK T cells failed to exert Th2-promoting functions. In this case, the authors assigned a major role to myeloid DC through their capacity to polarize iNK T cells towards the Th1 phenotype [23,24]. Our results extend this role of myeloid DC in humans. Indeed, we show that α-GC-pulsed myeloid DC completely block IgE and IgG production mediated by the CD4+ iNK T/α-GC-pulsed B cell interaction. Furthermore, the addition of myeloid DC enhanced the production of IFN-γ and decreased that of IL-4 by CD4+ iNK T cells co-cultured with α-GalCer-loaded B cells. Variation in the distribution of iNK T cell subsets together with the dominant inhibitory effect of myeloid DC might explain why iNK T cells can promote both Th2 and Th1 responses according to the immune conditions. Further investigations are needed to define the molecular events accounting for the pro-Th2 effect of CD4+ iNK T cells on B cells and its control by myeloid DC. It might be postulated that CD1d-expressing B cells play a specific and direct role as pro-Th2 stimulatory elements in situations in which Th1-mediated responses could become harmful. The opposite effect of B cells and myeloid DC on iNK T cell activation might result from different activation pathways, as documented in the mouse for CD28 and CD40L, which can exert opposite effects on iNK T cell functions [35]. It is also conceivable that IL-12 that can be produced by myeloid DC upon interaction with α-GC-activated iNK T cells plays a determinant role in the suppression of IgE responses [23]. Finally, some iNK T cell lines prepared with autologous DC presenting α-GC did not induce IgE and IgG production by the autologous B cells (data not shown, and manuscript in preparation) confirming the interference of DC in our model.
Another important point concerns the identity of the responder B cells. Although our data indicate that CD4+ but not DN iNK T cells are crucial for the accumulation of isotype-switched B cells in our model, it might be interesting in further experiments to study precisely the respective implication of naive and memory B cell subsets.
Because of their dual capacity to promote both Th1 and Th2 responses according to the immune conditions, iNK T cells should be taken into account as a potential tool of immuno-intervention in relevant diseases. The use of α-GC-pulsed B cells deserves consideration as a mean for selectively promoting Th2-mediated regulatory functions of iNK T cells.
Acknowledgments
This work was supported by grants from Poitiers University Hospital, the Ligue contre le Cancer, Comité Poitou-Charente and the Association pour la Recherche sur le Cancer (no. 5889). We are grateful to Elke Schneider (CNRS UMR 8147) for critical comments on this manuscript. We are especially indebted to the Pharmaceutical Research Laboratory, Kirin Brewery (Gunma, Japan) for providing α-GC (KRN 7000) to Albert Bendelac (Princeton University, Princeton, USA) and to Gregorio Aversa (Schering-Plough, Dardilly, France) for the gift of 20H2 hybridoma and the mAb89 MoAb, respectively.
References
- 1.Godfrey DI, Hammond KJL, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–83. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald HR. Development and selection of NKT cells. Curr Opin Immunol. 2002;14:250–4. doi: 10.1016/s0952-7915(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 4.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4–CD8– T cells. J Exp Med. 1994;180:1171–6. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4–CD8– T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, Mattner J, Cantu C, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 9.Mattner J, DeBord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, Xing GW, Poles MA, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351–6. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Paul W. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–9. [PubMed] [Google Scholar]
- 12.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by α-galactosylceramide treatments prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 14.Apostolou I, Takahama Y, Belmant C, et al. Murine natural killer T (NKT) cells contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc Natl Acad Sci USA. 1999;96:5141–6. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar H, Belperron SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 16.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–20. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 17.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–5. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 18.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdin N, Brossay L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NKT cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–25. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Hong S, Scherer DC, et al. Activation of NKT cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–7. [PubMed] [Google Scholar]
- 21.Kitamura H, Ohta A, Sekimoto M, et al. α-Galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Rogers KH, Lewis DB. Beta 2-microglobulin-dependent T cells are dispensable for allergen-induced T helper 2 responses. J Exp Med. 1996;184:1507–12. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J, Watanabe N, Kawano T, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J Exp Med. 1999;190:783–92. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–7. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Vliet HJJ, Nishi N, Koezuka Y, et al. Potent expansion of human natural killer T cells using α-galactosylceramide (KRN7000)-loaded monocyte-derived dendritic cells, cultured in the presence of IL-7 and IL-15. J Immunol Meth. 2001;247:61–72. doi: 10.1016/s0022-1759(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 26.Galli G, Nuti S, Tavarini S, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–7. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levan-Petit I, Lelievre E, Barra A, et al. T(h)2 cytokine dependence of IgD production by normal human B cells. Int Immunol. 1999;11:1819–28. doi: 10.1093/intimm/11.11.1819. [DOI] [PubMed] [Google Scholar]
- 30.Hameg A, Apostolou I, Leite-de-Moraes M, et al. A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules and autopresents the α-galactosylceramide antigen. J Immunol. 2000;165:4917–26. doi: 10.4049/jimmunol.165.9.4917. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki N, Antonenko S, Ho S, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–7. [PubMed] [Google Scholar]
- 33.Stern LJ, Wiley DC. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992;68:465–77. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- 34.Schofield L, McConville M, Hansen A, et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–9. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]