Abstract
Malnutrition compromises immune function, resulting in reduced resistance to infection. Recent animal and human studies have suggested that leptin is capable of modulating the immune response and that its levels, which are regulated by nutritional status, fall rapidly during starvation. Leptin deficiency is associated with impaired cell-mediated immunity, an increased incidence of infectious disease and an associated increase in mortality. The purpose of this study was to examine the effect of leptin on activation and cytokine production in peripheral blood T cells from malnourished children. The data obtained in the present study demonstrate that leptin produced an increase in the percentage of CD4+ and CD8+ cells producing interleukin (IL)-2 and interferon (IFN)-γ in 24-h cultures. Moreover, leptin decreased the percentage of CD4+ and CD8+ cells producing IL-4 and IL-10, and enhanced activation of circulating T cells when co-stimulated by phorbol 12-myristate 13 acetate (PMA)–ionomycin. Leptin enhanced the expression of activation markers CD69 and CD25 in both CD4+ and CD8+ cells after 5 h of stimulation. In conclusion, the results obtained show that leptin modulates CD4+ and CD8+ cell activation towards a T helper 1 (Th1) phenotype by stimulating the synthesis of IL-2 and IFN-γ. In contrast, leptin decreases IL-4 and IL-10 production. Moreover, leptin enhanced the expression of CD69 and CD25 on CD4+ and CD8+ cells after stimulation with PMA–ionomycin.
Keywords: activation, cytokines, infections, leptin, malnutrition
Introduction
Protein-calorie malnutrition represents a significant worldwide health problem. Malnutrition is an important cause of immune suppression and increases host susceptibility to infectious diseases [1]. Recent evidence indicates that leptin is capable of modulating the immune response, and that its levels are regulated by nutritional status. Leptin deficiency is associated with impaired cell-mediated immunity, an increased incidence of infectious disease and an associated increase in mortality [2,3].
Leptin, the product of the ob gene, is a 16-kDa non-glycosylated peptide hormone synthesized mainly by adipocytes [4,5]. Leptin has structural homology with members of the long chain helical cytokine family, which includes interleukin (IL)-6 and IL-11 [6], and the long isoform of the leptin receptor (OB-Rb) shares structural and functional similarities with the gp130 cytokine-receptor family [7,8]. Furthermore, the OB-Rb is distributed widely and expressed in both human and murine haemopoietic stem cell populations [5] and is also expressed in lymphoid tissues, where leptin has been demonstrated to play an important role during T cell-mediated immune responses [9].
Cells from ob/ob mice (leptin-deficient) show defective mixed lymphocyte reactions, with poor generation of interferon (IFN)-γ and increased IL-4. Leptin treatment of cells from the ob/ob mice in vitro reverses mixed lymphocyte reaction defects, leading to vigorous secretion of IFN-γ and blunting of IL-4 secretion [10]. It has been shown that exogenous administration of leptin during fasting protects mice from the lymphoid atrophy associated with starvation, indicating a role for leptin in the immune dysfunction associated with starvation [11]. In addition, leptin enhances the secretion of tumour necrosis factor (TNF)-α, IL-6 and IL-12 by peritoneal macrophages, as well as IFN-γ by T cells [12,13].
The observation that leptin deficiency may be partially responsible for the immune impairment that accompanies malnutrition [11,14], which is associated with an increased frequency of infection [3], has stimulated interest in the effects of leptin on immunity [2,9].
Previously, we have demonstrated that malnutrition causes severe impairment of IL-2 and IFN-γ production. In contrast, our data show increased IL-4 and IL-10 production by CD4+ and CD8+ cells of malnourished children. These data reveal that malnutrition alters the balance of type 1/type 2 immune responses. In addition, the activation capability of CD4+ and CD8+ cells is considerably decreased. These alterations may contribute to reduced immunological capacity and resultant increased sensitivity to infection associated with malnutrition [15]. The aim of the present study was to assess the effects of human leptin on cytokine synthesis and activation in peripheral CD4+ and CD8+ cells from malnourished infected children.
Materials and methods
Peripheral blood was collected in sterile tubes containing heparin from malnourished infected children aged 6–60 months. Samples were obtained from the Hospital General Gustavo Baz Prada and Hospital Infantil Iztapalapa. The study was approved by the Medical Ethics Committee of The General Direction of Medical Services. Informed consent was obtained from all parents of the participating children.
Subjects
Group 1 (WNI): well-nourished infected children
This group included 12 well-nourished children (six girls and six boys) hospitalized as a result of respiratory or gastrointestinal bacterial infections. Their ages ranged from 6 to 48 months, and all had adequate weight/height ratios according to their age (Table 1).
Table 1.
Clinical characteristics and nutritional status of well-nourished infected (WNI) and malnourished infected (MNI) children.
| Study group (n) | Age (months) | Weight (g) | Height (cm) | Weight deficit (%) | Infection type |
|---|---|---|---|---|---|
| WNI (12) | 21·5 (6–48) | 11·7 (7·3–18·5) | 85·4 (67–109) | < 10% | Respiratory (11) Sepsis (1) |
| MNI children with severe malnutrition (7) | 19·71 (6–36) | 6·35 (4·1–7·7) | 73 (62–90) | 40·0–55·0 | Respiratory (4) Gastrointestinal (3) |
| MNI children with second-degree malnutrition (5) | 31·6 (10–60) | 9·0 (5·9–13·6) | 89·75 (72–111) | 29·19–38·5 | Gastrointestinal (2) Respiratory (3) |
Group 2 (MNI): malnourished, infected children
This group consisted of 12 malnourished-infected children (MNI) of both sexes (five girls and seven boys), suffering from severe bacterial infection: respiratory (seven children) and gastrointestinal (five children) infections. Bacterial infections were diagnosed rigorously on the basis of clinical data and routine laboratory testing. The group included five children with second-degree malnutrition (weight/height deficit > 25% and < 40% according to age) and seven children with severe (third-degree) malnutrition marasmus showing severe weight/height deficit (> 40% for age).
Severity of malnutrition was assessed in all cases, based upon clinical signs and symptoms of malnutrition, as well as weight/height deficit according to the established values for Mexican children [16].
Reagents and antibodies
Antibodies utilized were peridinin chlorophyll (PerCP)-anti-CD4, PerCP-anti-CD8, fluorescein isothiocyanate (FITC)-anti-IL-2, FITC-anti-IFN-γ, phycoerythrin (PE)-anti-IL-4, allophycocyanin (APC)-anti-IL-10, PE-anti-CD25 and APC-anti-CD69 (Becton Dickinson Immunocytometry Systems, San José, CA, USA). Human recombinant leptin was from R&D Systems (Minneapolis, MN, USA). Phorbol 12-myristate 13 acetate (PMA), ionomycin (I), and brefeldin-A were from Sigma Chemical Company (St Louis, MO, USA). Fluorescence activated cell sorter (FACS) lysing solution and FACS permeabilizing solution were from Becton Dickinson.
Cell preparation and in vitro culture
In order to study the effects of leptin on cytokine production and capability of activation, whole blood from malnourished children was incubated for 19 h with or without leptin (10 ng/ml). Subsequently, whole blood from MNI and WNI children was either stimulated for 5 h at 37°C with PMA (25 ng/ml) and ionomycin (1 µg/ml) in the presence of brefeldin-A (10 µg/ml) and RPMI-1640 without l-glutamine (induces, or stimulates, cytokine production), or retained in identical medium without PMA and ionomycin but with brefeldin A (spontaneous, or unstimulated, cytokine production). No serum was added to the cultures. Stimulated and unstimulated (resting cells) cultures were aliquoted for staining.
Stain of cell surface antigens
Specific staining of respective cell-surface molecules was performed by anti-human CD4-PerCP or anti-human CD8-PerCP. One hundred µl of cell suspension were incubated with 10 µl of a fluorescence-conjugated antibody for 30 min at room temperature. As controls, FITC and PE-labellednon-specific mouse IgG1 antibodies were used to establish background fluorescence. After incubation, cells were washed with 1% bovine serum albumin (BSA) prepared in phosphate-buffered saline (PBS).
Detection of intracellular cytokines
After cell surface antigen stain, FACS lysing solution was added to each tube. After further incubation, samples were centrifuged and treated with FACS permeabilizing solution for 10 min at room temperature. Samples were washed with 1% BSA in PBS and incubated with fluorescently labelled anti-cytokine antibodies for 30 min. After incubation, cells were washed and finally fixed in 1% paraformaldehyde prior to analysis.
Flow cytometry analysis
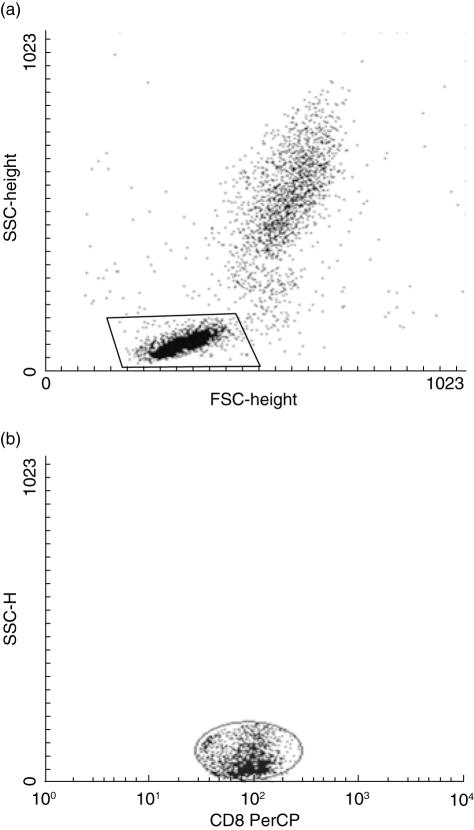
Four-colour flow cytometry was performed using a FACSCalibur flow cytometer. A minimum of 10 000 cells-gated events were acquired and analysed with cellquest™ (Becton Dickinson) software. Figure 1 shows how gates were set on the forward scatter (FSC)–side scatter (SSC) to acquire all lymphocytes, PerCP-SSC distribution for gate on the basis of anti-CD4 and anti-CD8 labelling (gate on CD4+ or CD8+ cells). In all cases, these gates were used to establish FITC-, PE- and APC-positive cells.
Fig. 1.
(a) The lymphocyte gate set in the forward scatter–side scatter distribution. (b) The peridinin chlorophyll-SSC distribution; this gate was set on to restrict the analysis to CD4+ or CD8+ cells. For the analysis of cytokine production and activation markers the cells were passed by windows (a) and (b).
Statistical analysis
Results are expressed as arithmetic means ± standard error. Differences between groups were analysed using the Mann–Whitney U-test for unpaired samples. Differences with P-values < 0·05 were considered significant.
Results
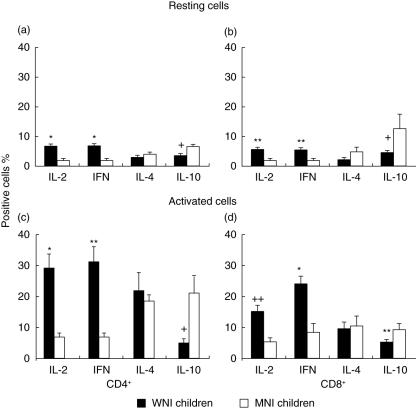
The percentage of CD4+ IL-2+ unstimulated cells in MNI children (2·21 ± 0·21%) was diminished significantly compared to WNI (6·95 ± 0·57%) children (P < 0·01). Also, the percentages of CD4+ IFN-γ+ cells were significantly higher in the WNI (7·04 ± 0·91%) group than in MNI children (2·18 ± 0·91%; P < 0·01). The WNI children showed a decreased percentage (3·18 ± 0·74%) of CD4+ IL-4+ cells compared to MNI (4·22 ± 0·46%) children. The percentages of CD4+ IL-4+ cells showed no statistically significant differences among groups. In contrast, the percentage of CD4+ IL-10+ cells was significantly higher in the MNI group (6·84 ± 1·45%) than in the WNI (3·78 ± 0·13%) group (P< 0·005) (Fig. 2a).
Fig. 2.
Effect of malnutrition on the percentages of cytokine-positive cells from well-nourished infected (WNI, n = 12) and malnourished (MN, n = 12) children. The percentage of interleukin (IL)-2-, interferon (IFN)-γ-, IL-4- and IL-10-producing resting cells in CD4+ and CD8+ cells is shown in the upper panel. The percentage of IL-2-, IFN-γ-, IL-4- and IL-10-producing activated cells in CD4+ and CD8+ cells is shown in the lower panel. Data are based upon flow cytometric analysis of 10 000 events and are means ± standard error. *P < 0·01; + +P < 0·05; +P < 0·005 and **P < 0·001; in all cases the difference was between WNI versus MNI group.
Figure 2b shows a decreased production of cytokines in CD8+ unstimulated cells from MNI children (IL-2+ cells, 1·73 ± 0·26% and IFN-γ+ cells, 2·36 ± 0·63%; P < 0·05) compared to cells from WNI (5·96 ± 0·89% and 5·63 ± 1·18%, respectively). Even though the percentage of CD8+ IL-4+ cells tended to be greater in MNI children than in WNI children (4·69 ± 1·95% and 2·45 ± 0·34%, respectively), the difference was not statistically significant. In contrast, the percentage of CD8+ IL-10+ cells was significantly higher in MNI children (12·60 ± 4·99%) than in WNI (4·92 ± 2·24%; P < 0·005) children.
The percentage of CD4+ IL-2+ and CD4+ IFN-γ+ stimulated cells was diminished significantly in malnourished children (7·14 ± 0·74 and 7·18 ± 1·32, respectively) compared to WNI (29·07 ± 4·55 and 30·99 ± 5·62) children (P < 0·01 and P < 0·001, respectively). The percentages of CD4+ IL-4+ cells showed no statistically significant differences between MNI and WNI groups (15·44 ± 2·05 and 18·85 ± 3·60, respectively). In contrast, the percentage of IL-10+ cells was significantly higher in the MNI group (21·12 ± 5·59) compared to the WNI (5·09 ± 1·53) group (Fig. 2c, P < 0·005).
Decreased production of type 1 cytokines was observed in CD8+ stimulated cells from MNI children (IL-2: 5·38 ± 0·81%, P < 0·05 and IFN-γ: 6·78 ± 2·32%, P < 0·001) compared to WNI (14·78 ± 2·02% and 23·10 ± 2·92%, respectively). Even though the percentage of CD8+ IL-4-producing cells tended to be greater in malnourished children than in WNI (11·85 ± 3·61% and 9·17 ± 2·21%, respectively), the difference was not statistically significant. In contrast, the percentage of CD8+ IL-10+ cells was significantly higher in MNI children (8·97 ± 1·95%) in comparison to WNI (5·14 ± 1·15%, P < 0·05), as shown in Fig. 2d.
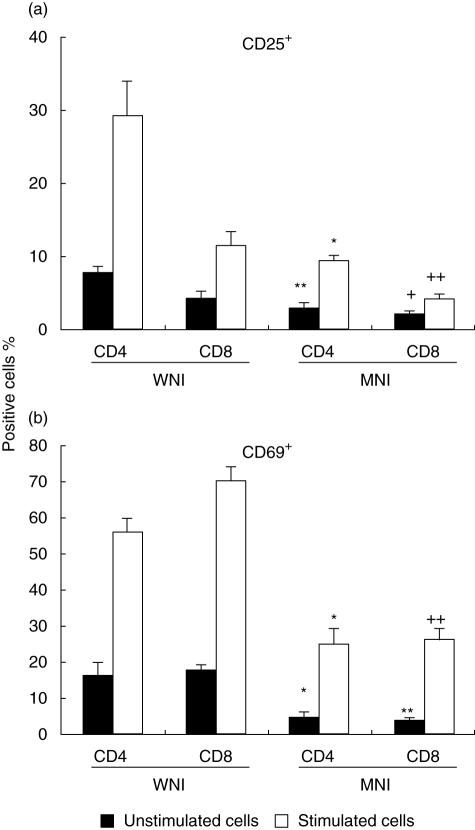
Furthermore, the percentage of positive cells exhibiting the activation markers CD69 and CD25 was analysed. Percentages of CD4+CD25+- and CD8+CD25+-stimulated cells were significantly higher in the WNI group in comparison to MNI children (Fig. 3a, P < 0·01). In addition, the percentages of CD4+CD25+- and CD8+CD25+-unstimulated cells were significantly higher in the WNI group in comparison to MNI children (Fig. 3a, P < 0·001).
Fig. 3.
Expression of activation antigen (a), CD25; (b), CD69 by CD4+ and CD8+ cells from unstimulated and stimulated peripheral blood cells. Cells were activated with phorbol 12-myristate 13 acetate (PMA)–ionomycin for 5 h at 37°C. Cells were stained and analysed as described in the Methods section. Data are based upon flow cytometric analysis of 10 000 events and are means ± standard error. Results are expressed as percentage of positive cells. (a) *P < 0·01 versus CD4+ stimulated cells from WNI children; **P < 0·001 versus CD4+ unstimulated cells from WNI children; +P < 0·05 versus CD8+ unstimulated cells from WNI children and ++P < 0·01 versus CD8+ stimulated cells from WNI children. (b) *P < 0·01 versus CD4+ stimulated and unstimulated cells from WNI children; **P < 0·001 versus CD8+ unstimulated cells from WNI children; ++P < 0·001 versus CD8+ stimulated cells from WNI children. All bars were significant different in stimulated versus unstimulated cells (P < 0·05).
In relation to CD69+ cells, the percentage of CD4+ and CD8+-unstimulated cells was significantly lower for the MNI group (Fig. 3b, P < 0·01 and P < 0·001, respectively). When cells were stimulated with PMA–I, the percentage of CD69+ CD4+ cells was significantly higher in comparison to cells from the MNI group (P < 0·01, Fig. 3b). Also, the expression of the CD69 activation marker in stimulated CD8+ cells was minimal in cells from the MNI group in comparison to the WNI group (P < 0·001, Fig. 3b).
Effect of leptin on spontaneous cytokine production
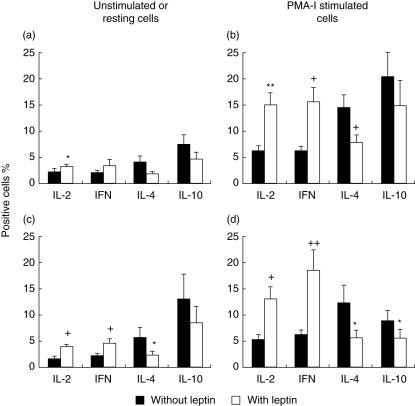
Percentages of IL-2+ CD4+ and IFN-γ+ CD4+ in the resting cell population were increased when cells were incubated with leptin (3·31 ± 0·46% and 3·45 ± 0·9%, respectively) in comparison to cells without leptin (2·21 ± 0·21% and 2·18 ± 0·41%, respectively) (P < 0·05 for IL-2+ cells). In contrast, leptin induced a decrease in the percentage of CD4+ cells producing IL-4: 4·22 ± 1·03% to as low as 1·92 ± 0·33%. The percentage of IL-10+ CD4+ cells (7·42 ± 1·93%) was higher in untreated cells in relation to cells treated with leptin, which also demonstrated a significant decrease in IL-10+ CD4+ cells (4·73 ± 1·26%; Fig. 4a).
Fig. 4.
Effect of leptin on the cytokines production in peripheral blood lymphocytes of malnourished infected children. The percentage of interleukin (IL)-2-, interferon (IFN)-γ-, IL-4- and IL-10-producing cells in CD4+ cells is shown in the upper panel (a, b). The percentage of IL-2-, IFN-γ-, IL-4- and IL-10-producing cells in CD8+ cells is shown in the low panel (c, d). Cells were activated with phorbol 12-myristate 13 acetate (PMA)–inomycin for 5 h at 37°C. Data are based upon flow cytometric analysis of 10 000 events and are means ± standard error. *P < 0·05, **P < 0·001, +P < 0·01, + +P < 0·005 versus cells without leptin treatment.
In CD8+ cells, the production of T helper 1 (Th1) cytokines was increased by leptin treatment (IL-2: 1·64 ± 0·26% to 4·00 ± 0·64% and IFN-γ: 2·23 ± 0·40% to 4·64 ± 0·87%, P < 0·01) (Fig. 4c). In contrast, the percentages of type 2 cytokines was higher in untreated cells (IL-4: 5·82 ± 1·95% and IL-10: 13·10 ± 4·89%) in comparison to leptin-treated cells (2·27 ± 0·59% and 8·44 ± 3·25%, respectively; P < 0·05).
Effect of leptin on induced cytokine production
Figure 4b shows that leptin produced an increase in the percentage of CD4+ cells producing IL-2 (15·15 ± 2·35%) in comparison to untreated controls (6·33 ± 0·74%, P < 0·001). Also, the percentage of IFN-γ+ cells was significantly higher in cells incubated with leptin (15·60 ± 2·79%) compared to without leptin treatment (6·27 ± 0·91%, P < 0·01). Cells incubated with leptin exhibited a lower percentage of type 2 cytokine-positive cells (IL-4 and IL-10) than cells without leptin treatment. Even though the percentage of CD4+ IL-10-producing cells tended to be greater in cell populations without leptin treatment compared to leptin-treated cells (22·12 ± 6·08% and 14·88 ± 4·75%, respectively), the difference was not statistically significant. In contrast, the percentage of CD4+ IL-4+ cells was significantly higher in cells without leptin (14·55 ± 2·24%) in comparison to leptin-treated cells (7·84 ± 1·33%, P < 0·01).
As shown in Fig. 4d, percentages of CD8+ IL-2+ and CD8+ IFN-γ+ cells were significantly lower in the absence of leptin treatment (5·38 ± 0·81% and 6·65 ± 2·70, respectively) compared to leptin-treated cells (13·12 ± 2·57% and 18·55 ± 3·87%, P < 0·01 and P < 0·005, respectively). The percentage of CD8+ IL-4+ cells was significantly higher in untreated cell populations (12·31 ± 3·48%) compared to leptin treatment (5·71 ± 1·22, P < 0·05). In addition, the percentage of IL-10+ CD8+ cells was significantly higher in untreated (13·10 ± 1·95%) compared to leptin-treated cells (5·61 ± 1·21%, P < 0·05).
Effect of leptin on expression of activation markers
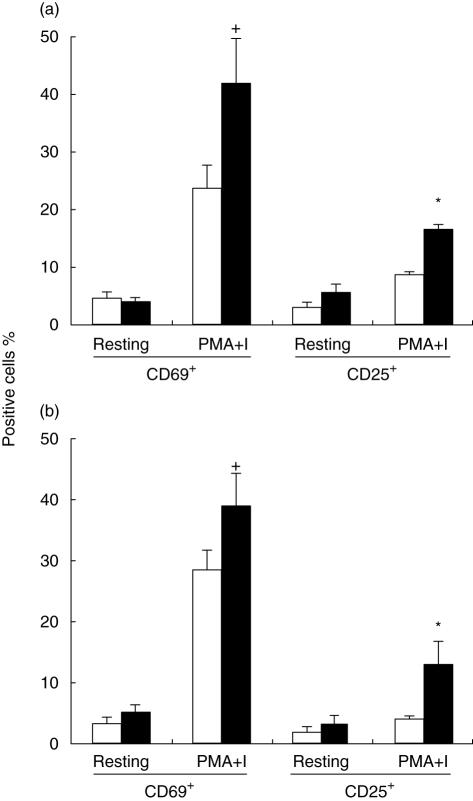
In order to study the effect of leptin on activation, whole blood was cultured in the presence of PMA–I with or without leptin and the expression of CD69 and CD25 in both CD4+ and CD8+ T cells was examined. As shown in Fig. 5, leptin potentiates the activation effect of PMA–I on CD4+ and CD8+ cells increasing the percentage of cells expressing both activation markers.
Fig. 5.
Leptin effects on activation antigens expression CD69 and CD25 by CD4+ (a) and CD8+ (b) cells from resting and activated peripheral blood cells from malnourished-infected children. Cells were activated with phorbol 12-myristate 13 acetate (PMA)–ionomycin for 5 h at 37°C. Cells were stained and analysed as described in the Methods section. Data are based upon flow cytometric analysis of 10 000 events and are means ± standard error. *P < 0·05 and +P < 0·01 versus cells without leptin treatment.
These data show that there was no significant effect of leptin on the activation (CD69+ cells) of CD4+ and CD8+ T resting cells. When cells were stimulated with PMA–I, the percentages of CD69+ CD4+ cells was significantly higher (42·24 ± 7·58%) in comparison to untreated cells (23·36 ± 4·74%, P < 0·01, Fig. 5a). Also, the expression of this activation marker in stimulated CD8+ cells was minor in cells without leptin treatment (28·58 ± 3·03% to 38·97 ± 5·23%, P < 0·01, Fig. 5b).
The percentage of CD4+ CD25+ and CD8+ CD25+ resting cells showed no statistically significant differences between cells treated and not treated with leptin. The percentage of CD25+ cells in the CD4+-and CD8+-activated cell population was lower in leptin-treated cells (3·17 ± 0·54% and 2·17 ± 0·40%, respectively) in comparison to untreated cells (5·66 ± 1·94% and 3·58 ± 1·3%, Fig. 3b). Further, the proportion of CD25+ CD4+-stimulated cells was significantly higher with leptin incubation (16·91 ± 2·48%) compared to without leptin (8·87 ± 1·61%, P < 0·05). Also, when CD8+ cells were incubated with leptin, the percentage was significantly greater (13·27 ± 3·49%) in comparison to cells stimulated without leptin treatment (4·25 ± 0·80%, P < 0·05).
Percentages of CD69+ and CD25+ cells were clearly higher in cultures treated with leptin. Additionally, it was clear that leptin alone had no effect on cell activation (CD69+ and CD25+ cells) in unstimulated whole blood CD4+ and CD8+ cells.
Discussion
In the present work we have studied the effect of leptin on cytokine production and activation of peripheral blood CD4+ and CD8+ cells from malnourished children. Cytokines play an important role in the nutrition–infection complex [17]. The generation of protective T cell responses against infectious agents is a complex process in which cytokines act as signals that direct the development of adaptive immunity [18].
Malnutrition is a pathological condition associated with decreased leptin levels [19]; therefore, diminished leptin concentrations in malnourished children may contribute to decreased cytokine production and increased susceptibility to infections. Mancuso et al. [20] reported that leptin-deficient mice exhibit increased susceptibility to Gram-negative bacterial pneumonia; this susceptibility to bacterial pneumonia could be restored by treating with exogenous leptin. Previously, alterations in the capacity to produce some cytokines have been reported in malnourished children. González et al. [21] observed that lymphocytes are unable to secrete normal quantities of cytokines. In addition, we recently demonstrated that malnutrition results in severe impairment of IL-2 and IFN-γ production. In contrast, our data show increases in IL-4 and IL-10 production by CD4+ and CD8+ cells of malnourished children [15].
Indeed, the results of this study demonstrated that incubation of resting peripheral blood CD4+ and CD8+ cells with leptin induced an increase in the production of IL-2 and IFN-γ and a decrease in the production of IL-4 and IL-10. We found no differences between resting cells treated with and without leptin in CD4+ cell cytokine production except for IL-2, which increased when cells were incubated with leptin. In contrast, we found significant differences between resting cells with and without leptin treatment in IL-2, IFN-γ and IL-4 production in CD8+ cells; however, leptin showed no effect on IL-10 synthesis in unstimulated CD8+ cells.
Our study reveals an imbalance between type 1/type 2 responses in cells from malnourished infected children, demonstrated by a trend toward a decrease in IFN-γ and an increase in IL-4 and IL-10 production. However, when cells were incubated with leptin, a significant enhancement of IFN-γ production associated with the suppression of IL-4 and IL-10 was shown. Thus, leptin could play an important role in the regulation of cytokine synthesis as well as in modulation of the immune response.
The data shown in the present study are in agreement with the observation that leptin plays an important role in T cell-mediated immune responses [9]. Specifically, it has been reported that leptin enhances cognate T cell responses, skewing cytokine responses towards a Th1 phenotype in mice [10]. In addition, leptin up-regulates the production of proinflammatory cytokines by murine macrophages [22].
Previously, Soliman et al. [23] demonstrated decreased leptin levels in severely malnourished infants. In this context, the low leptin levels were associated with suppression of the lymphoproliferative response [24]. Presumably, in infected children, spontaneous ex vivo cytokine production reflects in vivo stimulation and, thus, represents cell-specific activity in the context of a current infection (s) [25]. However, this response is very low in malnourished infected children in comparison to the immune response (cytokine production and activation capability) observed in well-nourished infected children [15].
Additionally, we were able to demonstrate that exogenous leptin restored in vitro activation capability of PMA–I-stimulated cells. However, leptin is not able to increase the expression of activation markers in resting (unstimulated) CD4+ and CD8+ cells from malnourished children. We have demonstrated previously that the activation capability of CD4+ and CD8+ cells from malnourished infected children is considerably decreased [15,26].
Previously, it has been reported that human leptin induces the expression of the activation marker CD69 in human monocytes [27,28]. Furthermore, human leptin alone is not able to activate human peripheral blood lymphocytes in vitro. However, when T lymphocytes are co-stimulated with PHA or concanavalin A, leptin enhances the proliferation and activation of cultured T lymphocytes [29]. Also, other data have shown that leptin modulates the proliferation of T cells stimulated with anti-CD3 [30]. These results agree with data observed here. Here we show that leptin is a potentiator of CD4+ and CD8+ cell activation when co-stimulated with a non-specific stimulus such as PMA–I.
No differences were observed regarding the degree of malnutrition and the type of infection. Further studies including a greater number of patients will be necessary to address the relationship between these factors.
The results of this study demonstrate that human leptin not only enhances cytokine production induced by PMA–I, but also modulates the activation of CD4+ and CD8+ cells from children. This may be due to the presence of the leptin receptor (Ob-R) on human circulating leucocytes [12] and on lymphocytes, as demonstrated previously in mice [10,22]. Also, the presence of both the short and long isoforms of the leptin receptor has been confirmed in human peripheral blood T lymphocytes (both CD4 and CD8) [29].
In conclusion, we have demonstrated in the present study that leptin modified the synthesis of cytokines from peripheral blood CD4+ and CD8+ cells. Specifically, leptin enhances the secretion of IL-2 and IFN-γ and inhibits the production of IL-4 and IL-10. The results of this study demonstrate that human leptin can also modulate the activation of CD4+ and CD8+ cells from malnourished infected children. Finally, it should be recognized that leptin did not fully restore cytokine synthesis and capability of activation; however, leptin may help in restoring immune responses in malnourished children.
Acknowledgments
This study was partially supported by FOMES, México, grant no. 98-35-28. The authors thank MD Luis Antonio Flores Niño de Rivera from Hospital General Gustavo Baz Prada Servicios de Salud del Gobierno del Estado de México, for his help in collecting blood samples, and MS Edith Cortés and Dr Cristina González for her assistance.
References
- 1.Faggioni R, Moser A, Feingold K, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781–7. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. 2000;11:7–14. [PubMed] [Google Scholar]
- 3.Ozata M, Ozdemir I, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 4.Faggioni R, Kenneth R, Feingold K, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–71. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–72. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–9. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia A. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 10.Lord GM, Matarese G, Howard J, Baker R, Bloom S, Lechler R. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 11.Howard J, Lord G, Matarese G. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–59. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarkesh-Esfahani H, Pockley G, Metcalfe R, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–99. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 13.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier C. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–91. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 14.Moore SE, Morgan G, Collison A, Swain J, O'Connell M, Prentice A. Leptin, malnutrition, and immune response in rural Gambian children. Arch Dis Child. 2002;87:192–7. doi: 10.1136/adc.87.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez L, González C, Flores L, Jiménez-Zamudio L, Granel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12:502–7. doi: 10.1128/CDLI.12.4.502-507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos-Galván R. Pediatric somatometry. Arch Inv Med (México) 1975;6(Suppl. 1):83–396. [PubMed] [Google Scholar]
- 17.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol. 1999;21:317–38. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 18.Hunter CA, Reiner SL. Cytokines and T cells in host defense. Curr Opin Immunol. 2000;12:413–18. doi: 10.1016/s0952-7915(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancuso P, Gottschalk A, Phare S, Peters-Golden M, Lukacs N, Huffnagle G. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 21.González C, Rodríguez L, Bonilla E, et al. Electrophoretic analysis of plasmatic and lymphocytes secreted proteins in malnourished children. Med Sci Res. 1997;25:643–6. [Google Scholar]
- 22.Loffreda S, Yang S, Lin H, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 23.Soliman AEI, Zalabany M, Salama M, Ansari B. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism. 2000;49:819–25. doi: 10.1053/meta.2000.6745. [DOI] [PubMed] [Google Scholar]
- 24.Palacio A, López M, Pérez-Bravo F, Monkeberg F, Schlesinger L. Leptin levels are associated with immune response in malnourished infants. J Clin Endocrinol Metab. 2002;87:3040–46. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 25.Walker D, Jason J, Wallace K, et al. Spontaneous cytokine production and its effect on induced production. Clin Diagn Lab Immunol. 2002;9:1049–56. doi: 10.1128/CDLI.9.5.1049-1056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nájera O, González C, Toledo G, et al. Early activation of T, B and NK lymphocytes in infected malnourished and infected well-nourished children. J Nutr Immunol. 2001;5:85–97. [Google Scholar]
- 27.Marzio R, Mauel J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565–82. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 28.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 30.Lord GM, Matarese G, Bloom S, Lechler R. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72:330–38. [PubMed] [Google Scholar]