Abstract
Chronic Chagas heart disease (cChHD), a chronic manifestation of the Trypanosoma cruzi infection, is characterized by high antibody levels against the C-terminal region of the ribosomal P proteins (i.e. peptide R13, EEEDDDMGFGLFD) which bears similarity with the second extracellular loop of β1-adrenergic receptor (β1-AR, peptide H26R HWWRAESDEARRCYNDPKCCDFVTNR). Because it has not been demonstrated clearly that IgGs from cChHD patients bind to native human β1-AR, the aim of this study was to investigate further the physical interaction between cChHD IgGs and the human β1-AR. Immunofluorescence assays demonstrated the binding of these antibodies to the receptor expressed on stably transfected cells, together with a β1-AR agonist-like effect. In addition, immunoadsorption of the serum samples from cChHD patients with a commercially available matrix, containing peptides representing the first and the second extracellular loop of the β1-AR, completely abolished reactivity against the H26R peptide and the physiological response to the receptor. The follow-up of this specificity after in vitro immunoadsorption procedures suggests that this treatment might be used to diminish significantly the serum levels of anti-β1-AR antibodies in patients with Chagas heart disease.
Keywords: autoantibodies, β1-adrenergic receptor, chronic Chagas heart disease, immunoadsorption, Trypanosoma cruzi
Introduction
Chronic Chagas heart disease (cChHD) is defined as a cardiomyopathy with focal or disseminated inflammatory infiltrates, cardiac muscle destruction and progressive fibrosis caused by chronic infection with the protozoan parasite Trypanosoma cruzi [1,2]. Autoimmune responses against the β1-adrenergic receptor (β1-AR) have been proposed to be involved in the pathogenesis of this cardiac disease [3–5]. Our findings indicate that antibodies directed against the ribosomal P2β protein of T. cruzi (TcP2β) were able to cross-react with and stimulate the β1-AR. This reactivity was attributed to the highly antigenic acidic epitope present on the C-terminal end of the parasite ribosomal protein, named R13 (EEEDDDMGFGLFD), which bears similarity to an acidic motif (AESDE) on the second extracellular loop of the β1-AR [6–8]. Indeed, the functional effect of these autoreactive antibodies has been demonstrated using a classic pharmacological assay, considered the gold standard for assessment of anti-cardiac receptor antibody specificities, based on primary culture of neonatal rat cardiomyocytes [7]. Because IgGs with strong anti-β1-AR reactivity are associated with ventricular arrhythmias (VA) in cChHD [9,10], it has been suggested that their catecholamine-like action may play a major role in the pathophysiology of cChHD [3,6–8]. In experimental models, mice immunized with recombinant TcP2β protein that, in most cases, elicited anti-R13 antibodies with concomitant β1-adrenergic stimulating activity presented supraventricular tachycardia accompanied by premature death [8,11]. The pathogenic effect of this type of antibodies was confirmed by passive transfer of an anti-R13 monoclonal antibody (MoAb 17·2) [7] and its recombinant version, scFv C5 [12]. Both antibodies induced supraventricular tachycardia in recipient animals [7,12].
The presence of antibodies against β1-AR has also been described in idiopathic dilated cardiomyopathy (IDC) [13,14]. Recently, Jahns et al. obtained evidence from experimental animal models implying a significant role of the cardiac β1-AR as a pathophysiologically and clinically relevant autoantigen in human IDC [15,16]. Interestingly, immunoadsorption of this type of antibody with a matrix containing peptides representing the first and the second extracellular loop of the β1-AR, namely the Coraffin column, led to restoration of cardiac function, suggesting that this type of treatment could be used as a novel therapeutic alternative for cChHD patients with VA [17,18].
In contrast to anti-β1-AR autoantibodies in IDC [15,16], it has not yet been demonstrated unambiguously that neither IgGs from cChHD patients nor human immunopurified anti-R13 antibodies interact physically with native human β1-AR. Accordingly, the aim of this study was to explore further the interaction between cChHD IgGs and this adrenoceptor. Our results demonstrate a clear binding of these antibodies to the mentioned receptor in a cellular setting, and show that they act as β1-AR agonists. The follow-up of this specificity after in vitro immunoadsorption procedures suggests that this treatment might be used in the future to decrease the serum levels of anti-β1-AR antibodies in patients with Chagas' disease.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM:F12 (F-12), geneticin (G418 sulphate), penicillin G, streptomycin sulphate, LipofectamineTM reagent and pcDNA-3·1 eukaryotic expression vector carrying the NEO gene were obtained from Invitrogen Gibco (ny, USA). Bradford reagent was purchased from Bio-Rad (Hercules, CA, USA). NitrocelluloseHybond C membranes, I-[4,6-propyl-3H]dihydroalprenolol [(DHA), 3·59 TBq/mmol, 97·0 Ci/mmol] (–)-[3H]CGP-12177 (1·92 TBq/mmol, 52·0 Ci/mmol) and cAMP enzyme immunoassay (EIA) were purchased from Amersham Pharmacia (London, UK). Coraffin matrix was obtained from Fresenius Medical Care Affina GmbH (Berlin, Germany). Peroxidase conjugated anti-human IgG (H + l), atropine, DL-propranolol hydrochloride (–)-isoproterenol (+)-bitartrate salt (ISO) and bisoprolol were purchased from Sigma-Aldrich (St Louis, MO, USA). Texas red-labelled goat anti-mouse IgG (H + l) and goat anti-human IgG labelled with fluorescein isothiocyanate (FITC) were purchased from Jackson ImmunoResearch (Baltimore, USA).
Patient population
Serum samples were obtained from 32 patients with cChHD and 20 healthy individuals recruited initially at the Ramos Mejia and Fernandez Hospitals, Buenos Aires, Argentina.
The patients were then classified according to the severity of heart disease. Group I consisted of 20 patients with ventricular arrythmia (VA), group II comprised 10 patients with other rhythm disturbances and group III included two asymptomatic patients. Healthy individuals (HI) made up the control group. The study protocol complied with the Helsinki Declaration and was approved by the Committee for Ethical and Legal aspects of Research (CELAR) of the Instituto de Investigaciones en Ingenieria Genetica y Biologia Molecular, Buenos Aires, Argentina.
Synthetic peptides
Peptides R13 (representing C-terminal region of TcP2β) and H26R (representing a region of the second extracellular loop of the human β1-AR) were synthesized as described previously [7].
Monoclonal antibodies
MoAb M16 raised against H26R peptide was prepared as described in Mobini et al. [19]. Both MoAbs 17·2 against R13 peptide and 40·14 against a central epitope of TcP2β were prepared as described by Mahler et al. [7].
Human IgG fractions
The IgG fractions from 32 cChHD patients and 20 healthy individuals (HI) were prepared as described by Mahler [10]. Protein concentration was determined by Bradford reagent and expressed as total IgG concentration (mg/ml).
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as described previously [5,10]. Briefly, T. cruzi epimastigote lysate (50 µg/well), recombinant glutathione S-transferase (GST)-TcP2β protein (2 µg/ml) and both R13-bovine serum albumin (BSA) (1 µM) or H26R (10 µM) peptides were coated overnight at 4°C in 0·05 M bicarbonate–carbonate buffer (pH 9·6). Bound IgG fractions (dilution 1/50–1/200) were detected with peroxidase-conjugated anti-human IgG (H + l) at 37°C for 1 h. Cut-off values were determined as described in [10]. Sera with ratio values above the cut-off line were considered positive for antigens.
For competition ELISA, IgG fractions in an appropriate dilution [yielding an optical density (OD) of 1·0] were preincubated with peptide concentrations ranging from 0 to 625 µM for 1 h at room temperature under agitation and then added to the R13-BSA or H26R-coated plate as described previously.
Characterization of IgG fractions from cChHD patients
Immunoreactivities of sera, IgG fractions and immunopurified antibodies were assessed against T. cruzi lysate, the recombinant ribosomal P protein TcP2β and its derived R13 peptide by ELISA. Table 1 shows the prevalence of antibodies in the different cChHD patient groups. All sera from patients included in groups I and III showed reactivity against T. cruzi lysate, TcP2β and R13 peptide, while only 40% of patients from group II presented reactivity against these antigens. No reactive antibodies were detected in HI.
Table 1.
Prevalence of anti-Trypanosoma cruzi antibodies present in IgG fractions from chronic Chagas heart disease (cChHD) patients.
| % Prevalence | |||
|---|---|---|---|
| T. cruzi | TcP2β | R13 | |
| AV cChHD (group I) (n = 20) | 100 | 100 | 100 |
| Other rhythm disturbances cChHD (group II) (n = 10) | 40 | 40 | 40 |
| cChHD asymptomatic (group III) (n = 2) | 100 | 100 | 100 |
| HI | 0 | 0 | 0 |
(n = 20). Reactivity pattern of IgGs from cChHD patients (n = 32) against T. cruzi lysate, P2β protein and its derivate R13 peptide. The prevalence of specific antibodies was determined by enzyme-linked immunosorbent assay, considering the optical density values obtained for sera of HI (n = 20) (see Materials and methods). HI: healthy individuals.
Affinity purification of anti-R13 antibodies
Anti-R13 antibodies from cChHD patients were purified with GST-TcP2β coupled to circular nitrocellulose Hybond C membranes, as described previously [10]. Antibody reactivity against TcP2β and R13 peptide was detected by ELISA.
Immunoadsorption
Immunoadsorption was performed in six sera from cChHD patients with strong anti-β1-AR reactivity. Sera were immunoadsorbed with 400 µl of Coraffin matrix (Fresenius Medical Care Affina GmbH) for 1 h at 4°C and the IgGs were eluted by glycine-HCl 1 M (pH 2·8). The remaining activity against H26R peptide was tested by ELISA. The Coraffin matrix contains two different peptide ligands: PDCM349 peptide is related to the first extracellular loop and PDCM075 peptide to the second extracellular loop of the β1-AR [20].
Stable transfection of COS-7 and CHO-K1 cells
COS-7 and CHO-K1 cells were cultured in DMEM and F12 supplemented with 10% fetal bovine serum (FBS) (Natocor, Cordobu, Argentina), penicillin G (100 units/ml) and streptomycin sulphate (100 µg/ml) and kept in a humidified 5% CO2/95% air atmosphere at 37°C.
pBC expression vectors containing the human β1-AR cDNA were kindly provided by Dr R. J. Lefkowitz (Duke University, Durham, NC, USA). The cDNA was subcloned into pcDNA-3·1 mammalian expression vector. One μg of β1-pcDNA3·1 plasmid was mixed with lipofectamine in accordance with the manufacturer's instructions. Cells were selected for resistance to Geneticin. Cell lines stably expressing β1-AR were obtained by limited dilution of resistant cultures and screening for β1-AR expression by indirect immunofluorescence (IIF) assay and radioligand binding on whole cells. For this study, we selected transfected COS-7 and CHO-K1 cell lines named hereafter β1-COS-A3 and β1-CHO-F10. Non-transfected COS-7 and CHO-K1 cells and cells transfected with the original transfection vector were used as negative controls.
Ligand binding assays on whole cells
The expression of β1-AR and KD values were determined by saturation binding assay on whole cells. Stably transfected β1-COS-A3 or β1-CHO-F10 cells were incubated with [3H]-DHA at different concentrations (0·023–60 nM) for 2 h at 4°C. After incubation, cells were washed with ice-cold phosphate-buffered saline (PBS) and then lysed by incubation with 0·25 M NaOH and 1% sodium dodecyl sulphate (SDS). Finally, the cells were assayed for [3H] and protein concentration.
Receptor density was 0·380 ± 0·032 pmol/mg of protein for COS and 0·412 ± 0·027 pmol/mg of protein for CHO cells, and for stably transfected cells receptor density was 1·230 ± 0·070 pmol/mg of protein for β1-COS-A3 and 1·540 ± 0·080 pmol/mg of protein for β1-CHO-F10. The KD values were 8·90 ± 1·80 nM and 29·50 ± 2·90 nM for β1-COS-A3 and β1-CHO-F10, respectively.
Binding competition experiments were carried out at a single concentration of [3H]-DHA (2·5 nM) or [3H]-CGP-12177 (2·5 nM) and with different concentrations of DL-propranolol ranging from 10−8 to 10−3 mol/l. As expected for the β1-AR, DL-propranolol displays a Ki value consistent with those reported by others [21,22] (data not shown). For both assays, non-specific binding was determined in the presence of 10 µM DL-propranolol and was less than 15% of total binding with [3H]-DHA (2·5 nM) and 10% with [3H]CGP-12177 (2·5 nM).
Immunofluorescence assay
The assay was performed on both monolayers β1-COS-A3 or β1-CHO-F10 cells grown on glass coverslips. Priorto immunofluorescence, cells were washed twice with ice-cold PBS pH 7·4 and fixed with formaldehyde 3·8% (v/v). Residual formaldehyde was quenched by addition of 0·02 M glycine in PBS pH 7·4. Each manipulation was preceded by washing the cells three times in PBS, 0·05% Tween 20. After blocking in PBS–BSA 5%, the cells were treated with: MoAb M16 (150 nM), MoAb 17·2 (150 nM), IgG fractions (150 nM) and immunopurified anti-R13 antibodies (150 nM). Bound antibodies were detected by fluorescence using FITC-labelled goat anti-mouse IgG (H + l) diluted 1 : 100 (v/v) for MoAbs and with a goat anti-human IgG labelled with FITC diluted 1 : 100 (v/v) for human IgGs. Images were obtained using a Zeiss confocal microscope LSM-510 system with a highly corrected objective (C-Apochromat × 40 numerical aperture 1·2 under water) and processed by Image Browser 3·0.
cAMP accumulation assay
β1-COS-A3 and β1-CHO-F10 cells (∼105/well) were seeded in 96-well culture plates 24 h before stimulation, washed with PBS and then incubated with 1 ml of Hanks' balanced medium buffer containing 10 mM HEPES plus 100 µM isobutylmethylxanthine (IBMX) to block 3′,5′-cyclic adenosine monophosphate (cAMP) hydrolysis for 30 min at 37°C. cAMP levels were measured after incubation with 1 µM isoproterenol (ISO), 150 nM MoAbs and 150 nM IgGs for different times at 37°C. The total cellular cAMP content was determined using the commercial cAMP enzyme immunoassay (Amersham Pharmacia). cAMP concentration was expressed as pmol of cAMP/mg of protein. Results were from duplicates of three independent experiments.
Internalization assay
β1-AR internalization was determined using [3H]CGP-12177, a hydrophilic β-AR ligand, as described by Hupfeld and Staehelin [23,24]. Briefly, transfected cells were treated with either 150 nM MoAbs and 150 nM IgG fractions for the indicated times at 37°C. The reaction was stopped by washing with ice-cold PBS. Thereafter, cells were incubated with 2·5 nM [3H]-CGP-12177 in binding buffer (DMEM, 20 nM HEPES) for 4 h at 4°C. Receptor internalization was defined as the percentage reduction in surface receptors following agonist stimulation, relative to matched control cells. For all assays, 10 µM DL-propranolol was used to define non-specific binding.
Measurement of beating frequency of neonatal rat cardiomyocytes
Cardiac myocytes were prepared from 2–4-day-old neonatal Sprague-Dawley female rat hearts by the enzymatic method and the functional assay was performed as described previously [10].
Data analysis
Unless indicated otherwise, all experiments were repeated at least three times. The results from each representative experiment are presented in the figures and tables. The data are given as mean ± standard deviation (s.d.) of the replicates. The maximal number of binding sites (Bmax) and KD were calculated by non-linear regression, with r > 0·90 as a criterion for acceptability of the data using Graphpad Prism (GraphPad Software Inc., San Diego, CA, USA).
Results
Physical interaction between IgGs and human β1-AR
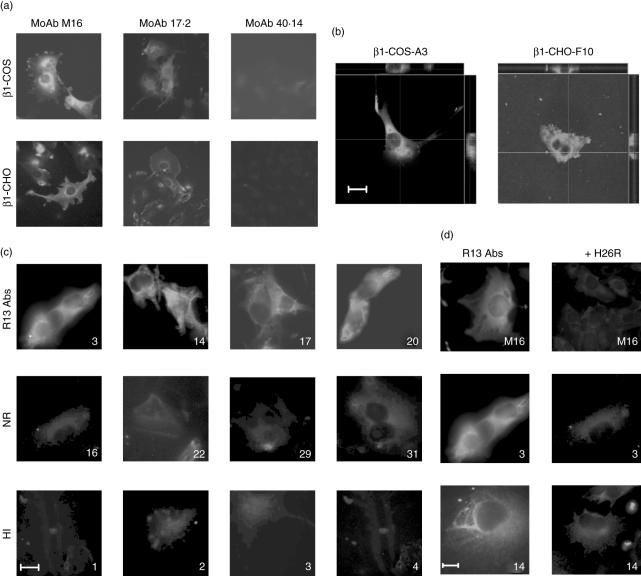
After transfection of β1-COS-A3 and β1-CHO-F10 cells with the human β1-AR, we used the anti-β1-AR MoAb M16 [19] to assess the stable expression and localization of the receptor by indirect immunofluorescence (IIF). Figure 1a,b shows that the human β1-AR was localized primarily on plasma membrane, forming a smooth rim around the cell and a punctuated labelling inside the cytoplasm, which may be due to receptor clustering and/or high receptor density in vesicles. MoAb 17·2, directed against the C-terminal epitope of TcP2β (R13 peptide), presented a similar pattern, albeit less intense, whereas MoAb 40·14, directed against an internal epitope of TcP2β, did not react with these cell lines (Fig. 1a). Staining was not observed in non-transfected cells (data not shown).
Fig. 1.
Immunofluorescence of β1-adrenergic receptor (AR) on stably transfected cells. (a) Micrographs analysis of stably transfected β1-COS and β1-Chinese hamster ovary (CHO) cells, showing the immunostaining pattern with monoclonal antibodies (MoAbs) M16, 17·2 and 40·14. (b) Selected clones, both β1-COS-A3 and β1-CHO-F10, stained with MoAb M16. Images taken by confocal microscopy and computer-generated cross-sections displayed on the top (x–z plane) and on the right (y–z plane) showed that β1-AR was distributed primarily on the plasma membrane of the selected clones. These images are representative of six separate experiments. (c) Physical interaction between IgGs from chronic Chagas heart disease (cChHD) patients with β1-AR. β1-CHO-F10 were incubated with immunopurified anti-R13 antibodies (R13 antibodies), IgGs that did not possess anti-β1-AR reactivity (NR) or IgG fractions from healthy individuals (HI). (d) Stably transfected β1-CHO-F10 cells were incubated with either MoAb M16 or immunopurified anti-R13 antibodies (p3, p14) in the presence or absence of H26R peptide. Bar, 10 µm.
In order to determine interaction between IgG fractions from cChHD patients with the β1-AR, IgGs were incubated with the transfected cells. The presence of anti-β1-AR IgGs was revealed by a staining pattern similar to that described above for MoAb M16 (Fig. 1a). Interestingly, 20 of 32 different IgG fractions showed a positive reaction with the β1-AR by immunofluorescence (Fig. 1c). These 20 IgG preparations also induced a positive chronotropic effect on neonatal rat cardiomyocytes, indicating complete agreement between both different tests (Table 2). On the other hand, only 16 of the 20 β1-AR reactive IgGs reacted with the H26R peptide in ELISA (Table 2).
Table 2.
Correlation between β1-adrenergic receptor (AR) recognition and functional effects induced by IgG fractions from chronic Chagas heart disease (cChHD) patients.
| Patient | ELISA H26R | IIF β1-COS-A3 and β1-CHO-F10 | Functional assay | |
|---|---|---|---|---|
| AV cChHD (group I) | p1 | + | + | β1-AR |
| p2 | + | + | β1-AR | |
| p3 | +++ | + | β1-AR | |
| p4 | – | + | β1-AR | |
| p6 | + | + | β1-AR | |
| p8 | ++ | + | β1-AR | |
| p9 | ++ | + | β1-AR | |
| p10 | – | + | β1-AR and M2-Ch | |
| p11 | + | + | β1-AR and M2-Ch | |
| p12 | ++ | + | β1-AR | |
| p13 | ++ | + | β1-AR | |
| p14 | +++ | + | β1-AR | |
| p15 | ++ | + | β1-AR and M2-Ch | |
| p17 | +++ | + | β1-AR and M2-Ch | |
| p18 | – | + | β1-AR and M2-Ch | |
| p20 | ++ | + | β1-AR | |
| p21 | + | + | β1-AR and M2-Ch | |
| p25 | – | + | β1-AR and M2-Ch | |
| p26 | + | + | β1-AR and M2-Ch | |
| p27 | + | + | β1-AR and M2-Ch | |
| Prevalence (%) | 80 | 100 | 100 | |
| Rhythms disturbance cChHD (group III) | p5 | – | – | M2-Ch |
| p7 | – | – | M2-Ch | |
| p16 | – | – | n.a. | |
| p19 | – | – | n.a. | |
| p22 | – | – | n.a. | |
| p23 | – | – | M2-Ch | |
| p24 | – | – | n.a. | |
| p28 | – | – | n.a. | |
| p29 | – | – | n.a. | |
| p30 | – | – | M2-Ch | |
| Prevalence (%) | 0 | 0 | 0 | |
| Asymptomatic cChHD (group III) | p31 | – | – | n.a. |
| p32 | – | – | n.a. | |
| Prevalence (%) | 0 | 0 | 0 |
Reactivity pattern of IgGs from cChHD patients (n = 32) against H26R peptide by enzyme-linked immunosorbent assay (ELISA), considering the optical density (OD) values obtained for sera of healthy individuals (HI) (n = 20) (see Materials and methods). –: OD < 0·2; +: 0·2–0·4; ++: 0·4–0·6; +++: > 0·6. Immunofluorescence on β1-AR stably transfected cells and functional assay on neonatal rat cardiomyocytes were performed as described in Materials and methods; n.a.: no activity.
In order to confirm that the physical interactions of cChHD IgGs with the β1-AR were due, at least in part, to the presence of antibodies against the C-terminal end of TcP2β, we immunopurified anti-R13 antibodies from four patients with high anti-R13 antibody levels (p3, p14, p17, p20). These immunopurified antibodies showed a strong receptor-specific immunostaining pattern on the surface of the cells, similar to those obtained with total IgG fractions (Fig. 1c). No staining was observed with IgGs from cChHD patients that do not possess anti-β1-AR reactivity, nor with IgG fractions from healthy individuals (Fig. 1c, Table 2).
Preincubation of either MoAb M16 or immunopurified anti-R13 antibodies (p3 and p14) with H26R peptide abolished the immunostaining on β1-COS-A3 cells, as shown in Fig. 1d, confirming the specificity of this interaction. Similar results were obtained with total IgG fractions from cChHD patients (data not shown).
cAMP accumulation induced by IgGs from cChHD patients
To substantiate further the ability of both IgG fractions and immunopurified anti-R13 antibodies to interact with β1-AR and activate adenylate cyclase, we measured receptor-dependent accumulation of cAMP in β1-COS-A3 and β1-CHO-F10 cells.
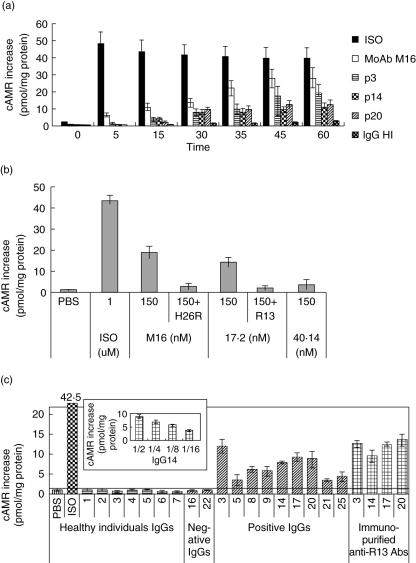
The long-lasting increment of cAMP induced by anti-β1-AR antibodies contrasts with the β1-agonist ISO effect, as ISO induced a fast AMPc accumulation (Fig. 2a). Maximal response to anti-β1-AR was obtained 60 min after stimulation with different IgG fractions and MoAb M16 (Fig. 2a).
Fig. 2.
3′,5′-cyclic adenosine monophosphate (cAMP) levels on β1-COS-7 cells treated with IgG fractions and immunopurified anti-R13 antibodies. (a) Time–course of cAMP accumulation: effect of IgGs from three patients (p3, p14 and p20) with high anti-β1-adrenergic receptor (AR) antibodies and healthy individual (HI) on cAMP production in β1-COS-A3 transfected cells, compared with isoprotenerol (ISO). (b) β1-COS-A3 cells were stimulated with monoclonal antibodies for 1 h at 37°C or with ISO for 5 min at 37°C. The reaction was inhibited by preincubation with specific peptides. (c) Cells were incubated with human IgG fractions from chronic Chagas heart disease (cChHD), healthy individuals or immunopurified anti-R13 antibodies for 1 h at 37°C. Inset: dose–response curve of IgG from p14 on cAMP accumulation. cAMP concentration was expressed as pmol of cAMP/mg protein. The results were normalized using cAMP content of untreated cells as 100%. Results are expressed as the mean ± s.d. of three independent experiments carried out in triplicate.
Figure 2b shows that both MoAb M16 and MoAb 17·2 induced an increase in cAMP that was abolished by preincubation of these MoAbs with H26R and R13 peptides, respectively, confirming the specificity of each interaction. As expected, no effect was observed with MoAb 40·14. Moreover, IgG fractions reactive against the β1-AR as assessed by IIF and the cardiomyocyte assay, also induced cAMP accumulation in β1-COS-A3 cells (Fig. 2c), confirming that these antibodies stimulate the β1-AR. Immunopurified anti-R13 fractions also induced an increase on basal cAMP levels. This indicates clearly that anti-R13 antibodies contribute to the functional effect of the IgG fractions described above. Moreover, it is noteworthy that these effects were dose-dependent (Fig. 2c and inset). No cAMP accumulation was observed for IgG fractions with no functional activity on cardiomyocytes, nor with IgG fractions from healthy individuals (HI, n = 20) (Fig. 2c). The response of β1-CHO-F10 cells to different stimuli was similar to that observed with β1-COS-A3 cells (data not shown).
β1-AR internalization
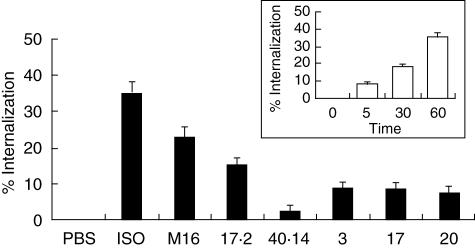
We examined the effect of antibodies on β1-AR internalization and compared it with the agonist-induced internalization. The number of β1-ARs remaining on the cell surface after stimulation was determined by hydrophilic antagonist [3H]-CGP-12177 binding on whole cells. When β1-CHO-F10 cells were stimulated with 1 µM ISO (5 min: 7 ± 1·8%, 60 min: 35·3 ± 2·9%) a substantial time-dependent decrease in the number of receptor molecules was registered (Fig. 3, inset). Stimulation of β1-CHO-F10 cells with MoAbs M16 and 17·2 also caused a decrease in the number of β1-AR: 23·1 ± 2·7% and 15·8 ± 1·5%, respectively (Fig. 3). No changes were observed in the total number of receptor molecules when incubated with MoAb 40·14 (Fig. 3). IgG fractions caused a decrease in cell surface β1-AR of 8 ± 2% (Fig. 3). Similar results were obtained using β1-CHO-F10 cells (data not shown).
Fig. 3.
Effect of IgGs on β1-adrenergic receptor (AR) internalization. β1-Chinese hamster ovary (CHO)-F10 cells (∼ 2 pmol of β1-AR/mg of protein) were treated with isoprotenerol (ISO), monoclonal antibody (MoAb) M16, MoAb 17·2 and IgG fractions for 1 h. The IgGs-promoted internalization was measured by assessing the proportion of β1-binding sites accessible to the hydrophilic ligand [3H]-CGP-12177 and expressed as percentage internalization, as described in Material and methods. Inset: β1-CHO-F10 cells were incubated in the presence or absence of ISO.
Immunoadsorption of anti-β1-AR antibodies from serum samples of cChHD patients
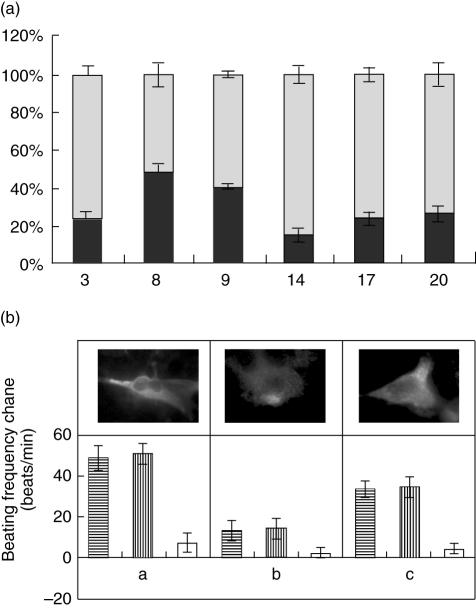
Because removal of autoantibodies against the β1-AR by immunoadsorption has been proposed as a potential therapy for improvement of left ventricular function in IDC [39,40], we tested the use of the Coraffin matrix (see Materials and methods) to deplete anti-β1-AR antibodies from serum samples of cChHD patients. Samples of six cChHD patients (p3, p8, p9, p14, p17, p20) were selected due to their strong reactivity against T. cruzi lysate. They presented a strong response to TcP2β protein, R13 and H26R peptides and a clear positive anti-β1-AR pattern as determined by IIF (Table 2). The reactivity of antibodies retained by the Coraffin matrix, as well as those in the flow-through fraction, were assessed. IgGs from the different flow-through fractions showed a strong decrease of reactivity against H26R peptide and had no ability to increase the beating rate of rat cardiomyocytes, and did not react with β1-COS-A3 and β1-CHO-F10 cells (Fig. 4). In contrast, IgGs eluted from the captured fractions possessed reactivity against H26R, as assessed by ELISA, IIF and functional assays, which were similar to those measured for the initial serum preparations (Fig. 4). This is shown for patient p3 in Fig. 4b. The depletion of β1-AR antibodies by treatment with the Coraffin matrix was demonstrated by IIF on β1-AR stably transfected cell lines and by cardiomyocyte assays.
Fig. 4.
β1-Immunoadsorption sera from chronic Chagas heart disease (cChHD) patients. (a) Coraffin immunoadsorption. Enzyme-linked immunosorbent assay reactivity of Coraffin immunoadsorbed sera from cChHD patients with strong anti-β1-adrenergic receptor (AR) reactivity. Results represent the difference between the total reactivity before immunoadsorption (100%), the flow-through fraction reactivity and the captured fraction reactivity of these IgGs. (b) Chronotropic effect of serum p3. The specificity of this effect was studied by subsequent addition of the M2-ChR antagonist atropine and the β1-AR antagonist bisoprolol. Upper panel, immunostaining pattern of serum p3: before immunoadsorption (a), flow-through fraction (b) and captured fraction (c).
Discussion
The existence of autoantibodies to surface structures of heart tissues has been a matter of controversy in the field of Chagas' disease [25,26]. Initial findings have been contested, although consistent results were obtained from pharmacological studies [27]. Direct molecular evidence for the presence of such autoreactive antibodies was difficult to obtain because tissues and cells used to record their presence contain a variable and/or not clearly defined population of different G protein-coupled receptors. In this work, we determined conditions in which stably transfected β1-AR-expressing cell lines, named β1-COS-A3 and β1-CHO-F10, could be used to detect anti-β1 antibody specificities. As shown in Fig. 1c, for the first time we were able to demonstrate direct binding and hence physical interaction of human IgG fractions from cChHD patients, including immunopurified anti-R13 antibodies with the human β1-AR in a cellular setting. Notably, the reactivity assessed by IIF was in complete agreement with results obtained for each of the IgG preparations using functional assays and performed better than ELISA using H26R peptide as reactive reagent (Table 2).
Stably expressing β1-COS-A3 and β1-CHO-F10 cells proved to be efficient tools to study the functional effects of antibodies from patients with cChHD. The transfected receptor maintained its biochemical and signal transduction properties as demonstrated after ISO treatment, cAMP assay (Fig. 2) [28–30]. Our results show that human IgG fractions and immunopurified anti-R13 antibodies possess the ability to increase cAMP levels in these cell lines, just as observed by incubation with murine antibodies such as MoAbs M16 and 17·2 (Fig. 2). Altogether, these data indicate that antibodies developed during Chagas' disease not only bind, but also induce activation of β1-AR by a mechanism involving the molecular signalization pathway elicited by β1-AR agonists.
Furthermore, these cells allowed an initial evaluation of receptor internalization levels induced by cChHD antibodies and murine MoAbs. Figure 3 shows that exposure of β1-COS-7-A3 cells to different MoAbs induced a lower internalization effect than the agonist ISO [31–33]. The low level of internalization shown by human IgG fractions seem to be in agreement with results obtained by Wallukat et al., who demonstrated the lack of short-term desensitization of beta adrenergic activation cascade by anti-β1-AR antibodies from IDC patients [34].
Previous experiments performed in mice, such as immunization with T. cruzi ribosomal P proteins, indicated that an antibody response against parasite epitopes may be pathogenic by exerting a direct adrenalin-like effect on cardiac rhythm [11,12]. Similarly, chronic adrenergic stimulation mediated by antibodies may have a cardiotoxic effect resembling that caused by catecholamines, which induce cardiac changes similar to those observed in Chagas' disease, namely induction of microfocal lesions associated with a mononuclear cell infiltrate with increased involvement of the left ventricle, and particularly the left ventricular apex [35]. Indeed, an immunological response against β1-AR provoked by immunization with peptides derived from the second extracellular loop of this receptor caused structural damage to myocardial cells and tissue [36,37]. Jahns et al. were able to demonstrate the potential pathogenic significance of autoantibodies targeting cardiac β1-AR in a passive transfer experiment performed with rats [38]. That these experimental models reflect the pathogenic relevance of this type of antibody is supported by the fact that in T. cruzi-infected individuals these antibody specificities correlate with cardiac rhythm disturbances and disorders [9,39]. Because immunoadsorption of circulating anti-β1-AR antibodies has been found to improve myocardial performance in patients with IDC [18,40,41], it can be hypothesized that removal of circulating antibodies may lead to an improvement of cardiac symptoms in cChHD patients. Our in vitro immunoadsorption experiments indicate that the commercially available Coraffin matrix, containing peptides representing the first and the second extracellular loop of β1-AR, was able to deplete anti-β1-AR antibodies from serum samples of cChHD patients and that β1-COS-A3 and β1-CHO-F10 cell lines are appropriate diagnostic reagents to follow this procedure. In general, our results demonstrate that IIF assays based on stably transfected cells are well suited for monitoring the anti-β1 adrenergic reactivity of sera from cChHD patients.
In summary, this work provides direct evidence that β1-AR was recognized specifically by antibodies developed during cChHD. This physical interaction may explain the pathogenic role of these antibodies and at least some of the electrical manifestations in the myocardium of chronically infected patients. We also showed that it may be possible to remove these antibodies by immunoadsorption with a commercially available matrix. Further experiments are needed to confirm that this procedure may be an efficient therapeutic tool for cChHD patients with cardiac complex arrhythmias and measurable levels of circulating β1-AR antibodies.
Acknowledgments
The authors express their gratitude to Dr Johan Hoebeke for interest and helpful criticism of the manuscript. The authors also acknowledge Dr R. Mobini for kindly sending and allowing the use of MoAb anti-β1-AR in these studies (Wallenberg Laboratory for Cardiovascular Research, Goteborg, Sweden). During the entire development of this work, M. J. L. was supported mainly by an International Research Grant from the Howard Hughes Medical Institute (Chevy Chase, MD, USA). This research was also supported by grants from the World Health Organization/Special Program for Research and Training in Tropical Diseases; University of Buenos Aires, Health and Social Action Office-fellowship Ramón Carrillo-Arturo Oñativia and the National Agency of Scientific and Technological Promotion (FONCYT BID 1201/OC-AR 01–14389). The support of CNRS-CONICET (2001–02) and INSERM-CONICET (2002–03) collaborative French–Argentinean research grants, as well as the ECOS-Sud project ‘Anticorps antiproteines ribosomales P de T. cruzi comme inhibiteur specifique de la traduction’ (France–Argentine, 2005–08) are acknowledged. This paper was written while M. J. L. was international professor (2005–06) of a Chaire Internationale de Recherche Blaise Pascal, Fondation Ecole Normale Superieure, Region Ile de France, Paris, France.
References
- 1.Rosenbaum MB. Chagasic cardiomyopathy. Prog Cardiovasc Dis. 1964;7:99–225. doi: 10.1016/s0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- 2.Elizari MV, Chiale PA. Cardiac arrhythmias in Chagas' heart disease. J Cardiovasc Electrophysiol. 1993;4:596–608. doi: 10.1111/j.1540-8167.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum MB, Chiale PA, Schejtman D, et al. Antibodies to α adrenergic receptors disclosing agonist-like properties in idiopathic dilated cardiomyopathy and Chagas' heart disease. J Cardiovasc Electrophysiol. 1994;5:367–75. doi: 10.1111/j.1540-8167.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 4.Masuda MO, Levin MJ, Farias de Oliveira S, et al. Functionally active cardiac antibodies in chronic Chagas'disease are specifically blocked by Trypanosoma cruzi antigens. FASEB J. 1998;12:1551–8. doi: 10.1096/fasebj.12.14.1551. [DOI] [PubMed] [Google Scholar]
- 5.Elies R, Ferrari I, Wallukat G, et al. Structural and functional analysis of the B cell epitopes recognized by anti-receptor autoantibodies in patients with Chagas' disease. J Immunol. 1996;157:4203–11. [PubMed] [Google Scholar]
- 6.Kaplan D, Ferrari I, Lopez Bergami P, et al. Antibodies to ribosomal P proteins of T. cruzi in Chagas' disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus. Proc Natl Acad Sci USA. 1997;94:10301–6. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahler E, Sepulveda P, Jeannequin O, et al. A monoclonal antibody against the immunodominant epitope at the ribosomal P2β protein of Trypanosoma cruzi interacts with the human beta 1 adrenergic receptor. Eur J Immunol. 2001;31:2210–6. doi: 10.1002/1521-4141(200107)31:7<2210::aid-immu2210>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Bergami P, Scaglione J, Levin MJ. Antibodies against the carboxyl-terminal end of the Trypanosoma cruzi ribosomal P proteins are pathogenic. FASEB J. 2001;15:2602–12. doi: 10.1096/fj.01-0132com. [DOI] [PubMed] [Google Scholar]
- 9.Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–71. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 10.Mahler E, Hoebeke J, Levin MJ. Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 beta protein in patients with chronic Chagas' heart disease. Clin Exp Immunol. 2004;136:527–34. doi: 10.1111/j.1365-2249.2004.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bergami P, Gomez KA, Levy GV, et al. The beta1 adrenergic effects of antibodies against the C-terminal end of the ribosomal P2beta protein of Trypanosoma cruzi associate with a specific pattern of epitope recognition. Clin Exp Immunol. 2005;142:140–7. doi: 10.1111/j.1365-2249.2005.02885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smulski C, Labovsky V, Levy G, et al. Structural basis of the cross-reaction between an antibody to the Trypanosoma cruzi ribosomal P2β protein and the human β1 adrenergic receptor. FASEB J. 2006;20:1396–406. doi: 10.1096/fj.05-5699com. [DOI] [PubMed] [Google Scholar]
- 13.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1998;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 14.Fu M, Matsui S. Cardiomyopathy an autoimmune disease? Keio J Med. 2002;51:208–12. doi: 10.2302/kjm.51.208. [DOI] [PubMed] [Google Scholar]
- 15.Jahns R, Boivin V, Siegmund C, et al. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1998;99:649–54. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 16.Jahns R, Boivin V, Lohse MJ. Beta(1)-adrenergic receptor function, autoimmunity, and pathogenesis of dilated cardiomyopathy. Trends Cardiovasc Med. 2006;16:20–4. doi: 10.1016/j.tcm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Wallukat G, Muller J, Hetzer R. Specific removal of β1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 18.Mobini R, Staudt A, Felix SB, et al. Hemodynamic improvement and removal of autoantibodies against β1-adrenergic receptor by immunoadsorption therapy in dilated cardiomyopathy. J Autoimmun. 2003;20:345–50. doi: 10.1016/s0896-8411(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 19.Mobini R, Fu M, Wallukat G, et al. A monoclonal antibody directed against an autoimmune epitope on the human beta1-adrenergic receptor recognized in idiopathic dilated cardiomyopathy. Hybridoma. 2000;19:135–42. doi: 10.1089/02724570050031176. [DOI] [PubMed] [Google Scholar]
- 20.Rönspeck W, Brinckmann R, Egner R, et al. Peptide based adsorbers for therapeutic immunoadsorption. Ther Apheresis Dialysis. 2003;7:91–7. doi: 10.1046/j.1526-0968.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 21.Marquardt DL, Wasserman SI. Characterization of the rat mast cell beta-adrenergic receptor in resting and stimulated cells by radioligand binding. J Immunol. 1982;129:2122–7. [PubMed] [Google Scholar]
- 22.Staehelin M, Simons P, Jaeggi K, et al. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983;258:3496–502. [PubMed] [Google Scholar]
- 23.Hupfeld CJ, Dalle S, Olefsky JM. β-Arrestin 1 down-regulation after insulin treatment is associated with supersensitization of beta 2 adrenergic receptor Galpha s signaling in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2003;100:161–6. doi: 10.1073/pnas.0235674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staehelin M, Simons P. Rapid and reversible disappearance of beta-adrenergic cell surface receptors. EMBO J. 1982;1:187–90. doi: 10.1002/j.1460-2075.1982.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girones N, Fresno M. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends Parasitol. 2003;19:19–22. doi: 10.1016/s1471-4922(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 26.Tarleton RL. Chagas disease: a role for autoimmunity? Trends Parasitol. 2003;19:447–51. doi: 10.1016/j.pt.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Kierszenbaum F. Where do we stand on the autoimmunity hypothesis of Chagas disease? Trends Parasitol. 2005;21:513–6. doi: 10.1016/j.pt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Gavi S, Yin D, Shumay E, et al. The 15-amino acid motif of the C terminus of the beta2-adrenergic receptor is sufficient to confer insulin-stimulated counterregulation to the beta1-adrenergic receptor. Endocrinology. 2005;146:450–7. doi: 10.1210/en.2004-0595. [DOI] [PubMed] [Google Scholar]
- 29.Iwatsubo K, Toya Y, Fujita T, et al. Ischemic preconditioning prevents ischemia-induced beta-adrenergic receptor sequestration. J Mol Cell Cardiol. 2003;35:923–9. doi: 10.1016/s0022-2828(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 30.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 31.McLean AJ, Milligan G. Ligand regulation of green fluorescent protein-tagged forms of the human β1- and β2-adrenoceptors; comparisons with the unmodified receptors. Br J Pharmacol. 2000;130:1825–32. doi: 10.1038/sj.bjp.0703506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiina T, Kawasaki A, Nagao T, et al. Interaction with beta-arrestin determines the difference in internalization behavor between beta1- and beta2-adrenergic receptors. J Biol Chem. 2000;275:29082–90. doi: 10.1074/jbc.M909757199. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Paquet M, Lau AG, et al. β1-adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2). Differential regulation of receptor internalization by MAGI-2 and PSD-95. J Biol Chem. 2001;276:41310–7. doi: 10.1074/jbc.M107480200. [DOI] [PubMed] [Google Scholar]
- 34.Wallukat G, Fu MLX, Magnusson Y, et al. Agonistic effects of anti-peptide antibodies and autoantibodies directed against adrenergic and cholinergic receptors: absence of desensitization. Blood Press. 1996;5:31–6. [PubMed] [Google Scholar]
- 35.Rosenbaum MB, Chiale PA, Schejtman D, et al. Antibodies to beta-adrenergic receptors disclosing agonist-like properties in idiopathic dilated cardiomyopathy and Chagas' heart disease. J Cardiovasc Electrophysiol. 1996;5:367–75. doi: 10.1111/j.1540-8167.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 36.Matsui S, Fu ML, Hayase M, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Cardiac Fail. 1999;5:246–54. doi: 10.1016/s1071-9164(99)90009-x. [DOI] [PubMed] [Google Scholar]
- 37.Matsui S, Fu MXL, Katsuda S, et al. Peptides derived from cardiovascular G-protein-coupled receptors induce morphological cardiomyopathic changes in immunized rabbits. J Mol Cell Cardiol. 1997;29:641–55. doi: 10.1006/jmcc.1996.0307. [DOI] [PubMed] [Google Scholar]
- 38.Jahns R, Boivin V, Hein L, et al. Direct evidence for a β1-adrenergic receptor directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiale PA, Garro HA, Schmidberg J, et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006;3:1182–6. doi: 10.1016/j.hrthm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Larsson L, Haugen E, et al. Effects of autoantibodies removed by immunoadsorption from patients with dilated cardiomyopathy on neonatal rat cardiomyocytes. Eur J Heart Fail. 2006;8:460–7. doi: 10.1016/j.ejheart.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Dorffel WV, Wallukat G, Dorffel Y, et al. Immunoadsorption in idiopathic dilated cardiomyopathy, a 3-year follow-up. Int J Cardiol. 2004;97:529–34. doi: 10.1016/j.ijcard.2004.03.001. [DOI] [PubMed] [Google Scholar]