Abstract
Recent studies had demonstrated that serum and mesangial immunoglobulin A1 (IgA1) in patients with IgA nephropathy (IgAN) were polymeric and deglycosylated. The current study was to investigate the binding characteristics of monomeric and polymeric normal human IgA1 on mesangial cells and the influence of in vitro deglycosylation of IgA1 molecules. The normal human IgA1 was desialylated and degalactosylated with specific enzymes, respectively. The monomeric IgA1 (mIgA1) and polymeric IgA1 (pIgA1) were separated by Sephacryl S-300 chromatography. The binding capacities of the mIgA1 and pIgA1 to primary human mesangial cells (HMC) were evaluated by classical radioligand assay. Both the native mIgA1 and pIgA1 could bind to HMC in a dose-dependent and saturable manner. The maximal binding capacity of the native pIgA1 were significantly higher than that of the native mIgA1 (P < 0·05). However, the affinity of the native mIgA1 was almost 100 times higher than that of the native pIgA1. After deglycosylation, binding of the two deglycosylated mIgA1 to HMC could not be detected. However, the maximal binding capacities of the two deglycosylated pIgA1 to HMC were increased significantly compared with that of native pIgA1. The affinity of the two deglycosylated pIgA1 was similar to that of native pIgA1 (P > 0·05). The current study suggests differential binding characteristics of native monomeric and polymeric IgA1 on mesangial cells. Glycosylation of IgA1 molecules could significantly affect the binding of IgA1 on HMC.
Keywords: binding capacity, deglycosylation, galactose, glycosylation, IgA nephropathy, IgA1, mesangial cell, radioligand, sialic acid
Introduction
Immunoglobulin A (IgA) nephropathy (IgAN), which leads to progressive renal failure in almost one-third of patients, is the most common glomerulonephritis. The disease is characterized by the deposition of polymeric IgA1 in glomerular mesangium [1,2]. The mechanisms responsible for the mesangial deposition remain unclear. Many studies have suggested that abnormal physicochemical properties of circulating IgA1, such as size, charge and glycosylation, might play a pivotal role in the pathogenesis of IgA nephropathy [3–9].
The IgA1 hinge region is a unique mucin-like O-linked glycopeptide. The core peptide has a proline-, serine- and threonine-rich amino acid sequence in which the five serines are able to carry O-linked oligosaccharides consisting of sialic acid, galactose and N-acetylgalactosamine [10]. Several studies have shown reduced galactosylation and sialylation of serum IgA1 from patients with IgAN [4,5,11,12]. Furthermore, two recent studies have been successful in examing the O-glycosylation of IgA1 eluted from the mesangium of kidneys from patients with IgAN. Both reports demonstrated that mesangial IgA1 had a more extreme abnormality of O-glycosylation than serum IgA1 in patients with IgAN, suggesting strongly that circulating IgA1 molecules with aberrant O-glycosylation deposited preferentially in the glomerular mesangium [13,14]. More importantly, Sano et al. found accumulation of under-glycosylated IgA1 molecules in rat kidney after injection with in vitro enzymatically desialylated (deSIgA1) or further degalactosylated IgA1 (deS/deGal IgA1) [8].
Increased serum macromolecular IgA was found in patients with IgAN and the glomerular IgA1 deposits were mainly polymeric [15,16]. Altered O-glycosylation of serum IgA1 might favour self-aggregation and the formation of an immune complex with IgG [17–20]. It has been found that under-glycosylated IgA1-containing immune complexes could bind more efficiently to mesangial cells than circulating immune complexes from healthy controls [18,21,22]. Furthermore, studies showed that IgA1 with different molecular weights might play different roles in IgAN. Leung et al. found that cultured human mesangial cells (HMC) could bind more IgA with molecular weights from 300 to 610 kDa isolated from patients with IgAN than that of controls [3]. Moura et al. also found that polymeric, not monomeric, IgA1 could interact with transferrin receptor on cultured human mesangial cells (HMC) and mediated internalization [23].
Our previous study demonstrated that in vitro heat-aggregated IgA1 (aIgA1) from patients with IgAN had a higher binding capacity to HMC and stronger biological effects than aIgA1 from healthy controls [24]. Furthermore, the binding capacities of deSIgA1 and deS/deGalIgA1 to HMC were significantly higher than that of native normal IgA1 [25]. The purpose of the current study was to investigate the binding characteristics of monomeric and polymeric normal human IgA1 on HMC and the influence of deglycosylation of IgA1 molecules.
Materials and methods
Isolation of normal human serum IgA1
IgA1 was isolated from pooled sera of healthy blood donors by jacalin affinity chromatography [26]. Briefly, the pooled sera were diluted 1 : 1 with 175 mM Tris-HCl (pH 7·4), filtered through a 0·22 µm Corning syringe filter (Corning Glass Works, Corning, NY, USA) and applied to a jacalin column prepared using commercially available jacalin immobilized on agarose (Pierce Chemical Company, Rockford, IL, USA) with an IgA1 binding capacity of more than 2 mg/ml of gel. The column was then washed with 175 mM Tris-HCl (pH 7·4) until the optical density at 280 nm was less than 0·1. IgA1 was eluted with 0·1 M melibiose (Sigma, St Louis, MO, USA) in 175 mM Tris-HCl in 2·0 ml fractions until the optical density returned to 0·1. The fractions were then pooled and concentrated by ultrafiltration with 10 kDa exclusion membranes (Millipore Corporation, Bedford, MA, USA). The concentrated sample was dialysed against distilled water for 24 h to remove melibiose and then the IgA1 was lyophilized. The study was approved ethically and informed consent was obtained.
Deglycosylation of normal serum IgA1
The purified serum IgA1 samples from healthy individuals were digested with neuraminidase from Clostridium perfringens (Sigma) and β-galactosidase from bovine testis (Sigma), respectively, according to the procedures described by Iwase et al. [27]. Three mg IgA1 samples were used; 1 mg IgA1 was dissolved in 100 µl 0·2 M sodium acetate buffer (SAB), pH 5·0, without adding enzymes as a control and 1 mg IgA1 was dissolved in 95 µl SAB with 5 µl neuraminidase to remove sialic acid; the third 1 mg IgA1 was dissolved in 79 µl SAB, 5 µl neuraminidase and 16 µl β-galactosidase to remove both sialic acid and galactose. The samples were incubated overnight at 37°C and then dialysed against 0·05 M phosphate-buffered saline (PBS). The non-treated, neuraminidase-treated and neuraminidase plus β-galactosidase-treated IgA1 were named as native IgA1, deSIgA1 and deS/deGalIgA1, respectively.
Evaluation of the efficacy of enzyme digestion
The efficacy of the enzymatic treatment was evaluated by enzyme-linked immunosorbent assay (ELISA) using elderberry bark lectin (Sambuscus nigra agglutinin, SNA; Vector Laboratories, Burlingame, CA, USA) and Vicia vilosa lectin (VVL; Vector Laboratories), which specifically bind the sialic acid and the terminal GalNAc residue, respectively. Rabbit anti-human IgA (Dako, Glostrup, Denmark) diluted to 5·5 µg/ml in 0·05 M bicarbonate buffer pH 9·6 were coated to the wells of one-half of a polystyrene microtitre plate (Costar, Cambridge, MA, USA). The wells in the other half were coated with bicarbonate buffer alone to act as antigen-free wells. The volumes of each well for this step and for subsequent steps were 100 µl, all incubations were carried out at 37·C for 1 h and the plates were washed three times with 0·01 M phosphate-buffered saline containing 0·1% Tween20 (PBST). The plates were then blocked with PBST containing 1% bovine serum albumin (BSA) (PBST/BSA). Monomer and polymer of native IgA1, deSIgA1 and deS/deGalIgA1 diluted to 5 µg/ml in PBST were added in duplicate to both antigen-coated and antigen-free wells, respectively. After incubation and washing, the 1 : 250 diluted biotinylated SNA or 1 : 200 diluted biotinylated VVL were added. The wells were then incubated with avidin–horseradish peroxidase diluted to 1 : 20000 (Sigma) for 40 min and then washed. All plates were exposed to an enzyme substrate consisting of 4 mg O-phenylenediamine in 10 ml 0·1 M citrate phosphate buffer (pH 5. 0) and 10 µl 3% H2O2 (added just before use). The reaction was stopped with 1 M H2SO4. The absorbance at 490 nm was recorded in an ELISA reader (Bio-Rad550, Tokyo, Japan). All the assays were repeated three times and the absorbance was expressed as mean ± standard deviation (s.d.).
To assess further the purity of IgA1 fractions and the influence of deglycosylation on the size of IgA1 heavy chain, the six IgA1 fractions (20 µg) were separated by a 12·5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel in reducing conditions.
Fractionation of IgA1 with different glycosylations
IgA1 with different glycosylations was dialysed against 0·05 M PBS overnight at 4°C, then diluted in 0·05 M PBS and filtered. Samples of IgA1 and the appropriate molecular markers (Gel Filtration HMW Calibration Kit; Amersham Bioscience, Buckinghamshire, UK) were separated by size-exclusion chromatography on a 16/60 S-300 Sephacryl column (Amersham Bioscience, Uppsala, Sweden) under the same conditions, respectively. The calibration profile of the standard molecular markers includes thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa) and aldolase (158 kDa). According to the standard molecular markers, IgA1 with a molecular weight over 440 kDa was collected as polymeric IgA1, while IgA1 with a molecular weight around 150 kDa was collected as monomeric IgA1.
Isolation and culture of primary HMC
Normal portion of human kidney cortex was obtained from nephrectomized specimens of patients with kidney tumours. Primary culture of HMC was performed using a standard sequential sieving technique [28]. Briefly, cortical tissue, bathed in Hank's balanced salt solution (HBSS) at 4°C, was minced and passed sequentially through 140, 80 and 220 mesh sieves. The retained glomeruli were washed with copious amounts of HBSS to remove tubular fragments, incubated for 3 min at 37°C with 1·0 mg/ml collagenase type Ia (Sigma) and centrifuged at 99·5 g for 5 min at 4°C to pellet the glomeruli. The pelleted glomeruli were resuspended in RPMI-1640 medium buffered with 25 mM HEPES at pH 7·4 and supplemented with 20% fetal calf serum (FCS) (Gibco, Brisbane, Australia), 30 mg/ml penicillin, 68 mg/ml streptomycin and 150 mg/ml glutamine, plated in 75-cm2 tissue culture flasks (Costar) and cultured in humidified 5% CO2 atmosphere at 37°C. Cells were passaged when confluent using 0·05% trypsin in 0·5 mM ethylenediamine tetraacetic acid (EDTA). Purity and identification of mesangial cells were evaluated by cell morphology, negative staining by immunohistochemistry with antibody to cytokeratin and factor VIII antibody (Sigma) and positive staining of vimentin and desmin. All experiments were performed on the third or fourth passage.
125I labelling of different IgA1 preparations
The pIgA1 and mIgA1 of native IgA1, deSIgA1 and deS/deGalIgA1 were radio-labelled with Na 125I (>15 mCi/mg Iodide, Amersham Corporation, Arlington Heights, IL, USA), respectively, using the idogen method [29] to a specific activity of 0·5 mCi/mg. The excess free Na 125I was separated from the protein by size-exclusion chromatography on a column of Sephadex G-50, as reported previously [24].
Binding of IgA1 to HMC
HMC suspended in medium was aliquoted into 12-well plates. The cells were grown to 60–80% confluency for about 36–48 h in RPMI-1640 medium with 20% FCS. Cell numbers were counted by a haemocytometer with an average of 1 × 105/well. Cells were washed three times with PBS and preincubated at 4°C for 60 min with 0·3 ml 0·5% BSA/PBS alone or in the presence of 0·3 ml 2 mg/ml unlabelled mIgA1 or pIgA1 of native IgA1, deSIgA1 or deS/deGalIgA1, respectively. After preincubation, cells were washed three times with 0·5% BSA/PBS and incubated at 4°C for 60 min with 125I-labelled pIgA1 or mIgA1 of native IgA1, deSIgA1 or deS/deGalIgA1 in 0·3 ml 0·5% BSA/PBS alone or in the presence of increasing amounts of non-radioactive mIgA1 or pIgA1 of native IgA1, deSIgA1 or deS/deGalIgA1 from 0 to 0·8 mg/ml. After incubation, the cells were washed three times with PBS and solubilized in 1·0 ml 1 M NaOH for 10 min. The lysates were transferred to gamma tubes and the radioactivity was determined in a Packard 5002 gamma counter (Downer's Grove, IL, USA). The experiments with triplicate wells were repeated three times and the data were analysed using the excel programs of Windows 2000 (Microsoft).
Statistical analyses
The results were expressed as mean ± s.d. Comparisons among means were performed using one-way analysis of variance (anova) and the S–N–K test in spss (SPSS Inc., Chicago, IL, USA) version 11·0 statistical analysis program. A P-value less than 0·05 was considered significant.
Results
Deglycosylation
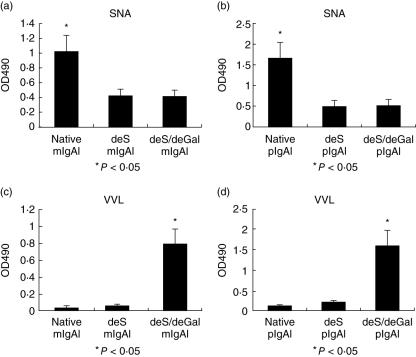
Serum IgA1 was digested with neuraminidase and neuraminidase plus β-galactosidase, and then deSIgA1 and deS/deGalIgA1 were produced. The mIgA1 and pIgA1 were separated by size-exclusion chromatography. As shown in Fig. 1, the terminal sialic acid in deSIgA1 and deS/deGalIgA1 was effectively removed. The sialic acid levels of monomeric native IgA1 (native mIgA1), monomeric deSIgA1 (deS mIgA1) and monomeric deS/deGalIgA1 (deS/deGal mIgA1) were 1·009 ± 0·225, 0·427 ± 0·087 and 0·435 ± 0·095, respectively. The sialic acid level of native mIgA1 was significantly higher than that of deS mIgA1 and deS/deGal mIgA1 (P < 0·05) (Fig. 1a). The sialic acid levels of polymeric native IgA1 (native pIgA1), polymeric deSIgA1 (deS pIgA1) and polymeric deS/deGalIgA1 (deS/deGal pIgA1) were 1·660 ± 0·317, 0·514 ± 0·14 and 0·551 ± 0·104, respectively. The sialic acid level of native pIgA1 was significantly higher than that of deS pIgA1 and deS/deGal pIgA1 (P < 0·05) (Fig. 1b).
Fig. 1.
Reactivity of the native immunoglobulin A1 (IgA1), desialylated (deSIgA1) and further degalactosylated IgA1 (deS/deGal IgA1) with Sambuscus nigra agglutinin (SNA) and Vicia vilosa lectin (VVL). SNA could specifically bind the terminal sialic acid. The terminal sialic acid of O-glycan in deSIgA1 and deS/deGalIgA1 was effectively removed. The sialic acid level of native mIgA1 was significantly higher than that of deS mIgA1 and deS/deGal mIgA1 (P < 0·05) (a). The sialic acid level of native pIgA1 was significantly higher than that of deS pIgA1 and deS/deGal pIgA1 (P < 0·05) (b). VVL could specifically bind the terminal GalNAc. The sialic acid and galactose were removed and the terminal GalNAc of O-glycan in deSIgA1/deGalIgA1 was highly exposed. The GalNAc level of deS/deGal mIgA1 was significantly higher than that of native mIgA1 and deS mIgA1 (P < 0·05) (c). The GalNAc level of deS/deGal pIgA1 was significantly higher than that of native pIgA1 and deS pIgA1 (P < 0·05) (d). Native mIgA1: monomeric native IgA1; deS mIgA1: monomeric deSIgA1; deS/deGal mIgA1: monomeric deS/deGalIgA1; native pIgA1: polymeric native IgA1; deS pIgA1: polymeric deSIgA1 and deS/deGal pIgA1: polymeric deS/deGalIgA1.
As shown in Fig. 1, the sialic acid and galactose were removed and the terminal GalNAc of deS/deGalIgA1 was highly exposed. The GalNAc levels of native mIgA1, deS mIgA1 and deS/deGal mIgA1 were 0·0311 ± 0·011, 0·066 ± 0·004 and 0·74 ± 0·23, respectively. The GalNAc level of deS/deGal mIgA1 was significantly higher than that of native mIgA1 and deS mIgA1 (P < 0·05) (Fig. 1c). The GalNAc levels of native pIgA1, deS pIgA1 and deS/deGal pIgA1 were 0·127 ± 0·055, 0·207 ± 0·090 and 1·594 ± 0·353, respectively. The GalNAc level of deS/deGal pIgA1 was significantly higher than that of native pIgA1 and deS pIgA1 (P < 0·05) (Fig. 1d).
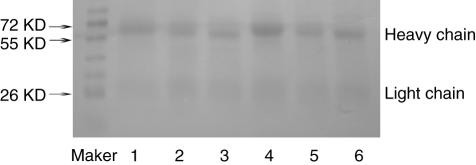
The SDS-PAGE gel showed that the six IgA1 fractions were highly pure and the size of the IgA1 heavy chain was slightly decreased after deglycosylation (Fig. 2).
Fig. 2.
The purity of the six immunoglobulin A1 (IgA1) fractions and changes of the size of the IgA heavy chain after deglycosylation. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis showed that the six IgA1 fractions were highly pure and the size of the IgA1 heavy chain was slightly decreased after deglycosylation. Lane 1: native monomeric IgA1 (mIgA1); lane 2: desialylated (deS) mIgA1; lane 3: deS/degalactosylated (deGal) IgA1; lane 4: native polymeric IgA1 (pIgA1); lane 5: deS pIgA1; lane 6: deS/deGal pIgA1.
Binding of IgA1 to HMC
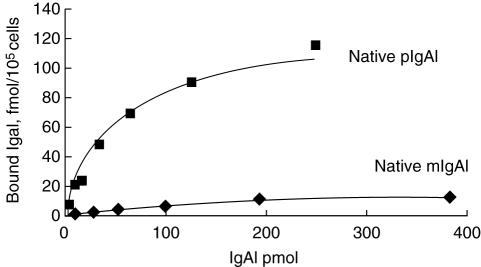
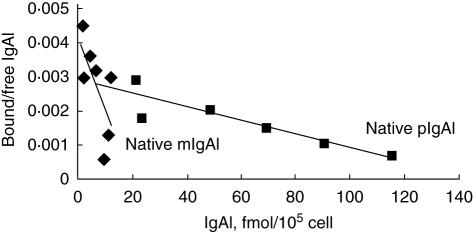
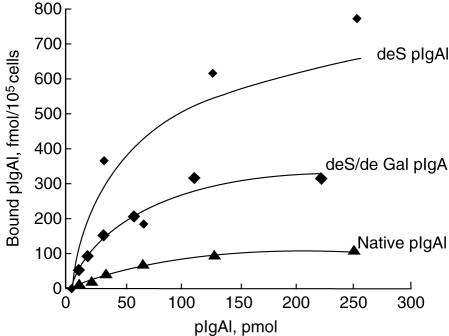
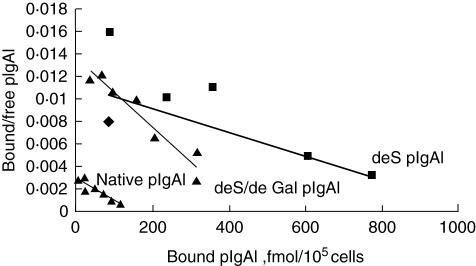
The native mIgA1 and native pIgA1 could bind to HMC in a dose-dependent, saturable manner (Fig. 3). The maximal binding capacity of native pIgA1 was significantly higher than that of native mIgA1 (190 ± 93·62 fmol/105 cell versus 10·53 ± 4·30 fmol/105 cell, P < 0·05). Based on one IgA1 receptor binding one IgA1 molecule only, the binding sites/cell of native pIgA1 was significantly higher than that of native mIgA1 (1·14 ± 0·56 × 106/cell versus 6·34 ± 2·59 × 104/cell, P < 0·05). However, the affinity of native mIgA1 was almost 100 times higher than that of native pIgA1, as Scatchard analysis revealed that dissociation constants (Kd) of native mIgA1 and native pIgA1 were 6·25 ± 4·33 × 10−9mol/l and 1·12 ± 0·54 × 10−7mol/l, respectively (Fig. 4).
Fig. 3.
The binding characteristics and binding capacities of the native monomeric immunoglobulin A1 (mIgA1) and polymeric IgA1 (pIgA1) to human mesangial cells (HMC). The binding of native mIgA1 and native pIgA1 to HMC was in a dose-dependent and saturable manner. The binding capacity of native pIgA1 was significantly higher than that of native mIgA1 (P < 0·05).
Fig. 4.
Scatchard analysis of Kd values of the native monomeric immunoglobulin A1 (mIgA1) and polymeric IgA1 (pIgA1) binding to human mesangial cells. Kd of native mIgA1 and native pIgA1 were 6·25 ± 4·33 × 10−9mol/l and 1·12 ± 0·54 × 10−7mol/l, respectively. The affinity of native mIgA1 was almost 100 times higher than that of native pIgA1.
After desialylation and degalactosylation in vitro, binding of the two deglycosylated mIgA1 to HMC could not bedetected (Fig. 5). The deS pIgA1 and deS/deGal pIgA1 could still bind to HMC in a dose-dependent, saturable manner (Fig. 6). However, the maximal binding capacities of deS pIgA1 and deS/deGal pIgA1 to HMC were increased significantly compared with that of native pIgA1 (566·67 ± 104·46 fmol/105 cell and 417·33 ± 45·98 fmol/105 cell versus 190 ± 93·62 fmol/105 cell, P < 0·05, respectively); the binding sites/cell of deS pIgA1 and deS/deGal pIgA1 to HMC were also increased significantly compared with that of native pIgA1 (3·41 ± 0·63 × 106/cell and 2·51 ± 0·28 × 106/cell versus 1·14 ± 0·56 × 106/cell, P < 0·05, respectively). The maximal binding capacities and the binding sites/cell of deS pIgA1 and deS/deGal pIgA1 were similar. The affinity of deS pIgA1 and deS/deGal pIgA1 was similar to that of native pIgA1, as the Kd of deS pIgA1 and deS/deGal pIgA1 were 3·10 ± 2·00 × 10−7mol/l and 3·44 ± 2·70 × 10−7mol/l, respectively (versus 1·12 ± 0·54 × 10−7 mol/l, P > 0·05) (Fig. 7).
Fig. 5.
The binding characteristics of deglycosylated monomeric immunoglobulin A1 (mIgA1) to human mesangial cells (HMC). The binding of desialylated (deS) mIgA1 and deS/degalactosylated (deGal) mIgA1 to HMC was linear.
Fig. 6.
The binding characteristics and binding capacities of three polymeric immunoglobulin A1 (IgA1) to human mesangial cells (HMC). The binding of the three polymeric IgA1 to HMC was in a dose-dependent and saturable manner. The binding capacities of the two deglycosylated polymeric IgA1 were significantly higher than that of the native polymeric IgA1 (P < 0·05, respectively).
Fig. 7.
Scatchard analysis of Kd values of three polymeric immunoglobulin A1 (pIgA1) binding to human mesangial cells (HMC). The affinity to HMC of the three polymeric IgA1 was similar. Kd of native pIgA1, desialylated (deS) pIgA1 and deS/degalactosylated (deGal) pIgA1 were 1·12 ± 0·54 × 10−7mol/l, 3·10 ± 2·00 × 10−7mol/l and 3·44 ± 2·70 × 10−7mol/l, respectively (P > 0·05).
Discussion
The mechanism leading to mesangial IgA deposition in patients with IgA nephropathy is still unknown. There was no direct evidence to suggest that the deposited IgA was directed against specific glomerular antigens [30]. Substantial evidence suggests that serum underglycosylated IgA1 from patients with IgA nephropathy might interact directly with putative mesangial cell IgA1 receptors.
In order to elucidate the effects of the size of IgA1 molecules on the binding characteristics on HMC, the native IgA1 molecules were separated into monomer and polymer. The present study revealed that binding of both the native mIgA1 and pIgA1 on HMC were dose-dependent and saturable, which suggested that the binding might be associated with receptors. Furthermore, although the binding capacity of the native mIgA1 to HMC was much lower than that of the pIgA1, the binding affinity of the native mIgA1 to HMC was about 100 times higher than that of the pIgA1. We speculated that there might be two different IgA1 receptors on mesangial cells which could bind the native mIgA1 and pIgA1, respectively. Polymerization and deglycosylation might change the spatial conformation of the pIgA1 and further affect the binding characteristic of the pIgA1 on HMC, creating lower affinity but higher binding capacity. However, these need to be investigated further.
To date, the known IgA receptors CD89, asialoglycoprotein receptor and polymeric immunoglobulin receptor could not be confirmed to express on HMC [26,31–34]. Other receptors have emerged recently as candidate receptors for binding of IgA-containing circulating immune complexes: CD71 (transferrin receptor) [35] and Fcα/µ receptor [36] and other unidentified Fcα receptors [33]. Moura et al. had addressed that CD71 could serve as a mesangial IgA1 receptor, but the failure to block IgA binding to HMC completely might indicate that a second type of an as-yet uncharacterized mesangial IgA receptor might exist [35]. They also showed that CD71 could bind IgA1 but not IgA2, which also suggested another receptor, because HMC could bind both IgA subclasses [35]. Furthermore, Leung et al. found that IgA1-induced macrophage migration inhibitory factor (MIF) synthesis of HMC could not be blocked by IgA receptors (CD89, asialoglycoprotein receptor, CD71, pIgR), which suggested that the pIgA-induced MIF production by HMC might be through other unidentified IgA receptors [37]. Barratt et al. have recently reported a novel IgA1 receptor, suggesting that the novel Fcα receptor was different from Fcα/µ receptor because IgM could not inhibit IgA1 binding to HMC through the novel Fcα receptor, while IgM could bind Fcα/µ receptor [33].
Previous studies, including ours, have demonstrated that patients with IgA nephropathy had altered glycosylations of circulating IgA1 [11,12,23], and the deglycosylated IgA1 existed only in the IgA1-containing macromolecules in sera from patients with IgA nephropathy [12]. Altered O-glycosylation of serum IgA1 might favour self-aggregation or immune complex formation [17–20], and it has been found that under-glycosylated IgA1-containing immune complexes could bind more efficiently to mesangial cells than circulating immune complexes from healthy controls [18,21,22]. However, different aberrantly glycosylated IgA1 simply represented various percentages of total human serum IgA1 in different individuals; the in vitro deglycosylated IgA1 fractions adopted in this study were extremely simplified in comparison to the O-glycosylation of IgA1 in IgA nephropathy, which changed much more subtly. Although this deglycosylation mode could represent only partially the actual condition in the body, it was very helpful to clarify the functional effects of different glycosylation of IgA1 molecules on the binding to HMC. Therefore, we employed in vitro enzyme digestion to produce exclusively deglycosylated IgA1 to investigate the functional effects of different glycosylation of IgA1 molecules on the binding to HMC [25].
In the present study, we have demonstrated that deglycosylation of IgA1 had diverse effects on mIgA1 and pIgA1. Both the desialylated and degalactosylated pIgA1 had an increased binding capacity in comparison with the normal pIgA1. This confirmed that the loss of sialic acid and galactose might enhance the binding of polymeric IgA1 to HMC. However, the deglycosylated monomeric IgA1 could not bind to HMC in a dose-dependent, saturable manner, which suggested that sialic acid and galactose might be essential for native monomeric IgA1 binding to HMC through a receptor.
Oligosaccharides at the hinge region carry negatively charged sialic acid that is large and bulky compared with the protein backbone. Lack of terminal sialic acid might lead to alterations in electrostatic charge and tertiary structure, which might potentially affect interactions with proteins and receptors [31,38]. It has been shown that IgA1 with reduced galactosylation had an increased capacity for self-aggregation in vitro, perhaps favouring the deposition of macromolecular IgA1 [39]. Recent analysis has also demonstrated that degalactosylated IgA1 was a major component of circulating immune complexes in patients with IgA nephropathy, suggesting that the abnormal O-glycosylation might imply the classical immune complex deposition model [12]. The current study found that the polymeric deglycosylated IgA1 had increased binding capacity compared to native polymeric IgA1, which suggested that the deglycosylation and autoaggregation of IgA1 molecules might make spatial conformation of deglycosylated pIgA1 the most suitable structure for the binding to HMC. Therefore, it was suggested that the spatial conformation of the deglycosylated pIgA1 might play a crucial role in mesangial IgA1 deposition. Our current study suggests strongly that sugars might be of importance for the correct folding of IgA1 [23], and that abnormalities of O-linked glycans of IgA1 with spatial organization of IgA1 molecules clustered in circulating immune complex might influence their binding to HMC [21]. Finally, although we employed in vitro enzyme digestion to remove the glycans of IgA1, the in vitro-made deglycosylated IgA1 might be not completely deS/deGal IgA1. Moreover, it could not represent completely the serum IgA1 molecules in patients with IgAN; therefore, we need to investigate future the binding capacity with IgA1 isolated from patients with IgAN.
Conclusion
The current study suggests that the binding characteristics of the native monomeric and polymeric IgA1 on mesangial cells were different. Glycosylation of IgA1 molecules could affect significantly the binding of IgA1 on HMC.
Acknowledgments
We thank doctor Chun-li Zhang for excellent technical assistance. This work was supported by a grant from the Ministry of Health, People's Republic of China (2004230003), a grant from the ‘985’ project from Peking University and a grant from the National Natural Science Foundation of China (30570852).
References
- 1.Donadio JV, Grande JP. Immunoglobulin A nephropathy: a clinical perspective. J Am Soc Nephrol. 1997;8:1324–32. doi: 10.1681/ASN.V881324. [DOI] [PubMed] [Google Scholar]
- 2.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 3.Leung JC, Tsang AW, Chan LY, Tang SC, Lam MF, Lai KN. Size-dependent binding of IgA to HepG2, U937, and human mesangial cells. J Lab Clin Med. 2002. pp. 398–406. [DOI] [PubMed]
- 4.Hiki Y, Tanaka A, Kokubo T, et al. Analyses of IgA1 hinge glycopeptides in IgA nephropathy by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol. 1998;9:577–82. doi: 10.1681/ASN.V94577. [DOI] [PubMed] [Google Scholar]
- 5.Allen AC, Bailey EM, Barratt J, Buck KS, Feehally J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol. 1999;10:1763–71. doi: 10.1681/ASN.V1081763. [DOI] [PubMed] [Google Scholar]
- 6.Leung JC, Tang SC, Lam MF, Chan TM, Lai KN. Charge-dependent binding of polymeric IgA1 to human mesangial cells in IgA nephropathy. Kidney Int. 2001;59:277–85. doi: 10.1046/j.1523-1755.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 7.Iwase H, Katsumata T, Itoh A, et al. Detection of enriched Thomsen-Friedenrich antigen on IgA1 from IgA nephropathy patients. J Nephrol. 2002;15:703–8. [PubMed] [Google Scholar]
- 8.Sano T, Hiki Y, Kokubo T, Iwase H, Shigematsu H, Kobayashi Y. Enzymatically deglycosylated human IgA1 molecules accumulate and induce inflammatory cell reaction in rat glomeruli. Nephrol Dial Transplant. 2002;17:50–6. doi: 10.1093/ndt/17.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Hashim OH, Shuib AS, Chua CT. Neuraminidase treatment abrogates the binding abnormality of IgA1 from IgA nephropathy patients and the differential charge distribution of its alpha-heavy chains. Nephron. 2001;89:422–5. doi: 10.1159/000046114. [DOI] [PubMed] [Google Scholar]
- 10.Kerr MA. The structure and function of IgA. Biochem J. 1990;271:285–96. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu LX, Zhao MH. Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int. 2005;68:167–72. doi: 10.1111/j.1523-1755.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu LX, Yan Y, Zhang JJ, Zhang Y, Zhao MH. The glycans deficiencies of macromolecular IgA1 is a contributory factor of variable pathological phenotypes of IgA nephropathy. Clin Exp Immunol. 2005;142:569–75. doi: 10.1111/j.1365-2249.2005.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–73. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 14.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–85. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro RC, Halbwachs-Mecarelli L, Roque-Barreira MC, Noel LH, Berger J, Lesavre P. Charge and size of mesangial IgA in IgA nephropathy. Kidney Int. 1985;28:666–71. doi: 10.1038/ki.1985.181. [DOI] [PubMed] [Google Scholar]
- 16.Trascasa ML, Egido J, Sancho J, Hernando L. IgA glomerulonephritis (Berger's disease): evidence of high serum levels of polymeric IgA. Clin Exp Immunol. 1980;42:247–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y, Xu LX, Zhang JJ, Zhang Y, Zhao MH. Self-aggregated deglycosylated IgA1 with or without IgG were associated with the development of IgA nephropathy. Clin Exp Immunol. 2006;144:17–24. doi: 10.1111/j.1365-2249.2006.03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–16. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 19.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwase H, Yokozeki Y, Hiki Y, et al. Human serum immunoglobulin G3 subclass bound preferentially to asialo-, agalactoimmunoglobulin A1/Sepharose. Biochem Biophys Re Commun. 1999;264:424–9. doi: 10.1006/bbrc.1999.1369. [DOI] [PubMed] [Google Scholar]
- 21.Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M. Interaction of human mesangial cells with IgA and IgA-containing immune complexes. Kidney Int. 2002;62:465–75. doi: 10.1046/j.1523-1755.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura I, Iwase H, Arai K, et al. Detection of gender difference and epitope specificity of IgG antibody activity against IgA1 hinge portion in IgA nephropathy patients by using synthetic hinge peptide and glycopeptide probes. Nephrology. 2004;9:26–30. doi: 10.1111/j.1440-1797.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 23.Moura IC, Arcos-Fajardo M, Sadaka C, et al. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol. 2004;15:622–34. doi: 10.1097/01.asn.0000115401.07980.0c. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zhao MH, Zhang YK, Li XM, Wang HY. Binding capacity and pathopyhysiological effects of IgA1 from patients with IgA nephropathy on human glomerular mesangial cells. Clin Exp Immunol. 2004;136:168–75. doi: 10.1111/j.1365-2249.2004.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JJ, Xu LX, Zhang Y, Zhao MH. Binding capacity of in vitro deglycosylated IgA1 to human mesangial cells. Clin Immunol. 2006;119:103–9. doi: 10.1016/j.clim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Westerhuis R, Van Zandbergen G, Verhagen NA, Klar-Mohamad N, Daha MR, van Kooten C. Human mesangial cells in culture and in kidney sections fail to express Fc alpha receptor (CD89) J Am Soc Nephrol. 1999;10:770–8. doi: 10.1681/ASN.V104770. [DOI] [PubMed] [Google Scholar]
- 27.Iwase H, Tanaka A, Hiki Y, et al. Estimation of the number of O-linked oligosaccharides per heavy chain of human serum IgA1 by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) analysis of the hinge glycopeptide. J Biochem. 1996;120:393–7. doi: 10.1093/oxfordjournals.jbchem.a021425. [DOI] [PubMed] [Google Scholar]
- 28.Troyer DA, Kreisberg JI. Isolation and study of glomerular cells. Meth Enzymol. 1990;191:141–52. doi: 10.1016/0076-6879(90)91012-u. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood FC, Hunter WM, Glover JS. The preparation of 125I labeled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114–23. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppo R, Amore A, Roccatello D, et al. IgA antibodies to dietary antigens and lectin-binding IgA in sera from Italian, Australian and Japanese IgA nephropathy patients. Am J Kidney Dis. 1991;17:480–7. doi: 10.1016/s0272-6386(12)80644-5. [DOI] [PubMed] [Google Scholar]
- 31.Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA. 1982;79:6229–31. doi: 10.1073/pnas.79.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diven SC, Caflisch CR, Hammond DK, Weigel PH, Oka JA, Goldblum RM. IgA induced activation of human mesangial cells. independent of Fc alpha R (CD89) Kidney Int. 1998;54:837–47. doi: 10.1046/j.1523-1755.1998.00054.x. [DOI] [PubMed] [Google Scholar]
- 33.Barratt J, Greer MR, Pawluczyk IZ, et al. Identification of a novel Fc alpha receptor expressed by human mesangial cells. Kidney Int. 2000;57:1936–48. doi: 10.1046/j.1523-1755.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 34.Leung JC, Tsang AW, Chan DT, Lai K. Absence of CD89, polymeric immunoglobulin receptor, and asialoglycoprotein receptor on human mesangial cells. J Am Soc Nephrol. 2000;11:241–9. doi: 10.1681/ASN.V112241. [DOI] [PubMed] [Google Scholar]
- 35.Moura IC, Centelles MN, Arcos-Fajardo M, et al. Identification of the transferring receptor as a novel immunoglobulin (Ig) A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194:417–25. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald KJ, Cameron AJ, Allen JM, Jardine AG. Expression of Fc α/μ receptor by human mesangial cells: a candidate receptor for immune complex deposition in IgA nephropathy. Biochem Biophys Res Commun. 2002;290:438–42. doi: 10.1006/bbrc.2001.6218. [DOI] [PubMed] [Google Scholar]
- 37.Leung JC, Tang SC, Chan LY, Tsang AW, Lan HY, Lai KN. Polymeric IgA increases the synthesis of macrophage migration inhibitory factor by human mesangial cells in IgA nephropathy. Nephrol Dial Transplant. 2003;18:36–45. doi: 10.1093/ndt/18.1.36. [DOI] [PubMed] [Google Scholar]
- 38.Gomez Guerrero C, Gonzalez E, Egido J. Evidence for a specific IgA receptor in rat and human mesangial cells. J Immunol. 1993;151:7172–81. [PubMed] [Google Scholar]
- 39.Kokubo T, Hiki Y, Iwase H, et al. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol. 1998;9:2048–54. doi: 10.1681/ASN.V9112048. [DOI] [PubMed] [Google Scholar]