Abstract
An aberrant T cell population is the basis for diagnosis of refractory coeliac disease and determines the risk of enteropathy-associated T cell lymphoma. This disease is serious with a poor survival. Pathogenetic mechanisms sustaining aberrant T cell proliferation remain unknown. Recently, alemtuzumab has been proposed as a promising new approach to treat these patients. Only few single cases have been tested at present; nevertheless, in all the cases a clinical improvement was observed. However, whether intraepithelial lymphocytes have been targeted effectively by alemtuzumab is still debated. This study reports, using two-dimensional difference gel electrophoresis (2D DIGE), hyperexpressed proteins associated specifically with aberrant T cells found in a patient with coeliac disease by comparison of the protein expression of this sample with that of patients with coeliac disease and polyclonal T cells or with control subjects. The data demonstrated a significantly higher expression of IgM, apolipoprotein C-III and Charcot–Leyden crystal proteins in a duodenal biopsy specimen of the patient with clonal T cells compared with that of other patients. These preliminary results allow hypothesizing different clinical effects of alemtuzumab in patients with coeliac disease and aberrant T cell proliferation, because as well as the probable effect on T cells, alemtuzumab could exert its effect by acting on inflammatory associated CD52+ IgM+ B cells and eosinophil cells, known to produce IgM and Charcot–Leyden crystal proteins, that we demonstrated to be altered in this patient. The results also emphasize the possible association of apolipoprotein with aberrant T cell proliferation.
Keywords: coeliac disease, immunology, MALDI-TOF analysis, protein profile, T cell receptor
Introduction
Coeliac disease (CD) is usually a benign disorder. However, some patients fail to improve or develop villous atrophy in spite of a strict gluten-free diet. This is termed coeliac disease, which is again divided into two categories. Type II has an aberrant clonal population of premalignant intraepithelial T lymphocytes (IEL), whereas type I does not. Type II will often develop into enteropathy-associated T cell lymphoma (EATL), a condition with a poor prognosis [1]. Patients with type II also have a poor prognosis with a 5-year survival of well below 50%.
T cells play an essential role in the pathogenesis of CD and refractory CD/EATL. The genesis and expansion of monoclonal T cells involve both inappropriate immune responses to gluten and acquisition of genetic abnormalities. Through the activity of the enzyme transglutaminase, glutamine residues in gluten are converted into glutamic acid [2]. Subsequently, a multitude of gluten-derived peptides is generated that, when bound to either uman leucocyte antigen (HLA)-DQ2 or DQ8, can induce T cell responses in CD patients [3]. A particular glutamine- and proline-rich 33-mer α-gliadin peptide, that contains six different T cell stimulatory sequences and is resistant to gastric and duodenal proteolysis, might be a primary initiator of the inflammatory response to gluten. In the large majority of patients, inflammatory T cell responses to other gluten peptides are also observed, implicating multiple gluten peptides in the disease process. Although oligo- or monoclonal IELs in patients with CD are pathological and can also be detected in the large majority of refractory CD patients who do not develop an EATL, they are not cytogenetically abnormal and do not form tumour masses which differentiate these patients from EATL patients, in addition to the absence of radiological and bone marrow evidence of lymphoma [1]. Moreover, only a fraction of patients with HLA-DQ2 or DQ8 haplotype develop CD, and only a fraction of them show an oligo- or monoclonal IEL; thus the real pathogenetic mechanisms inducing and sustaining these aberrant T cell remain largely unknown.
Refractory type II (RCD-II) CD is usually resistant to any known immunosuppressive therapy. Recently, alemtuzumab (Camptah-1H) has been proposed as an alternative to steroid therapy in type II CD [4–6]. However, its use is limited to few cases and, although an effect on clinical improvement had been observed, its role on aberrant T cell intraepithelial lymphocytes is controversial. Alezutumab is a humanized monoclonal antibody directed against the CD52 antigen, whose exact function remains unknown; CD52 antigen is expressed on the surface of all lymphocytes, monocytes, macrophages and eosinophils. It appears to be very active in chronic lymphocytic leukaemia as well as T cell lymphomas, while its activity in B cell non-Hodgkin's lymphoma (NHL) appears limited [7,8].
In this study we consider the information pertaining to investigation of the proteins associated with a patient with CD and aberrant T cell population by the two-dimensional difference gel electrophoresis (2D DTGE) approach. The aim is to understand more clearly the pathogenetic mechanisms associated with CD and clonal T cell proliferation to understanding of the mechanism of this specific disease and to discern the possible targets of alemtuzumab clinical effects.
Materials and methods
Patients
For proteomic analyses, duodenal biopsies and peripheral blood samples were selected from five patients positive to IgA and IgG anti-transglutaminase antibody assays, attending the Centro di Riferimento Oncologico, IRCCS. Patients were informed of the purpose of the study and informed consent was obtained. Patients' characteristics are shown in Table 1. Histological evaluation was performed according to Marsh–Oberhuber classification [9]. HLA-DQB1 polymerase chain reaction (PCR) amplification and nucleotide sequences were carried out as reported previously [10]. Clonal T cell receptor gamma chain variable regions (TCR-γ) were assessed previously by fluorescence multiplex PCR developed within the Biomed-2 concerted action and then subjected to capillary electrophoresis on an abi prism 3100. Data were analyzed using 3100 GeneScan 3·7 software [11].
Table 1.
Patients' characteristics.
| Patients | Age/sex | Anti-transglutaminase* (TG IgG; Tg IgA) | Modified Marsh grade | DQ2/8 variants | TCR-γ and TCR-β | Months from diagnosis | Months gluten-free diet | Familiarity |
|---|---|---|---|---|---|---|---|---|
| 1 | 41/F | + (n.a./18)† | 3C† | DQ2 | n.a. | 25 | 17 | No |
| – (n.a./4·4)‡ | 1‡ | Clonal T cell | ||||||
| 2 | 24/F | + (94·6; n.a.) | 3B | DQ2 | Polyclonal | 0 | 0 | No |
| 3 | 55/F | + (n.a.; 8·1) | 0 | DQ2 | Polyclonal | 0 | 0 | No |
| 4 | 19/F | + (52·2/n.a.) | 0 | – | Polyclonal | 0 | 0 | No |
| 5 | 34/F | + (37/06) | 0 | – | Polyclonal | 0 | 0 | No |
Antibodies anti-Tg IgG c.o. 26 UA/ml; IgA c.o. 7 UA/ml.
At first diagnosis; 25 months ago
now, after gluten-free diet. TCR: T cell receptor; n.a.: not evaluated.
Sample preparation for 2D-polyacrylamide gel electrophoresis (PAGE) analysis
To compare proteins expressed in intestinal cells we selected one patient with clonal TCR (patient 1), two patients with DQ2 heterozygosity, TCR polyclonal pattern, grade 0 and 3B modified Marsh classification, respectively (patients 2 and 3) and two patients with no DQ2 or DQ8 variants associated with CD, a polyclonal TCR and grade 0 Oberhuber–Marsh classification (patients 4 and 5). As shown in Table 2, we adopted a dye-swapping strategy to avoid dye labelling-bias; therefore the Cy3 and Cy5 dyes were interchangeable.
Table 2.
Experimental design for two-dimensional difference gel electrophoresis.
| Gel number | Cy2 | Cy3 | Cy5 |
|---|---|---|---|
| 1 | Pooled standard | Patients 2 and 3 | Patient 1 |
| 2 | Pooled standard | Patient 1 | Patients 4 and 5 |
Proteins were extracted from gut biopsies with a sample grinding kit (GE Healthcare, Milan, Italy) and 200 µl of lysis buffer containing 7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate hydrate (CHAPS) and 30 mM Tris-HCl pH 8·5. The cell lysates were prepared for 2D DIGE using a 2D clean-up kit (GE Healthcare) and resuspended in 7 M urea, 2 M thiourea and 4% CHAPS. Protein concentration was determined by Bio-Rad protein assay. For DIGE minimal labelling, 50 µg of protein sample were mixed with 100 pmol CyDye (GE Healthcare) and incubated on ice in the dark for 30 min.
The labelled samples were combined and mixed with rehydration buffer [7 M urea, 2 M thiourea, 4% CHAPS, 40 M dithiothreitol (DTT), 0·5% immobilized pH gradient (IPG) buffer] and subjected to overnight rehydration on 11 cm immobilized pH gradient strips [pH 3–10 non linear (NL)] (Bio-Rad, Milan, Italy).
2D DIGE and image analysis
Isoelectric focusing was carried out on a Bio-Rad Protean isoelectrofocusing (IEF) cell. The following voltage programme was used for first dimension separation: 250 V for 15 min, a slow voltage ramp to 8000 V over 2·5 h and a final focusing step for a total of 35 000 V/h. Focused IPG strips were stored at −80°C before equilibration and application to sodium dodecyl sulphate (SDS)-PAGE.
For the second dimension, IPG strips were equilibrated in 7 M urea, 2 M thiourea, 2% SDS, 30% glycerol, 50 mM Tris-HCl pH 8·8, reduced with 65 mM DTT and alkylated with 135 mM iodoacetamide. The second dimension was run on Criterion IPG + 1 Comb 8–16% precast gels (Bio-Rad). Gels were scanned on a Typhoon trio scanner (GE Healthcare).
Only those spots with greater than 1·5-fold changes in signal after normalization between the two populations were defined as altered.
Protein identification by mass spectrometric analysis
Proteins of interest were excised from colloidal Coomassie-stained preparative gel and destained with 25 mM ammonium bicarbonate in 50% acetonitrile. After overnight trypsin digestion, peptides were extracted with 1% triflouroacetic acid (TFA) and then subjected to ZipTip clean-up (Millipore SPA, Milan, Italy). Peptide mass fingerprinting was performed on a Voyager-DE PRO Biospectrometry Workstation mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA). Database searching was performed in parallel with the mascot search engine and the Aldente peptide mass fingerprinting tool (http://www.expasy.org/tools/aldente) with a 100 parts per million (p.p.m.) mass tolerance error.
Results
Patients and TCR molecular features
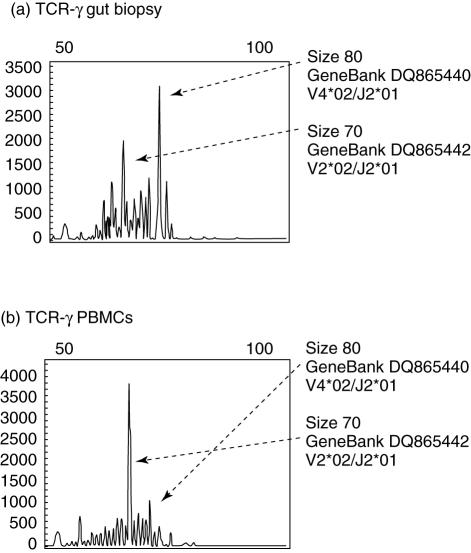
A clonal TCR-γ gene rearrangement was found in patient 1, a 41-year-old female with grade I villous transformation. TCR-γ gene rearrangements had been shown both in duodenal biopsy and in the peripheral blood (Fig. 1). Grade 1 modified Marsh histological classification was found, although the patient was submitted to a gluten-free diet. Marsh grade 0 and HLA-DQ2/8 negativity excluded CD in controls (patients 4 and 5).
Fig. 1.
Comparison of the fluorescence-labelled T cell receptor–polymerase chain reaction (TCR–PCR) products by GeneScan analysis. The x-axes represent molecular size (base pairs) and the y-axes fluorescence intensity. (a) Duodenal tissue; (b) peripheral blood mononuclear cells of patient 1. In the duodenal sample PCR results show two overexpanded TCR-γ clones, while in peripheral blood mononuclear cells a single TCR-γ clone was found. Base pair size, NCBI GeneBank accession number and the corresponding V, D, J germline segments are reported next to the corresponding TCR-γ clonal peaks.
Expression levels of IgM, apolipoprotein (apo) C3 and eosinophil proteins are higher in RCD-II compared to other samples
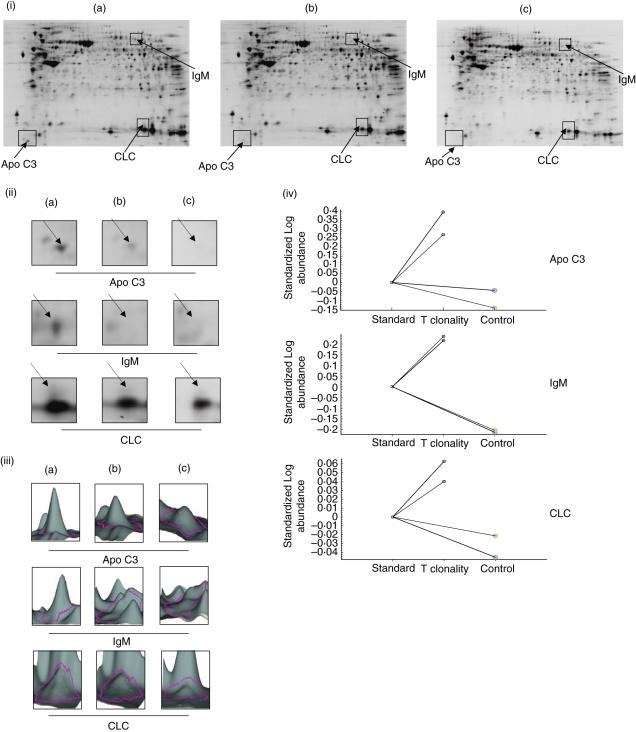
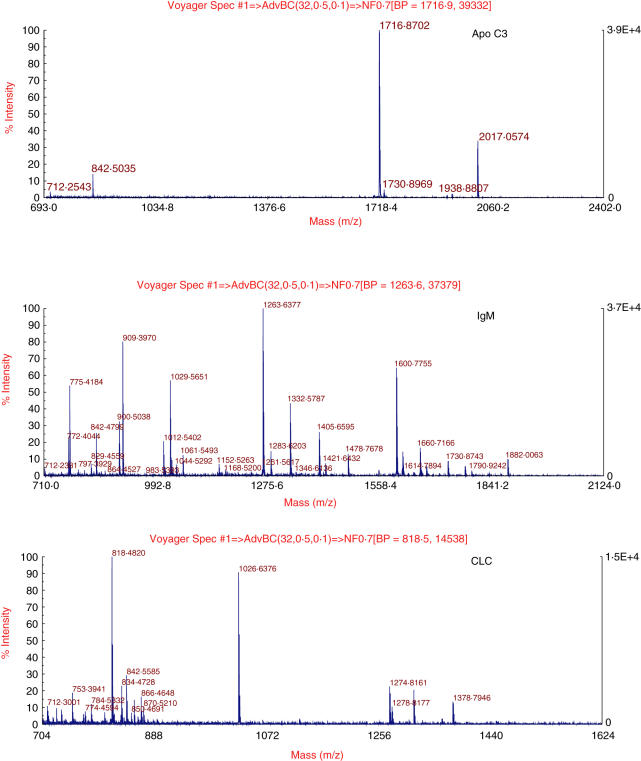
We compared protein expression from the only patient with clonal T cell proliferation (patient 1) with that of a patient with CD and a polyclonal T cell (patients 2 and 3) or of control subjects (patients 4 and 5). The experimental design is shown in Table 2. Following SDS-PAGE, gels were scanned by Typhoon trio and analysed with DeCyder 6·5 software (GE Healthcare). Spots with a more than 1·5-fold change in signal (patient 1 versus patients 2 and 3, gel 1; patient 1 versus patients 4 and 5, gel 2) were considered as altered (Fig. 2). The protein spots were identified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) peptide mass fingerprinting as apo C III (apo C3), IgM and Charcot–Leyden crystal protein (LCL) (Fig. 3).
Fig. 2.
Two-dimensional (2D) difference gel electrophoresis between refractory type II CD (RCD-II) and controls. (i) 2D pattern from gut biopsy of (a) an RCD-II patient; (b) coeliac disease (CD) patients and (c) patients with no DQ2/8 variants. Proteins were separated on the basis of pI (x-axis) and molecular mass (y-axis). Highlighted in rectangles are those areas where consistent alterations of protein expression were identified. (ii) Enlarged portions and (iii) 3D view of regions highlighted in (i). Each protein was identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), and shown as abbreviations on the gels. (iv) Graphical representation of the intergel distribution of apo C3, IgM and Charcot–Leyden crystal protein (CLC) expression levels in RCD-II patient and in CD and normal patients determined with bva version 6·5 software.
Fig. 3.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) identifying apo C-3, IgM and Charcot–Leyden crystal protein (CLC) proteins. Matching peptides are listed in Table 3.
Table 3.
Upregulated proteins in refractory type II (RCD-II) CD patient.
| Protein name | Average volume ratio | Swiss protein accession no. | P-value | Matching peptides |
|---|---|---|---|---|
| Apolipoprotein C-III | +2·7 | P02656 | 1·1e−9 | SEAEDASLLSFMQGYMK |
| TAKDALSSVQESQVAQQAR | ||||
| DALSSVQESQVAQQAR | ||||
| Ig μ chain C region | +2·72 | P01871 | 2e−11 | GFPSVLR |
| VSVFVPPR | ||||
| DGFFGNPR | ||||
| LICQATGFSPR | ||||
| QIQVSWLR | ||||
| QTISRPK | ||||
| GVALHRPDVYLLPPAR | ||||
| EQLNLR | ||||
| GQPLSPEK | ||||
| YVTSAPMPEPQAPGR | ||||
| Eosinophil lysophospholipase | +1·75 | Q05315 | 2·6e−10 | EYGAWK |
| IKPEAVK | ||||
| MVQVWR | ||||
| FNVSYLKR |
Discussion
The action of alemtuzumab and mechanisms that induce and sustain aberrant T cell proliferations in CD are still unknown. Our study is the first preliminary investigation of proteins associated with CD and clonal T cell proliferation using a 2D DIGE approach. Among differentially expressed proteins, we identified three proteins expressed more highly (greater than 1·5-fold) in the subject with CD and clonal T cell proliferation than in either subjects with CD and polyclonal T cells or in those with no CD. Proteins were identified by peptide mass fingerprinting as the heavy chain of IgM, apo C3 and CLC protein (galectin 10).
For decades it has been known that a potent humoral immune response occurs in untreated CD. It is predominantly of IgA and IgG classes in serum (systemic immunity) and of IgA and IgM classes in jejunal aspirate and gut lavage fluid (mucosal immunity); moreover, only the lavage fluid IgM concentration remains higher in patients with treated CD compared to controls [12–18]. Enhanced local secretion of IgA (P < 0·05) and IgM (P < 0·001) with respect to controls has also been demonstrated in CD patients using in vitro lymphocyte culture [19]. The high levels of spontaneous mucosal IgM secretion contrast with the reduced serum IgM levels observed; thus, mucosal sequestration of IgM rather than a specific defect in IgM production has been suggested. Because IgG and IgM (but not IgA) antibodies can activate complement, it is suggested that they might elicit damage following an encounter with antigens (e.g. gluten) penetrating the gut epithelium [20,21]. This could explain why patients with untreated CD show C3 hypocomplementaemia and have circulating immune complexes that disappear after a gluten-free diet is started, but reappear along with C3 split products shortly after gluten challenge [22]. The immunofluorescence staining intensity of immune deposits in gut tissue sections from adult patients with coeliac disease also correlate well with the degree of villous atrophy [21]. Moreover, mucosal complement activation may explain both the local release of prostaglandin E2 observed shortly after gluten challenge [23] and the release of cytotoxic granule components from neutrophils and eosinophils seen in untreated CD [24–26]. The striking crypt hyperplasia may therefore be complement-mediated regenerative change, and not only a result of T cell activation.
Our data, obtained using a different approach, confirm the concept of IgM segregation in the gut, and indicate that IgMs are also probably important in the immunopathogenesis of CD, especially in the course of aberrant T cell proliferation. The raised mucosal production of IgM and local IgM cell number [13,15,16,18,21], but not the concentrations of IgM in serum, emphasize the importance of studying the expression of proteins in the gut itself.
Apo C3 hyperexpression in the gut tissue of a CD patient is demonstrated here for the first time. It is known that the metabolism of circulating triglyceride-rich particles is affected strongly by the content of apo C3. Apo C3 contributes to hypertriglyceridaemia and may play a significant role in the expression of the small, dense lipoprotein (LDL) phenotype, as apo C3 is an inhibitor of lipoprotein lipase [27]. Besides a role in lipid metabolism, connections between lipoproteins and the innate immune system have been highlighted in several studies; moreover, infections and inflammations are accompanied by similar cytokine-induced release to that found in lipid and lipoprotein metabolism alterations [28,29]. Moreover, low-density lipoproteins have been found recently to be associated with the presence of two unexpected proteins, calgranulin A and lysozyme C, pointing to the possibility that LDL may play another role(s) in innate immunity and inflammation than known hitherto [30]. It is mainly apo A-1 which is known to affect cell types implicated in immunosurveillance [e.g. natural killer (NK), T and endothelial cells]. It is known to modify the function of these cell types [31–33], and during infection and inflammation there is a marked decrease in serum levels of high-density lipoproteins and apo A-I [34–36]. The apo A-I gene resides in an apo cluster with the apo C3 and apo A-IV genes and mRNA levels of apo A-I, C3 and A-IV are co-regulated in the intestine [37]. Moreover, it has been demonstrated recently that a fraction of T cells, associated with CD pathogenesis, can function as NK cells [38,39]. A subset of human NK T cells can recognize lipid antigens presented by the CD1d molecule [40] and apolipoproteins also seem to be implicated in antigen recognition by AA cells [41–43].
CLC is an eosinophil-specific granule protein. The extracellular deposition and formation of crystals in tissues and body fluids is known to be associated with inflammation [44]. Mucosal eosinophils increase in gastrointestinal diseases that are often associated with altered epithelial barrier function, including food-allergic enteropathies and inflammatory bowel diseases [45,46]. Moreover, while a variety of data indicate that numerous eosinophils are in an activated state [26] and that the local release of neutrophil and eosinophil granule components are enhanced in the gut tissue from patients with CD and Crohn's disease [24,45], a differential pattern of eosinophil cytokine production [i.e. interleukin (IL)-5] has been associated with CD but not Crohn's disease [47,48]. Because IL-5 could contribute to paracrine interaction with T and B cells, and in autocrine fashion participates locally in eosinophil differentiation and activation, we think that eosinophils may also contribute to the RCD-II phenotype.
Overall, our results indicate that a different expression pattern in IgM, apo C3 and CLC is associated specifically with clonal T cell because (i) it was not found in patient 3 with CD grade 3B; (ii) patient 1 is not suffering from any other disease; and (iii) these three proteins are known to be involved in the immune response which, directly or indirectly, are associated with CD. Although we cannot exclude that these high-protein expressions are due to a specific inflammation status due to CD and aberrant T cell proliferation, apo C3 may be involved particularly in sustaining T cell proliferation.
In conclusion, it seems more appropriate to assume that T cells, IgM-producing cells, eosinophils and apo-activated effector T cells may, overall, cause tissue damage in established CD with aberrant T cells. In this contest, the clinical effectiveness of alemtuzumab in refractory CD should derive from its effect on several cellular pathways as expression of CD52 antigen is ubiquitous on the surface of all lymphocytes, monocytes, macrophages and eosinophils. Based on these observations, further investigations of the in vivo action of alemtuzumab on IgM-B cells, lipid metabolism and eosinophilis in CD patients by comparative proteomic and immunological molecular approaches are warranted.
Finally, as receptor recognition which is involved in NK function is as yet completely uncharacterized, further study is warranted to elucidate whether apo C3 has an effectiverole in CD-associated pathogenesis, and especially in non-conventional T cell population immunoserveillance.
Acknowledgments
We thank Dr Alessandra Marzotto for technical help. This study was supported by ‘Associazione Italiana per la Ricerca sul Cancro’ (AIRC); Lega Italiana Tumori (LILT).
References
- 1.Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 3.Vader W, Stepniak D, Kooy Y, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc Natl Acad Sci USA. 2003;100:12390–5. doi: 10.1073/pnas.2135229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundin KE, Farstad IN, Raki M, et al. Alemtuzumab treatment of refractory celiac disease type II. Gastroenterology. 2006;130(Suppl. 2):A–666. [Abstract] [Google Scholar]
- 5.Verbeek WH, Mulder CJ, Zweegman S. Alemtuzumab for refractory celiac disease. N Engl J Med. 2006;355:1396–7. doi: 10.1056/NEJMc061784. [DOI] [PubMed] [Google Scholar]
- 6.Vivas S, Ruiz de Morales JM, Ramos F, Suarez-Vilela D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T cell lymphoma. N Engl J Med. 2006;354:2514–15. doi: 10.1056/NEJMc053129. [DOI] [PubMed] [Google Scholar]
- 7.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 8.Lundin J, Osterborg A, Brittinger G, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin's Lymphoma. J Clin Oncol. 1998;16:3257–63. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 9.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 10.De Re V, Caggiari L, De Vita S, et al. Genetic insights into the disease mechanisms of type II mixed cryoglobulinemia induced by hepatitis C virus. Dig Liver Dis. in press. [DOI] [PubMed]
- 11.De Re V, De Vita S, Sansonno D, et al. Type II. mixed cryoglobulinaemia as an oligo rather than a mono B-cell disorder: evidence from GeneScan and MALDI-TOF analyses. Rheumatology. 2006;45:685–93. doi: 10.1093/rheumatology/kei278. [DOI] [PubMed] [Google Scholar]
- 12.Wood GM, Shires S, Howdle PD, Losowsky MS. Immunoglobulin production by coeliac biopsies in organ culture. Gut. 1986;27:1151–60. doi: 10.1136/gut.27.10.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony S, Arranz E, Barton JR, Ferguson A. Dissociation between systemic and mucosal humoral immune responses in coeliac disease. Gut. 1991;32:29–35. doi: 10.1136/gut.32.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood GM, Howdle PD, Trejdosiewicz LK, Losowsky MS. Jejunal plasma cells and in vitro immunoglobulin production in adult coeliac disease. Clin Exp Immunol. 1987;69:123–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster-Smith M, Joyce S, Kumar P. Immunoglobulins in the jejunal mucosa in adult coeliac disease and dermatitis herpetiformis after the reintroduction of dietary gluten. Gut. 1977;18:887–91. doi: 10.1136/gut.18.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster-Smith M, Packer S, Kumar PJ, Harries JT. Cellular infiltrate of the jejunum after re-introduction of dietary gluten in children with treated coeliac disease. J Clin Pathol. 1976;29:587–91. doi: 10.1136/jcp.29.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kett K, Scott H, Fausa O, Brandtzaeg P. Secretory immunity in celiac disease: cellular expression of immunoglobulin A subclass and joining chain. Gastroenterology. 1990;99:386–92. doi: 10.1016/0016-5085(90)91020-7. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P, Halstensen TS, Kett K, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562–84. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree JE, Heatley RV, Losowsky ML. Immunoglobulin secretion by isolated intestinal lymphocytes: spontaneous production and T cell regulation in normal small intestine and in patients with coeliac disease. Gut. 1989;30:347–54. doi: 10.1136/gut.30.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott BB, Scott DG, Losowsky MS. Jejunal mucosal immunoglobulins and complement in untreated coeliac disease. J Pathol. 1977;121:219–23. doi: 10.1002/path.1711210405. [DOI] [PubMed] [Google Scholar]
- 21.Halstensen TS, Hvatum M, Scott H, Fausa O, Brandtzaeg P. Association of subepithelial deposition of activated complement and immunoglobulin G and M response to gluten in celiac disease. Gastroenterology. 1992;102:751–9. doi: 10.1016/0016-5085(92)90155-r. [DOI] [PubMed] [Google Scholar]
- 22.Moorthy AV, Zimmerman SW, Maxim PE. Dermatitis herpetiformis and celiac disease: association with glomerulonephritis, hypocomplementia, and circulating immune complexes. JAMA. 1978;239:2019–20. doi: 10.1001/jama.239.19.2019. [DOI] [PubMed] [Google Scholar]
- 23.Lavo B, Knutson L, Loof L, Hallgren R. Gliadin challenge-induced jejunal prostaglandin E2 secretion in celiac disease. Gastroenterology. 2006;99:703–7. doi: 10.1016/0016-5085(90)90958-4. [DOI] [PubMed] [Google Scholar]
- 24.Hallgren R, Colombel JF, Dahl R, et al. Studies on the secretion rate and immunohistochemical localization of granulocyte granule constituents. Am J Med. 1989;86:56–64. doi: 10.1016/0002-9343(89)90230-1. [DOI] [PubMed] [Google Scholar]
- 25.Kristjansson G, Hogman M, Venge P, Hallgren R. TI − gut mucosal granulocyte activation precedes nitric oxide production: studies in coeliac patients challenged with gluten and corn. Gut. 2005;54:769–74. doi: 10.1136/gut.2004.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombel JF, Torpier G, Janin A, Klein O, Cortot A, Capron M. Evidence for a local release of major basic protein. Gut. 1992;33:1190–4. doi: 10.1136/gut.33.9.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauger JF, Couture P, Bergeron N, Lamarche B. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J Lipid Res. 2006;47:1212–8. doi: 10.1194/jlr.M500455-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Khovidhunkit W, Kim MS, Memon RA, et al. TI − effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. TI − oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003;14:437–45. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson H, Leanderson P, Tagesson C, Lindahl M. Mapping of proteins in low-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:551–65. doi: 10.1002/pmic.200300938. [DOI] [PubMed] [Google Scholar]
- 31.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 32.Yasumasu T, Takahara K, Sadayasu T, et al. Effect of plasma lipoproteins on natural killer cell activity in the elderly population. J Gerontol A Biosci Med Sci. 2006;58:561–5. doi: 10.1093/gerona/58.6.m561. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Paulsson G, Stemme S, Hansson GK. TI − hypercholesterolemia is associated with a T helper (Th)1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–25. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabana VG, Siegel JN, Sabesin SM. TI − effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989;30:39–49. [PubMed] [Google Scholar]
- 35.Feingold KR, Hardardottir I, Memon R, et al. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res. 2006;34:2147–58. [PubMed] [Google Scholar]
- 36.Sammalkorpi K, Valtonen V, Kerttula Y, Nikkila E, Taskinen MR. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 2006;37:859–65. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 37.Metzger S, Levy Y, Arnon R, Chajek-Shaul T. TI − co-regulation of apo A-I, apo C-III and apo A-IV gene expression in human intestinal biopsies. Eur J Clin Invest. 1996;26:71–5. doi: 10.1046/j.1365-2362.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- 38.Meresse B, Curran SA, Ciszewski C, et al. TI − reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med. 2006;203:1343–55. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hue S, Mention JJ, Monteiro RC, et al. TI − a direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:361–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Gumperz JE, Roy C, Makowska A, et al. TI − murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 41.Champagne E, Martinez LO, Vantourout P, Collet X, Barbaras R. TI − role of apolipoproteins in gammadelta and NKT cell-mediated innate immunity. Immunol Res. 2005;33:241–55. doi: 10.1385/ir:33:3:241. [DOI] [PubMed] [Google Scholar]
- 42.Scotet E, Martinez LO, Grant E, et al. TI − tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Bonneville M, Scotet E. TI − synergism and complementarity between human CD1 and MHC-restricted T cells, two lymphoid subsets directed against distinct antigenic worlds. Front Biosci. 2005;10:596–607. doi: 10.2741/1556. [DOI] [PubMed] [Google Scholar]
- 44.Ackerman SJ, Liu L, Kwatia MA, et al TI –. Charcot–Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J Biol Chem. 2002;277:14859–68. doi: 10.1074/jbc.M200221200. [DOI] [PubMed] [Google Scholar]
- 45.Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:890–7. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 46.Lombardi C, Passalacqua G. Clinical revision of 1862 cases. Arch Intern Med. 2003;63:1371–3. doi: 10.1001/archinte.163.11.1371-b. [DOI] [PubMed] [Google Scholar]
- 47.Desreumaux P, Janin A, Colombel JF, et al. TI − interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992;175:293–6. doi: 10.1084/jem.175.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamkhioued B, Gounni AS, Aldebert D, et al. TI − synthesis of type 1 (IFN gamma) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Ann NY Acad Sci. 1996;796:203–8. doi: 10.1111/j.1749-6632.1996.tb32582.x. [DOI] [PubMed] [Google Scholar]