Abstract
Tuberculosis remains one of the most important infectious diseases worldwide. Several studies have suggested that genetic factors may affect susceptibility to tuberculosis, but the specific genes involved have not yet been fully characterized. NRAMP1/SLC11 A1 and P2X7 genes have been linked to increased risk for tuberculosis in some African and Asiatic populations. To explore the potential role of these genes in the susceptibility to pulmonary tuberculosis in a Mexican mestizo population, we evaluated the association of D543N and 3′-UTR polymorphisms in NRAMP1/SLC11 A1 and − 762 and A1513C polymorphisms in P2X7 genes with the risk for tuberculosis. Polymerase chain reaction (PCR) amplification of genomic DNA followed by restriction fragment length polymorphism analysis, and allelic-specific PCR was employed. We found no significant differences in allelic frequency in NRAMP1/SLC11 A1 gene polymorphisms in 94 patients with tuberculosis compared to 100 healthy contacts. Similarly, no significant association of the P2X7−762 gene polymorphism with tuberculosis was detected. In contrast, the P2X7 A1513C polymorphism was associated significantly with tuberculosis (P= 0·02, odds ratio = 5·28, 95% CI, 0·99–37·69), an association that had not been reported previously. However, when the function of P2X7 was assessed by an l-selectin loss assay, we did not find significant differences in patients compared to healthy contacts or between PPD+ and PPD– control individuals. This study further supports the complex role of P2X7 gene in host regulation of Mycobacterium tuberculosis infection, and demonstrates that different associations of gene polymorphisms and tuberculosis are found in distinct racial populations.
Keywords: genetic polymorphisms, NRAMP1, P2X7, SLC11 A1, tuberculosis
Introduction
Tuberculosis is the second cause of death of infectious disease in the world, with an estimated 8–9 million new cases occurring annually. In addition, it is estimated that one-third of world population is infected with Mycobacterium tuberculosis [1]. Among those who are infected, only approximately 5–10% will develop clinical disease [2]. Only a minority of these patients have identifiable risk factors such as diabetes or HIV infection. In other patients, both genetic predisposition and environmental factors may contribute to the development of tuberculosis [3]. Thus, it is expected that the identification of host genetic factors for susceptibility to tuberculosis would greatly aid the global control of tuberculosis.
It is well known that M. tuberculosis is a facultative intracellular pathogen and that macrophages are the main reservoirs of this agent, as well as the primary immune effector cells regulating their growth and viability. As other intracellular infectious agents, M. tuberculosis enters into host macrophages by phagocytosis and is encapsulated in the phagosome. These organelles acquire microbicidal activity through a maturation process that involves the formation of the phagolysosome. It has been described widely that M. tuberculosis survives into host cells by arresting the process of phagosomal maturation [4,5].
Different molecules are involved in the killing process of intracellular pathogens by phagocytes. In the mouse, a single gene on chromosome 1, designated natural resistance-associated macrophage protein (Nramp) [6], controls the natural resistance to infection with unrelated intracellular parasites. The human Nramp homologue gene (NRAMP1/SLC11 A1) maps to chromosome 2q35 and is composed of 15 exons, and the corresponding mRNA encodes a 550 amino acid long polypeptide [7]. Human NRAMP1/SLC11 A1 protein is expressed by monocytes, macrophages and polymorphonuclear neutrophils [8,9]. Upon phagocytosis, NRAMP1/SLC11 A1 is recruited to the membrane of the phagosome and remains associated with this organelle during its maturation to phagolysosome. Although the precise function of this molecule has not been characterized fully, different reports indicate that NRAMP1/SLC11 A1 controls the replication of intracellular parasites by altering the intravacuolar environment of the microbe-containing phagolysosome [9,10]. In this regard, it has been found that NRAMP1/SLC11 A1 mediates the flux of divalent cations into lysosome and that promotes, through the Fenton reaction, the generation of toxic hydroxyl radicals, which contributes significantly to the killing of intracellular pathogens [11–14]. As a proton/divalent cation anti-porter, NRAMP1/SLC11 A1 also has an important role in the modulation of phagosomal pH [15,16].
The P2X7 gene, located at position 12q24, encodes for a 595-aa long polypeptide with two transmembrane stretches [17]. It has been found that P2X7 is a ligand-gated cation channel that is expressed in the cell membrane and that is activated mainly by adenosine-5′-triphosphate (ATP) [18]. Stimulation of cells through P2X7 opens a cation channel which allows Ca2+ influx and K+ efflux, phenomena involved in the induction of apoptosis and the release of mature interleukin (IL)-1β, respectively [19,20]. In addition, P2X7 activation initiates a number of downstream events including the activation of a membrane metalloproteinase, which causes the shedding of l-selectin (CD62L) in both lymphocytes and monocytes [21]. Although chemoattractants such as IL-8 can induce the rapid shedding of l-selectin in neutrophils, extracellular ATP is the only natural mediator described so far that can cause l-selectin shedding in monocytes and lymphocytes [21,22]. Furthermore, the sustained activation of the P2X7 purinergic receptor, a plasma membrane ATP-gated ion channel, induces both the formation of membrane pores permeable to hydrophilic solutes and apoptosis [23]. In addition, it has been described that the induction of apoptosis of M. tuberculosis-infected macrophages through P2X7 also results in the killing of the mycobacteria [24,25].
The P2X7 gene is highly polymorphic in humans, and several single nucleotide polymorphisms (SNPs) have been described [26]. A common SNP in exon 13 (A1513C) results in the substitution of glutamic acid at position 496 by alanine (E496A) [27]. This polymorphism occurs in the region of the gene that encodes the carboxyterminal tail of the protein, and different studies have demonstrated that homozygosity for the C allele (C/C) leads to almost complete loss of the P2X7 function with lack of ATP-induced mycobacterial killing in these individuals [28–30]. In addition, a SNP in the promoter region of P2X7 gene at nucleotide position − 762 (C/T) has been described that has a protective association with tuberculosis [31]. On the other hand, the human NRAMP1/SLC11 A1 gene has been reported to be involved in susceptibility to infectious diseases, such as tuberculosis, leprosy and HIV infection, as well as to some autoimmune diseases [32]. Genetic variations in the human NRAMP1/SLC11 A1 gene have been found to be associated with tuberculosis in some Asiatic and African populations, mainly the D543N and 3′-UTR polymorphisms [33,34]. In this work, we decided to assess whether NRAMP1/SLC11 A1 and P2X7 gene polymorphisms are associated with susceptibility to M. tuberculosis infection in a Mexican mestizo population. For this purpose, we performed a case–control study comparing the frequency of several polymorphisms in these genes in patients with pulmonary tuberculosis and healthy contacts.
Materials and methods
Patients
Ninety-four patients with pulmonary tuberculosis from the south-east region of México were recruited for this study. Diagnosis was based on clinical and laboratory data. In all cases, the clinical picture and X-ray examination were highly indicative of tuberculosis, and M. tuberculosis was cultured from the sputum of all patients. Most patients were under therapy with different anti-tuberculous drugs. Forty-eight patients were male and 46 female. One hundred healthy contacts were studied as controls. An informed written consent was obtained from all individuals, and this study was approved by the ethics committee of the School of Medicine, UASLP.
Blood samples and DNA isolation
Peripheral blood mononuclear cell (PBMC) samples were obtained and were isolated by density gradient centrifugation over Ficoll-Hypaque cushions (Sigma Chemical Co., St Louis, MO, USA). Cells then were washed with phosphate-buffered saline (PBS) and resuspended at 2 × 106 cells/ml in RPMI-1640 culture medium, supplemented with 10% fetal bovine serum (FBS) and 2 mM l-glutamine. Genomic DNA was extracted by using the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA), and the DNA was then dissolved in sterile distilled water. Quantity and purity were determined by spectrophotometry (SmartSpec Plus Bio-Rad Laboratories, Inc., Hercules, CA, USA).
NRAMP1/SLC11 A1 DNA genotyping
Polymerase chain reactions (PCR) were performed in a total volume of 50 µl of a solution containing 100 ng genomic DNA, 5·0 µl free Mg2+ 10× buffer (Invitrogen Life Technologies, Carlsbad, CA, USA), 200 µM dNTPs, 2·0 mM MgCl2 and 1·0 U of recombinant Taq DNA polymerase (Invitrogen). Thermal cycling was performed on a TC-412 device (Techne, Cambridge, UK). For D545N and 3′-UTR NRAMP1/SLC11 A1 polymorphisms, cycling conditions were: 94°C for 5 min, 30 cycles of 94°C for 30 s, 68°C for 30 s and 72°C for 45 s, with a final 5-min extension at 72°C. Primers for this PCR were 5′-CTCTGGCTGAAGGCTCTCC-3′ (forward) and 5′-AACTGTCCCACTCTATTCCTG-3′ (reverse) [31]. A region of 244 base pairs (bp) was amplified, and genotyping was performed using restriction fragment length polymorphism (RFLP) analysis. In this regard, the PCR product was digested at 37°C for 24 h with Ava II or Fok I (Amersham Pharmacia Biotech UK Limited, Piscataway, NJ, USA) in a 20-µl reaction mix containing 1× restriction enzyme buffer and bovine serum albumin (BSA). Digested products were run on 1·5% agarose gel or 8·0% polyacrylamide gels, which were stained with ethidium bromide and visualized using an UV transilluminator.
P2X7 DNA genotyping
The − 762 polymorphism of P2X7 was genotyped by an allele-specific PCR. Cycling conditions were: 94°C for 5 min, 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 45 s, with a final 5-min extension at 72°C. The following primers were used: forward 5′-GCAGGCCAGGGAGTCA-3′, reverse 5′-ATCAGATTTGCAGGGGTAGG-3′; allele T 5′-GTCCCTCACTGAATAGGTCAAT-3′; allele C 5′-GTCCCTCACTGAATAGGTCAAC-3′ [29]. PCR products were run on 1·5% agarose gel or 8·0% polyacrylamide gels, which were stained with ethidium bromide and visualized using an UV transilluminator.
The A1513C SNP of P2X7 was genotyped by PCR/RFLP using the following primers: forward 5′-AGACCTGCGATGGACTTCACAG-3′, reverse 5′-GCCAGGTGGCGTAGCACCTG-3′ [29]. Cycling conditions were: 94°C for 5 min, 30 cycles of 94°C for 30 s, 68°C for 30 s and 72°C for 45 s, with a final 5-min extension at 72°C. PCR products were digested at 37°C for 16 h with 5·0 U of HaeII (Promega). Digested products were run on 1·5% agarose gel, which was stained with ethidium bromide and visualized using an UV transilluminator.
l -selectin loss assay
PBMC (1 × 106) were incubated for 5 min in RPMI-1640 at 37°C in the presence or not of 3·0 mM ATP, and then washed with 1 ml of cold PBS and centrifuged at 300 g for 5 min. Afterwards, cells were washed once with PBS, incubated with a fluorescein isothiocyanate (FITC)-conjugated anti-l-selectin monoclonal antibody (Becton Dickinson Biosciences, San Jose, CA, USA) for 30 min at 4°C, and then washed in PBS with 1% FBS twice, and the mean fluorescence intensity of l-selectin expression was determined in a fluorescence activated cell sorter (FACSCalibur) flow cytometer (Becton Dickinson). Results were expressed as the percentage of l-selectin loss expression induced by ATP.
Analysis of P2X7 expression
PBMC (2 × 106) were washed with PBS and centrifuged. Cells were then fixed for 10 min in 4% paraformaldehyde, washed in PBS and permeabilized for 10 min with absolute methanol. Cells were then washed in 2% BSA in PBS, and incubated with a rabbit polyclonal anti-P2X7 antibody (Becton Dickinson) directed against the C terminus region, washed and labelled with an FITC-conjugated goat anti-rabbit antibody (Calbiochem, La Jolla, CA, USA) for 30 min at 4°C. Finally, cells were washed twice, fixed in 1% paraformaldehyde and analysed by flow cytometry.
Analysis of mRNA NRAMP1/SLC11 A1 expression
Total RNA was isolated from PBMC with Trizol (Invitrogen) according to the manufacturer's recommendations. RNA integrity was monitored by agarose gel electrophoresis, and concentration was measured by spectrophotometry. For cDNA synthesis, 1·0 µg total RNA was mixed with 0·5 µg oligo(dT) primer (Invitrogen), heated for 5 min a 70°C, and then 1× reverse transcriptase buffer, 0·5 mM deoxyribonucleoside triphosphate (dNTP) mix (Promega), 0·01 M dithiothreitol, 40 U RNasin (Promega) and 200 units of Moloney murine leukaemia virus reverse transcriptase (M-MLV RT, Promega) were added. The tube was then incubated for 10 min at room temperature (RT), 90 min at 37°C and heated to 94°C to inactivate the reverse transcriptase. To amplify exons 13–15, we used forward primer 5′-AGCATGCCCACCCTGATGCAGG-3′ and reverse primer 5′-AGTGCTTAACCTCCATGGCAAG-3′. PCR was performed in a 25-µl reaction mixture containing 1× PCR buffer, 2 mM MgCl2, 200 µM dNTPs, 20 pmol of each primer and 1 U Taq DNA polymerase (Invitrogen), and 1 µl cDNA. The PCR was carried out as follows: initial denaturation at 94°C for 5 min, and then 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 45 s; the final extension step was performed at 72°C for 5 min. PCR products were detected in a 1·5% agarose gel with ethidium bromide staining. The size of the PCR product was determined with a 100-bp DNA molecular weight ladder (Invitrogen), and quantified by densitometric analysis, using the 1D Image Analysis software (Kodak Digital Science, Rochester, NY, USA).
Data analysis
Overall genotype frequencies were compared with the use of a 2 × 2 χ2 test. The Hardy–Weinberg equilibrium, which indicates the absence of discrepancy between genotype and allele frequency, was determined in controls and patients by the arlequin program. Statistical analyses were carried out using GraphPad prism software (GraphPad Software Inc., San Diego, CA, USA). Student's t-test and one-way anova were used to compare data from two groups or four groups, respectively.
Results
P2X7 gene polymorphisms in patients with pulmonary tuberculosis
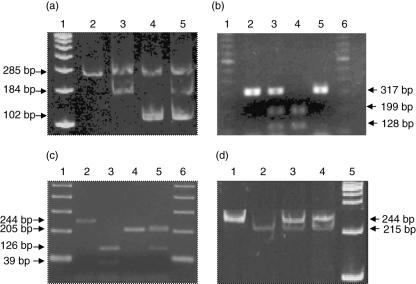
We first analysed the allelic frequencies of two different SNPs of the P2X7 gene. As shown in Fig. 1a, we were able to detect the − 762 T/C polymorphism by an allelic-specific PCR and found that the frequency of the T and C alleles was in Hardy–Weinberg equilibrium (Table 1). The frequency of the − 762T allele in patients with tuberculosis was 0·7447, whereas that of − 762C was 0·2553, with no significant differences when compared to those observed in healthy contacts (P= 0·174 and 0·075, respectively).
Fig. 1.
Detection of single nucleotide polymorphisms of P2X7 and NRAMP1 genes. Genomic DNA from 94 patients with pulmonary tuberculosis and 100 healthy contacts was isolated, and single nucleotide polymorphisms (SNPs) of P2X7 and NRAMP1 genes were determined as follows: (a) the − 762 T/C polymorphism of P2X7 was detected by an allelic-specific polymerase chain reaction (PCR) and its product was run on 8% polyacrylamide gel. Lane 1, 100 base pairs (bp) marker; lane 2, control DNA; lane 3, wild-type; lane 4, mutant type; lane 5, heterozygote. (b) The A1513C polymorphism of P2X7 was detected by restriction fragment length polymorphism (RFLP) analysis, as stated in Materials and methods. The PCR product was digested with HaeII and run on 2% agarose gel. Lane 1, 100 bp marker; lane 2, no digested; lane 3, heterozygote; lane 4, mutant; lane 5, wild-type. (c) The D543N polymorphism of NRAMP1 was detected by RFLP, as described in Materials and methods. Lane 1, 100 bp marker; lane 2, no digested; lane 3, wild-type; lane 4, mutant; lane 5, heterozygote. (d) The -TGTGdel polymorphism in the 3′-UTR region of NRAMP-1 gene was detected by RFLP, as stated in Materials and methods. Lane 1, 100 bp marker; lanes 2 and 3, heterozygotes; lane 4, wild-type; lane 5, mutant.
Table 1.
Frequency of the − 762T/C polymorphism of the P2X7 gene in patients with tuberculosis and healthy contacts.
| Patients | Control subjects | |||
|---|---|---|---|---|
| Genotype or allele | n | Frequency | n | Frequency |
| Genotype | ||||
| TT | 52 | 0·5745 | 51 | 0·4636 |
| TC | 32 | 0·3404 | 44 | 0·4 |
| CC | 8 | 0·0851 | 15 | 0·1364 |
| Total | 92 | 110 | ||
| Allele | ||||
| T | 140 | 0·7447 | 146 | 0·6636 |
| C | 48 | 0·2553 | 74 | 0·3363 |
| Total | 188 | 220 | ||
The polymorphism at position 1513 in exon 13 of the P2X7 gene was studied by RFLP by using the restriction enzyme HaeII (Fig. 1b). When the genotype distribution was analysed by χ2 test a significant difference was detected [P= 0·0242 and 0·0248, uncorrected and corrected, respectively, odds ratio (OR) for the C allele of P2X7 A1513C SNP of 5·28, 95% confidence interval (CI) = 0·99–37·69, Table 2], indicating an association of this SNP with tuberculosis that had not been found previously, suggesting a dominant effect of this gene variant.
Table 2.
Frequency of the A1513C polymorphism in the P2X7 gene exon 13 in patients with tuberculosis and healthy contacts.
| Patients | Control subjects | |||
|---|---|---|---|---|
| Genotype or allele | n | Frequency | n | Frequency |
| Genotype | ||||
| AA | 53 | 0·759 | 70 | 0·6364 |
| AC | 33 | 0·2289 | 38 | 0·3455 |
| CC | 8 | 0·012 | 2 | 0·0182 |
| Total | 94 | 110 | ||
| Allele | ||||
| A | 139 | 0·8735 | 178 | 0·8091 |
| C | 49 | 0·1265 | 42 | 0·1909 |
| Total | 188 | 220 | ||
Polymorphisms of NRAMP1/SLC11 A1 gene in tuberculosis patients
The allelic frequencies of the D543N and 3′-UTR variants of NRAMP1/SLC11 A1 gene were studied by RFLP using the Ava II and Fok I restriction enzymes, respectively. We found a very similar frequency of these gene polymorphisms in patients with pulmonary tuberculosis and healthy contacts, with no significant differences in the genotype distribution in both cases (P= 0·5178 and 0·9207, respectively, Tables 3 and 4).
Table 3.
Frequency of the D543N polymorphism in the NRAMP1 gene exon 15 in patients with tuberculosis and healthy contacts.
| Patients | Control subjects | |||
|---|---|---|---|---|
| Genotype or allele | n | Frequency | n | Frequency |
| Genotype | ||||
| GG | 54 | 0·5745 | 67 | 0·6091 |
| GA | 36 | 0·383 | 40 | 0·3637 |
| AA | 4 | 0·0425 | 3 | 0·0273 |
| Total | 94 | 110 | ||
| Allele | ||||
| G | 144 | 0·7659 | 174 | 0·7909 |
| A | 44 | 0·234 | 46 | 0·2091 |
| Total | 188 | 220 | ||
Table 4.
Frequency of the -TGTGdel 3′-UTR polymorphism in the NRAMP1 gene in patients with tuberculosis and healthy contacts.
| Patients | Control subjects | |||
|---|---|---|---|---|
| Genotype or allele | n | Frequency | n | Frequency |
| Genotype | ||||
| TGTG+/+ | 42 | 0·5753 | 48 | 0·5783 |
| TGTG+/– | 20 | 0·0274 | 23 | 0·2771 |
| TGTG–/– | 11 | 0·1507 | 12 | 0·1446 |
| Total | 73 | 83 | ||
| Allele | ||||
| TGTG | 104 | 0·7123 | 119 | 0·7169 |
| delTGTG | 42 | 0·2877 | 47 | 0·2831 |
| Total | 146 | 166 | ||
Expression of NRAMP1/SLC11 A1 and P2X7 in mononuclear cells (MNC) from tuberculosis patients
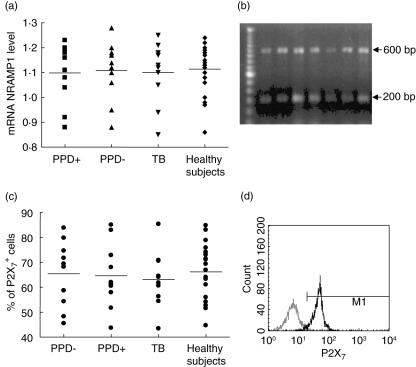
The expression of NRAMP1/SLC11 A1 and P2X7 genes in patients with tuberculosis was studied by reverse transcription–polymerase chain reaction (RT–PCR) (for NRAMP1/SLC11 A1) and flow cytometry (for P2X7). For this purpose, four groups were analysed: PPD+ and PPD– healthy contacts, tuberculosis patients and healthy individuals with no apparent exposure to M. tuberculosis. As shown in Fig. 2a, a similar expression of NRAMP1/SLC11 A1 gene at mRNA levels was found in the four groups, with no significant differences among them (P= 0·98, one-way anova analysis). In addition, no apparent association between NRAMP1/SLC11 A1 gene polymorphisms and its level of mRNA expression by monocytes was found in any group studied (data not shown).
Fig. 2.
Expression of P2X7 and NRAMP1 genes in mononuclear cells (MNC) from patients with pulmonary tuberculosis and healthy contacts. (a) The expression of NRAMP1 gene at mRNA level was analysed by reverse transcription–polymerase chain reaction (RT–PCR) and results were normalized to β-actin mRNA level, as stated in Materials and methods. Horizontal bars correspond to the arithmetic mean values of each group. No significant differences among the four groups studied were detected. (b) Representative results of NRAMP-1 mRNA expression in three patients with tuberculosis (lanes 2–4) and four healthy contacts (lanes 5–8). The 600 base pairs (bp) bands correspond to NRAMP1 and the 200 bp bands to β-actin. (c) Analysis of expression of P2X7. Peripheral blood mononuclear cells (PBMC) were immunostained for P2X7 and then analysed by flow cytometry. No significant differences were found among the four groups studied. Horizontal bars correspond to the arithmetic mean values of each group. (d) A representative histogram of P2X7 expression in PBMC from a patient with tuberculosis is shown. The thin line histogram corresponds to the negative control, and the thick line histogram to cells stained with a specific antibody to P2X7.
As in the case of NRAMP-1, we did not detect significant differences in the membrane expression of P2X7 by monocytes from patients with tuberculosis compared with other groups (P= 0·93, Fig. 2c). However, some tuberculosis patients tended to show higher levels of P2X7 expression compared to healthy contacts (Fig. 2d). In addition, no apparent association between the genotype of P2X7 and its level of expression in the different groups studied was found (P > 0·05 in all cases, data not shown).
Function of P2X7 in MNC from patients with tuberculosis
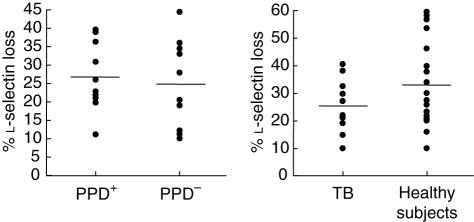
It has been reported widely that the stimulation of MNC through P2X7 induces the membrane shedding of l-selectin [26]. Therefore, we analysed by flow cytometry the loss of l-selectin induced by ATP in cells from tuberculosis patients and controls. Although some patients with tuberculosis tended to show a diminished response to ATP, no significant differences in this parameter were found among the groups studied (P < 0·05 in any case, Fig. 3). As expected [27], those individuals (patients or controls) bearing the 1513C allele show a diminished (heterozygous) or absent (homozygous) response (l-selectin loss) to ATP (data not shown).
Fig. 3.
Analysis of P2X7 function. Peripheral blood mononuclear cells (PBMC) from healthy contacts, PPD+ and PPD– (left panel) as well as from patients with pulmonary tuberculosis, and healthy subjects without apparent exposure to Mycobacterium tuberculosis (right panel) were incubated or not with 3 mm of adenosine-5′-triphosphate (ATP) for 5 min, and then analysed for l-selectin expression by flow cytometry, as stated in Materials and methods. No significant differences were detected among the groups studied.
Discussion
Tuberculosis remains as the seventh most important cause of global premature mortality and disability [1]. Currently, there are 8–9 million new cases and 2–3 million deaths annually. In this regard, it is well known that approximately one-third of the world's population is infected with M. tuberculosis. However, most of the 90% of infected individuals remain healthy, indicating the effectiveness of the different immune mechanisms in resistance against this mycobacterium. Although both acquired and innate mechanisms contribute to the killing of M. tuberculosis, the precise mechanisms that confer resistance against this infection have not been elucidated fully. Nevertheless, it seems evident that different genes are involved in susceptibility towards M. tuberculosis infection [34,35]. In this work, we have explored the possible association betweenpolymorphisms of P2X7, NRAMP1/SLC11 A1 genes and pulmonary tuberculosis.
NRAMP1/SLC11 A1 is a proton/divalent cation anti-porter located mainly in the lysosome membrane that fluxes, for example, Fe2+ and Mg2+ to either side of the membrane, depending on pH. In late endolysosomes, NRAMP1/SLC11 A1 delivers divalent cations from the cytosol into this acidic compartment [10]. Then, through the Fenton reaction, toxic hydroxyl radicals are generated, which contributes significantly to the killing of intracellular pathogens [13,36]. In addition, it has been described that the expression of inducible macrophage type nitric oxide synthase (iNOS), and the generation of nitric oxide and their toxic derivatives may be influenced by NRAMP1/SLC11 A1 [37]. Furthermore, mutations of NRAMP1/SLC11 A1 gene impair phagosomal acidification, which also contribute to the killing of intracellular pathogens into the phagolysosome [16].
The reports on the association of NRAMP1/SLC11 A1 gene polymorphisms with M. tuberculosis infection are controversial [38]. In this regard, Liu et al. have reported a positive association between the 3′-UTR but not theINT4 polymorphism of the NRAMP1/SLC11 A1 gene and pulmonary tuberculosis in a Chinese population [39]. In a meta-analysis performed recently by Li et al. [40], it was found that the four gene polymorphisms that have been studied for NRAMP1/SLC11 A1 [3′-UTR, D543N, INT4 and 5′-(GT)n] do not show a significant association with tuberculosis in a European population. In contrast, a variable but significant association among most of these variants has been found in Asian and African subjects [32,33,41]. Our results show that in a Mexican mestizo population there is no apparent association between the D543N and 3′-UTR variants of NRAMP1/SLC11 A1 and pulmonary tuberculosis. Therefore, it is evident that the genetic background exerts a decisive role on the possible influence of this gene in susceptibility to infection by M. tuberculosis. In this regard, it has been reported that the protein encoded by NRAMP1/SLC11 A1 seems to have a role in the control of growth of bacilli and progression of disease rather, than in susceptibility to M. tuberculosis infection [42,43]. It could be speculated, therefore, that in European and Mexican mestizo populations the possible protective role of NRAMP1/SLC11 A1 in M. tuberculosis infection can be replaced by other genes/molecules involved in the resistance against intracellular pathogens.
P2X7 is a ligand-gated cation channel with a trimeric structure that is expressed in the cell membrane and that is activated mainly by ATP [44]. It has been described that activation of this purinergic receptor results in the opening of cation-specific channels and non-specific pores that allow the passage of solutes of less than 900 Da [45]. Furthermore, stimulation of infected macrophages through this receptor induces activation of phospholipase D, an increase in intracellular calcium concentration and the killing of M. tuberculosis[46]. In addition, other intracellular phenomena induced through P2X7 have been described, including phosphorylation of extracellular-regulated kinase (ERK)1/2, activation of Src tyrosine kinases and triggering of programmed cell death [47]. Additional data indicate that this receptor could be involved in tissue damage, mediating the release of proinflammatory cytokines IL-1β and tumour necrosis factor (TNF)-α [19,48]. All these data suggest strongly that P2X7 may play a relevant role in the pathogenesis of M. tuberculosis infection. In this regard, we have performed previously a study on the expression and function of this receptor in patients with pulmonary tuberculosis. We found no apparent abnormalities in the expression of P2X7 and different functions induced by it in cells from these patients [49]. However, when the expression of different genes related to apoptosis and cytokines in response to ATP was analysed, it was evident that the PBMC from tuberculosis patients showed a different pattern of gene expression compared to healthy contacts [49]. In the present work, we decided to explore further the role of P2X7 in these patients. We found that the Glu496Ala SNP in exon 13 of the P2X7 gene showed a significant association with pulmonary tuberculosis, further supporting that this receptor plays a relevant role in the pathogenesis of the infectious process. In this regard, it has been described that the Glu496Ala mutation of P2X7 has an important effect on the function of this channel, and that the homozygosis for this polymorphisms led to almost complete loss of P2X7 function [46,50]. However, there are no previous reports on the association of this SNP with tuberculosis or other infections by intracellular bacteria. Therefore, this work is the first demonstration of an association between a genetic polymorphism of P2X7 and enhanced susceptibility to tuberculosis, suggesting the importance of carrying out similar studies in populations with different genetic backgrounds.
In contrast with the above results, we have not found a significant association between the − 762T/C variant of P2X7 and pulmonary tuberculosis. This finding also contrasts with the report of Li et al. on the protective association against tuberculosis of the CC or C allele genotype of this SNP in a Gambian population [30]. Although the putative mechanism of protection by these genotypes has not been elucidated, we consider our apparent discrepant results to be of interest. It is feasible that in a Gambian population the − 762T/C variant of P2X7 has an important effect on the regulation of expression of this gene, and that in a Mexican mestizo population this does not occur. This possibility is supported by our results on P2X7 detection in MNC by flow cytometry, showing no apparent relationship between the level of expression of P2X7 and the − 762T/C variant of this gene. Therefore, we believe that it would be of interest to determine the possible effect of − 762T/C genotypes on P2X7 expression in those populations showing a protective effect against tuberculosis.
In summary, our data indicate that the D543N and 3′-UTR variants of NRAMP1/SLC11 A1 gene are not significantly associated, as occurs in European ascendant populations, with pulmonary tuberculosis in Mexican mestizos. In contrast, our results suggest strongly that in this population the Glu496Ala mutation of P2X7, but not its − 762T/C variant, is associated with increased susceptibility for M. tuberculosis infection. The precise mechanism(s) accounting for this association, as well as its possible clinical relevance, remain interesting points to be explored.
Acknowledgments
This work was supported by grant 45933-M from CONACYT, México (to RG-A). Perla Niño-Moreno was a recipient of a scholarship from CONACYT (México).
References
- 1.World Health Organization. World Health Organization Report 2006 (WHO/HTM/TB/ 2006362) Beijing, China: WHO; 2006. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 2.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 3.Cooke GS, Hill AVS. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–77. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 4.Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Rusell DG. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci USA. 2004;101:13642–7. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas M, Barrera LF, Puzo G, Garcia LF. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–61. [PubMed] [Google Scholar]
- 7.Blackwell JM, Barton CH, White JK, et al. Genomic organization and sequence of the human NRAMP gene: identification and mapping of a promoter region polymorphism. Mol Med. 1995;1:194–205. [PMC free article] [PubMed] [Google Scholar]
- 8.Cellier M, Govoni G, Vidal S, et al. Human natural resistance-associated macrophage protein: cDNA cloning, chromosomal mapping, genomic organization, and tissue-specific expression. J Exp Med. 1994;180:1741–52. doi: 10.1084/jem.180.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruenheid S, Pinner E, Desjardins M, Gros P. Natural resistance to infection with intracellular pathogens: the Nramp 1 protein is recruited to the membrane of the phagosome. J Exp Med. 1997;4:717–30. doi: 10.1084/jem.185.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackam D, Rotstein OD, Zhang WJ, Gruenheid S, Gros P, Grinstein S. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp 1) impairs phagosomal acidification. J Exp Med. 1998;188:351–64. doi: 10.1084/jem.188.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter-Koltunoff M, Ehrlich S, Dror N, et al. NRAMP1-mediated innate resistance to intraphagosomal pathogens is regulated by IRF-8, PU.1, and Miz-1. J Biol Chem. 2003;278:44025–32. doi: 10.1074/jbc.M307954200. [DOI] [PubMed] [Google Scholar]
- 12.Goswami T, Bhattacharjee A, Babal P, et al. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem J. 2001;354:511–9. doi: 10.1042/0264-6021:3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwilling BS, Kuhn DE, Wikoff L, Brown D, Lafuse W. Role of iron in NRAMP1-mediated inhibition of mycobacterial growth. Infect Immun. 1999;67:1386–92. doi: 10.1128/iai.67.3.1386-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulero V, Searle S, Blackwell JM, Brock JH. Solute carrier 11a1 (Slc11a1; formerly Nramp1) regulates metabolism and release of iron acquired by phagocytic, but not transferring-receptor-mediated, iron uptake. Biochem J. 2002;363:89–94. doi: 10.1042/0264-6021:3630089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167:3300–7. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 16.Jabado N, Cuellar-Mata P, Grinstein S, Gros P. Iron chelators modulate the fusogenic properties of Salmonella-containing phagosomes. Proc Natl Acad Sci USA. 2003;100:6127–32. doi: 10.1073/pnas.0937287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buell GN, Talabot F, Gos A, et al. Gene structure and chromosomal localization of the human P2X7 receptor. Receptor Channels. 1998;5:347–54. [PubMed] [Google Scholar]
- 18.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–44. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 19.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1β release. J Immunol. 2003;170:5728–38. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 20.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-κB-driven protein synthesis. J Immunol. 2005;175:7611–22. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 21.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and 1-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–51. [PubMed] [Google Scholar]
- 22.Kushimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and Mel-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 23.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–8. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 24.Shemon AN, Sluyter R, Fernando SL, et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–86. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 25.Canaday DH, Beigi R, Silver RF, Harding CV, Boom WH, Dubyak GR. ATP and control of intracellular growth of mycobacteria by T cells. Infect Immun. 2002;70:6456–9. doi: 10.1128/IAI.70.11.6456-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boldt W, Klapperstück M, Büttner C, Sadtler S, Schmalzing G, Markwardt F. Glu496Ala polymorphism of human P2X7 receptor does not affect its electrophysiological phenotype. Am J Physiol Cell Physiol. 2003;284:C749–56. doi: 10.1152/ajpcell.00042.2002. [DOI] [PubMed] [Google Scholar]
- 27.Gu BJ, Worthington RA, Sluyter R, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–42. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 28.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. J Immunol. 2004;172:3399–405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 29.Cabrini G, Falzoni S, Forchap SL, et al. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–9. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- 30.Li CM, Campbell SJ, Kumararatne S, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis. 2002;186:1458–62. doi: 10.1086/344351. [DOI] [PubMed] [Google Scholar]
- 31.Blackwell JM, Goswami T, Evans CA, et al. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–84. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellamy R, Ruwende C, Corrah T, McAdam K, Whittle HC, Hill AVS. Variations in the NRAMP 1 gene and susceptibility to tuberculosis in West Africans. New Engl J Med. 1998;338:640–4. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 33.Ryu S, Park YK, Bai GH, et al. 3′UTR polymorphisms in the NRAMP1 gene are associated with susceptibility to tuberculosis in Koreans. Int J Tuberc Lung Dis. 2000;4:577–80. [PubMed] [Google Scholar]
- 34.Delgado CJ, Baena A, Thim S, Goldfeld AE. Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis. 2002;186:1463–8. doi: 10.1086/344891. [DOI] [PubMed] [Google Scholar]
- 35.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochem Biophys Acta. 1763;2006:609–20. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Fritsche G, Dlaska M, Barton H. Nramp1 functionality increases inducible nitric oxide synthase transcription via stimulation of IFN regulatory factor 1 expression. J Immunol. 2003;171:1994–8. doi: 10.4049/jimmunol.171.4.1994. [DOI] [PubMed] [Google Scholar]
- 38.Huang JH, Oefner PJ, Adi V, et al. Analyses of the NRAMP1 and IFN-γR1 genes in women with Mycobacterium avium-intracellular pulmonary disease. Am J Respir Crit Care Med. 1998;157:377–81. doi: 10.1164/ajrccm.157.2.9706012. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Cao WC, Zhang CY, et al. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case–control study. Int J Tuberc Lung Dis. 2004;8:428–34. [PubMed] [Google Scholar]
- 40.Li HT, Zhang TT, Zhou YQ, Huang QH, Huang J. SLC11A1 (formerly NRAMP1) gene polymorphisms and tuberculosis susceptibility: a meta-analysis. Int J Tuberc Lung Dis. 2006;10:3–12. [PubMed] [Google Scholar]
- 41.Liaw YS, Tsai-Wu JJ, Wu CH, et al. Variations in the NRAMP1 gene and susceptibility of tuberculosis in Taiwanese. Int J Tuberc Lung Dis. 2002;6:454–60. [PubMed] [Google Scholar]
- 42.Zhang W, Shao L, Weng X, et al. Variants of the natural resistance-associated macrophage protein 1 gene (NRAMP1) are associated with severe forms of pulmonary tuberculosis. Clinic Infect Dis. 2005;40:1232–6. doi: 10.1086/428726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malik S, Abel L, Tooker H, et al. Alleles of the NRAMP1 gene are risk factors for pediatric tuberculosis disease. Proc Natl Acad Sci USA. 2005;102:12183–8. doi: 10.1073/pnas.0503368102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. J Biol Chem. 1997;272:5482–6. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 45.Smart ML, Gu B, Panchal RG, et al. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem. 2003;278:8853–60. doi: 10.1074/jbc.M211094200. [DOI] [PubMed] [Google Scholar]
- 46.Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–88. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 47.Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006;177:2842–50. doi: 10.4049/jimmunol.177.5.2842. [DOI] [PubMed] [Google Scholar]
- 48.Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. General Immun. 2004;5:588–91. doi: 10.1038/sj.gene.6364127. [DOI] [PubMed] [Google Scholar]
- 49.Franco-Martinez S, Niño-Moreno P, Bernal-Silva S, et al. Expression and function of the purinergic receptor P2X7 in patients with pulmonary tuberculosis. Clin Exp Immunol. 2006;146:253–61. doi: 10.1111/j.1365-2249.2006.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of Mycobacteria. J Immunol. 2003;171:5442–6. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]