Abstract
The poor prognosis associated with ovarian carcinoma (OVCA) is linked to the high incidence of local recurrence. There is a pressing need to identify factors that can play a role in OVCA growth and spread. Here, we focused on CD40, a member of the tumour necrosis factor (TNF) receptor superfamily with important functions in immune response. The expression of CD40 has been reported on various types of carcinoma cells, but its biological role is still poorly understood. The aim of the present study was to investigate the expression and function of the CD40 in OVCA cell lines. Detectable CD40 levels ranging from low to very high were found on the cell surface of several OVCA cell lines by flow cytometry analysis. Co-culture with a murine cell line transfected with CD40 ligand (CD40L) inhibited cell growth and up-regulated the secretion of proinflammatory cytokines interleukin (IL)-6, IL-8 and TNF-α in high-level CD40-expressing OVCA cell lines. Similarly, an increase of IL-6 and IL-8 release could be obtained by adding a soluble form of CD40L to the OVCA cultures. These results suggest that CD40–CD40L interaction is an important pathway affecting growth regulation and cytokine production in OVCA.
Keywords: cytokines/interleukins, ovarian carcinoma, phenotype/cell markers
Introduction
CD40 is a membrane protein belonging to the tumour necrosis factor (TNF) receptor family. This molecule is expressed mainly by dendritic cells, macrophages and B cells [1]. Its ligand, CD40L (CD154), is expressed transiently on T cells following their activation via the T cell receptor. CD40 binding on antigen-presenting cells (APC) up-regulates co-stimulatory molecules CD80 (B7·1) and CD86 (B7·2) and the production of cytokines such as interleukin (IL)-12 or TNF-α. These effects lead cooperatively to T cell differentiation with polarization of T helper (Th) cells into the Th1 phenotype [2]. Interestingly, it was demonstrated that CD40 is also present in melanoma and in several types of carcinoma, including breast, ovary and nasopharynx carcinoma [3,4]. In contrast to haematopoietic cells, where the role of CD40–CD40L interactions has been well investigated, the relevance of CD40 function in human tumours remains unclear. Whereas some studies have suggested that CD40 activation on tumour cells induces proliferation or inhibits CD95-mediated apoptosis, others have shown that CD40 ligation results in growth inhibition and apoptosis [3,5,6].
Ovarian carcinoma (OVCA) is a significant cause of cancer-related death among women. The rate of relapse is high and the overall 5-year survival is very low: less than 30% [7]. At present, there is an increasing amount of evidence that the cytokine-rich inflammatory microenvironment of tumours may contribute to cancer growth and spread rather than immune response to cancer [8]. OVCA cells produce various cytokines such as IL-6, IL-8 or TNF-α, which could significantly affect tumour biology [9,10]. Here, we have analysed expression of CD40 in ovarian carcinoma and searched for a possible functional significance. The effect of CD40 ligation on ovarian carcinoma cell growth and cytokine production was studied using a mouse fibroblastic cell line transfected with the human CD40L gene.
Materials and methods
Tumour cell lines and primary tumour cells
The OVCA cell lines O65, O114 and O170 (derived from malignant ascites) and O135 (derived from a solid tumour) were generated in our laboratory. The initiation of these cell lines was performed from single cell suspensions obtained from malignant ascites or after the enzymatic dissociation of solid tumour, as described previously [9]. The cell lines O65, O135 and O170 were used at about 20 passages and the cell line O114 was used at about 70 passages. Cryopreserved single cell suspensions freshly dissociated from three solid biopsies (patients O113, O136 and O151) were thawed for phenotype analysis. The established human ovarian carcinoma cell line, OVCAR-3, and the breast carcinoma cell line, MCF-7, were purchased from the American Type Cell Collection (ATCC, Manassas, VA, USA). The IGROV1 cell line generated from a solid ovarian carcinoma was a gift from Dr J. Bénard (Institut Gustave Roussy, Villejuif, France). The epithelial origin of cultured OVCA cell lines was verified by morphological studies, as well as immunostaining with monoclonal antibodies (mAbs) directed against epithelial markers, epithelial cell adhesion molecule (EpCAM) and cytokeratin (data not shown).
Mouse fibroblastic L cells transfected with the human CD40L gene (CD40L+ L cells) was kindly provided by Dr Francine Brière (Schering Plough, Levallios-Perret Cedex, France), as well as CDw32/FcγRII transfected L cells used as a control (control L cells) [11].
All cell lines were cultured in 25 cm2 flasks (Cellstar®; Greiner Labortechnik, Frankfurt, Germany) in a humidified 5% CO2 incubator and RPMI-1640 medium plus 10% fetal calf serum (FCS) (Gibco, Life Technology, Eggenstein, Germany) supplemented by 100 µg/ml penicillin, 100 IU/ml streptomycin and 5 mMH HEPES, and defined as complete medium.
Flow cytometric detection of CD40 expression
Flow cytometry was used to assess the expression of cell surface markers on tumour cell lines, freshly isolated OVCA cells and L cells. Cells were stained with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated mAbs directed against CD40 (clone mAB89; Immunotech Marseille, France), anti-CD40L (clone B-B29; Diaclone, Besançon, France) or epithelial cell adhesion molecule (EpCAM, clone VU-1D9; StemCells, Grenoble, France). Direct cell surface staining was performed by incubating cells in tubes at 2·5–5 × 105 cells in 50 µl of phosphate-buffered saline (PBS) supplemented with 0·5% bovine serum albumin (BSA) with mAbs for 30 min at 4°C. In parallel, isotype-matched murine PE or FITC-conjugated immunoglobulins were used as negative controls. Data were acquired using a fluorescence activated cell sorter (FACS)Calibur cytometer (Becton Dickinson, Mountain View, CA, USA) and analysis was performed using CellQuest software.
[3H]-thymidine uptake proliferative assay
Human tumour cells (i.e. OVCA and MCF-7 cells) at confluence were trypsinized and resuspended in complete medium at 2·5–10 × 104 cells/ml. One hundred µl of the cell suspension were distributed onto 96-well flat-bottomed plates (Falcon®; Becton Dickinson, Franklin Lakes, NJ, USA). Triplicate samples were incubated with 100 µl of media alone or co-incubated with 100 µl of irradiated (75 Gy) CD40L+ or control L cells (1·25–5 × 104/ml) for 48 and 72 h. The final ratio of human tumour cells over L cells was 2:1. [3H]-thymidine (specific activity 25 Ci/mmol; Amersham, Les Ulis, France) was added at 1 µCi/well for the last 15 h of culture. Cells were harvested on a Filtermate 196 Harvester (Packard Instruments, Rungis, France) and incorporation of [3H]-thymidine was determined by liquid scintillation counting (TopCount, Packard Instruments). Results are expressed in counts per minute (cpm) [mean of triplicates ± standard deviation (s.d.)] and then translated into percentage of growth inhibition according to the formula:
Quantitative measurements of cytokine production
OVCA cells were cultured in complete medium at 7·5–20 × 104 cells/well in 12-well culture plates together with irradiated (75 Gy) CD40L+ or control L cells (7·5–20 × 104 cells/well) or media alone in a final volume of 1 ml. In some experiments, OVCA cells were incubated with 1 µg/ml soluble recombinant human CD40L (sCD40L; Alexis Biochemicals, Coger, France). After 72 h of culture, supernatants were collected, centrifuged and frozen at −80°C until analysis. IL-6 and IL-8 were assayed using a specific enzyme-linked immunosorbent assay (ELISA) (Diaclone) according to the manufacturer's instructions. A bead-based immunoassay kit, combined with flow cytometry [cytometric bead array (CBA); Becton Dickinson], was also used for quantification of IL-1β, IL-6, IL-8, IL-10 and TNF-α. Briefly, 50 µl of mixed bead populations coated with capture antibodies specific for each cytokine were mixed with 50 µl of test samples or recombinant standards and 50 µl of (PE)-conjugated detection antibodies, and maintained for 3 h at room temperature. Beads were washed with 1 ml of buffer by centrifugation for 5 min at 200 g. Supernatants were discarded carefully and beads were resuspended in 300 µl of buffer before FACS analysis. Calibration curves (0–5000 pg/ml) established with standard human recombinant cytokines were used for determining the concentration of each cytokine. Data were acquired with a FACSCalibur cytometer, analysed automatically with BD CBA analysis software and expressed in pg/ml.
Statistical analysis
Statistical analyses were performed using the nonparametric Mann–Whitney ranking test. Values of P < 0·05 were considered statistically significant.
Results
Expression of CD40 on tumour cells
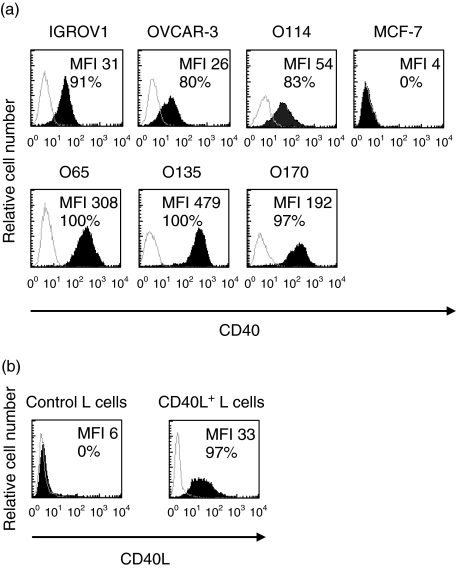
CD40 molecules were detected at the cell surface of all OVCA cell lines (n = 6) studied by flow cytometric analysis (Fig. 1a). The level of expression, as represented by median fluorescence intensity (MFI) conferred by fluorochrome-conjugated mAbs, was moderate for IGROV1, OVCAR3 and O114 cell lines (MFI ranging from 26 to 54) and high in three other cell lines, O65, O135 and O170 (ranging from 192 to 479). The breast carcinoma cell line MCF-7 did not express the CD40 molecule and was used as a control. Expression of the CD40 ligand on CD40L+ L cells was satisfactory and untransfected L cells (control L cells) did not express CD40 ligand (Fig. 1b).
Fig. 1.
Expression level of CD40 by ovarian carcinoma (OVCA) cell lines and CD40L by transfected L cells. Cell surface expression of CD40 on human tumour cells [i.e. OVCA and breast carcinoma cell line (MCF-7) cells] and CD40L on L cells were studied separately using anti-CD40 (a) or anti-CD40L monoclonal antibodies (mAbs) (b) (filled curve). Background staining is indicated (open curve). Results are expressed in histogram form as the number of cells by channel of fluorescence intensity. Median fluorescence intensity (MFI) is indicated in each histogram as well as the proportions of cells expressing the cell surface marker.
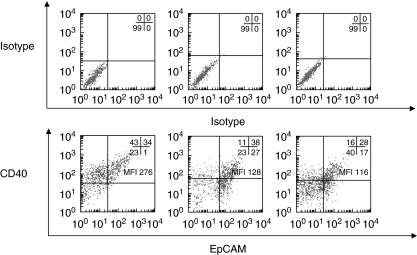
Expression of CD40 was also examined on OVCA cells dissociated freshly from three solid biopsies. Because single-cell suspension from tumour biopsies may contain mixed cell populations consisting of epithelial cells and stromal cells, the epithelial marker EpCAM was used to identify OVCA cells [13]. Double-staining revealed that a significant proportion of EpCAM-positive OVCA cells expressed the CD40 receptor with a range of 28–38% of double-positive (EpCAM/CD40) cells (Fig. 2). High levels of CD40 were detected with a MFI ranging from 116 to 226.
Fig. 2.
Expression of CD40 and epithelial cell adhesion molecule (EpCAM) by isolated ovarian carcinoma (OVCA) primary cells. Cryopreserved isolated OVCA primary cells from three patients (O113, O136 and O151) were thawed for analysis. Cell surface expression of CD40 and EpCAM on isolated OVCA primary cells was studied by double-staining using anti-CD40 and anti-EpCAM monoclonal antibodies (mAbs) (lower panel). Background staining is indicated (upper panel). Results are expressed in dot blot form. The proportions of cells expressing the cell surface marker in each quadrant are indicated as well as median fluorescence intensity (MFI) for the CD40 marker.
The effects of CD40 ligation on ovarian tumour cell growth
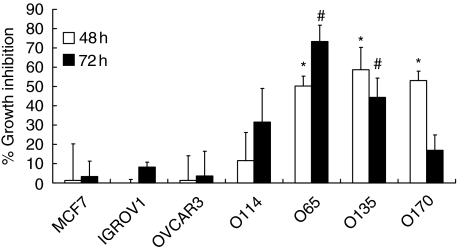
To examine CD40-mediated effects on growth of OVCA and MCF-7 cells, the proliferation of tumour cells co-incubated with irradiated CD40L+ was determined by [3H]-thymidine incorporation after 48 and 72 h of co-culture. Neither growth of CD40low cell lines IGROV1 and OVCAR3 nor the CD40-negative cell line MCF-7 was affected by L cells expressing CD40L+ (Fig. 3).
Fig. 3.
Effects of CD40 ligation on growth of tumour cells. Proliferation of human tumour cells [i.e. ovarian carcinoma (OVCA) and breast carcinoma cell line (MCF-7) cells] co-incubated with irradiated CD40L+ or control L cells was determined by [3H]-thymidine incorporation after 48 h and 72 h of co-culture. Data were expressed as percentage of growth inhibition as described in Materials and methods. Data are from three independent experiments (mean ± standard deviation). *Significantly different from MCF-7, OVCAR3, IGROV1 and O114 (P < 0·05); #significantly different from MCF-7, OVCAR3 and IGROV1 (P < 0·05).
In contrast, the ligation of CD40 resulted in a major decrease in growth for the CD40high cell lines O65, O135 and O170, which exhibited approximately 50% inhibition at 48 h. It was noted that the O170 cell line was insensitive to CD40L after 72 h of co-culture. Proliferation of the O114 cell line, which expresses a weak level of CD40, was slightly affected, with 30% inhibition at 72 h.
Cytokine production of OVCA cell lines after CD40 binding
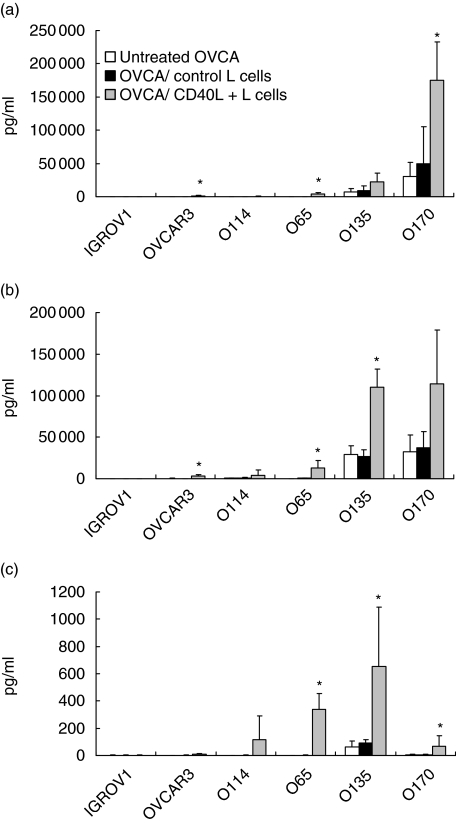
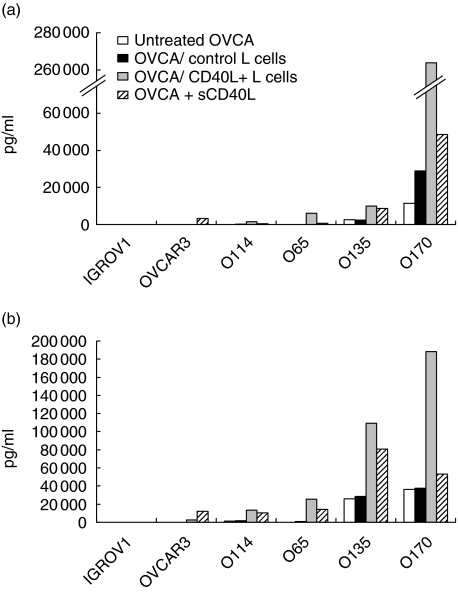
The effects of CD40 ligation on secretion of IL-6, IL-8 and TNF-α by the OVCA cell lines were studied after checking that transfected CD40L cells and control L cells did not produce any of these cytokines (data not shown). CD40L+ L cells up-regulated secretion of IL-6, IL-8 and TNF-α by CD40high OVCA cell lines (O65, O135 and O170). This effect was specific for CD40L, as control L cells had no effect (Fig. 4a,b,c). For the O135 and O170 cell lines, secretion increased twofold for IL-6 and three- to fourfold for IL-8. The constitutively low release of IL-6 and IL-8 by the O65 cell line (< 0·1 ng/ml) was highly increased by CD40 and reached 4 ng and 13 ng for IL-6 and IL-8, respectively. CD40 activation of the OVCAR-3 cell line weakly enhanced the release of IL-6 and IL-8. In addition, CD40L+ cells enhanced TNF-α production by the O135 cell line and induced de novo secretion by the O65 cell line (339 ± 117 pg/ml). TNF-α secretion was weakly increased in O170 cell lines. The secretion patterns of IGROV1 and O114 cell lines, which constitutively produced low or undetectable amounts of IL-6, IL-8 and TNF-α, was not modified by CD40L cells. CD40 engagement failed to induce IL-10 or IL-1β production in all cell lines studied (data not shown).
Fig. 4.
Effects of membrane-bound CD40L on cytokine production of ovarian carcinoma (OVCA) cells. OVCA cells were co-cultured with irradiated CD40L+ or control L cells, or incubated with medium alone in 12-well plates. After 72 h of culture, supernatants were collected and interleukin (IL)-6 (a), IL-8 (b) and tumour necrosis factor-α (c) were assayed by cytometric beads array or enzyme-linked immunosorbent assay. Concentrations are expressed in pg/ml. Data are from at least three independent experiments (mean ± standard deviation). *Significantly different from OVCA co-cultured with control L cells (P < 0·05).
Comparative experiments were carried out with a soluble human recombinant CD40L (sCD40L). In this condition, ligation of CD40 also led to the up-regulation of IL-6 and IL-8 secretion compared to basal levels (Fig. 5). The magnitude of sCD40L-mediated effects was less pronounced than with CD40L+ L cells. sCD40L failed to induce TNF-α secretion in any of the OVCA cell lines tested (data not shown).
Fig. 5.
Effects of soluble CD40L on cytokine production of ovarian carcinoma (OVCA) cells. OVCA cells were co-cultured with irradiated CD40L+ or control L cells, or incubated with either sCD40L (1 µg/ml) or medium alone in 12-well plates. After 72 h of culture, supernatants were collected and interleukin (IL)-6 (a) and IL-8 (b) were assayed by cytometric beads array. Concentrations are expressed as pg/ml.
Discussion
CD40 is a co-stimulatory molecule well known for its participation in the regulation of immune responses by enhancing the antigen-presenting function of APC [1]. Here, we focused on the CD40 molecule and its role in the biology of ovarian carcinoma. Our results show that CD40 is present at high levels on ovarian carcinoma (OVCA) cells dissociated freshly from surgical specimens, suggesting that CD40 may contribute to the pathogenesis of OVCA. Cytofluorometry analysis also revealed that this molecule was present at varying degrees on all OVCA cell lines tested: two established cell lines and four others generated in our laboratory from tumour biopsies. These results are consistent with recent data obtained by Gallagher et al., who additionally found CD40 expression on OVCA tumour tissue biopsies as assessed by immunohistochemistry [14]. We were able to demonstrate that CD40 on OVCA cells is functional, as CD40 ligation by the CD40L-transfected cell line delivered a signal which resulted in growth inhibition. Interestingly, sensitivity to the inhibitory signal delivered by CD40L was linked to the level of CD40 expression. The mechanisms underlying the growth inhibitory effects in epithelial cells are still unclear, but nuclear factor-kappa B (NF-kB) and c-jun N-terminal kinase (JNK) pathways may be involved [15]. The effects of CD40 stimulation on cell growth are variable and seem to depend upon tumour type. For example, engagement of CD40 receptor in breast carcinoma delivers a pro-apoptotic signal, while in nasopharyngeal and bladder carcinoma CD40 ligation results in increased survival [5,16,17].
Our results also show that stimulation by membrane-bound CD40L can enhance IL-6, IL-8 and TNF-α secretion in certain OVCA cell lines. Similarly, up-regulation of cytokine release was associated with a higher level of CD40 expression. In addition, treatment of OVCA cell lines with a soluble form of CD40L resulted in an increase of IL-6 and IL-8 production but did not affect TNF-α release. This finding suggests that membrane-bound CD40L is able to deliver a more sustained signal than soluble CD40L [14].
Distinct signalling pathways may be activated by CD40 ligation. This possibility is supported by our observations with the CD40low OVCAR3 cell line: the growth of this cell line was not inhibited following CD40 engagement, whereas secretion of IL-6 and IL-8 was enhanced.
The in vitro direct anti-tumour effect (i.e. growth inhibition) by CD40L supports the hypothesis that treatment targeting CD40 could be effective against CD40-positive tumours in vivo. Indeed, there is experimental evidence that growth of human breast carcinoma cell lines injected into severe combined immunodeficient (SCID) mice can be inhibited by recombinant CD40L treatment [16]. Conversely, it has been reported that CD40 activation can promote Kaposi's sarcoma growth in SCID mice by stimulating neoangiogenesis and tumour survival [18]. Data from the literature report that tumour development may represent the net balance between the effects of inhibitor and activator cytokines produced in the local tumour environment [19,20]. On this basis, our data showing that CD40 ligation up-regulates IL-6, IL-8 and TNF-α production are of major interest. Indeed, IL-6 may contribute to metastasic spread by improving the attachment and migration properties of OVCA cells, as was shown in vitro[21,22]. Moreover, IL-8/CXCL8 is a angiogenic factor that correlates with disease progression in OVCA [23]. Similarly, TNF-α has been described as a promoting-tumour factor inducing production of angiogenic factors and metalloprotease and favouring the neovascularization and tissue remodelling necessary for tumour progression [20]. Analysis of our findings must be cautious, as the same cytokine may produce opposing effects on tumour growth according to the context.
Taken together, the present data suggest that CD40–CD40L interaction may be one of the major pathways affecting growth regulation and cytokine production in ovarian carcinoma.
Acknowledgments
We are grateful to F. Brière for providing CD40L transfected L cells. We also thank C. Thomas de la Pintière for technical assistance. This work was supported by grants from l'Association Contre Le Cancer (ARC) and la Ligue Nationale Contre Le Cancer (comité d'Ille et Vilaine et du Morbihan).
References
- 1.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 2.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 3.Thomas WD, Smith MJ, Si Z, Hersey P. Expression of the co-stimulatory molecule CD40 on melanoma cells. Int J Cancer. 1996;68:795–801. doi: 10.1002/(SICI)1097-0215(19961211)68:6<795::AID-IJC18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Eliopoulos AG, Gallagher NJ, Dawson CW. CD40 and epithelial cells: across the great divide. Immunol Today. 1998;19:502–6. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 5.Sbih-Lammali F, Clausse B, Ardila-Osorio H, et al. Control of apoptosis in Epstein–Barr virus-positive nasopharyngeal carcinoma cells: opposite effects of CD95 and CD40 stimulation. Cancer Res. 1999;59:924–30. [PubMed] [Google Scholar]
- 6.Alexandroff AB, Jackson AM, Paterson T, et al. Role for CD40–CD40 ligand interactions in the immune response to solid tumours. Mol Immunol. 2000;37:515–26. doi: 10.1016/s0161-5890(00)00079-1. [DOI] [PubMed] [Google Scholar]
- 7.Byrom J, Davies Q. Cancer of the ovary. Curr Obstet Gynaecol. 2003;13:88–94. [Google Scholar]
- 8.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 9.Toutirais O, Chartier P, Dubois D, et al. Constitutive expression of TGF-beta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. Eur Cytokine Netw. 2003;14:246–55. [PubMed] [Google Scholar]
- 10.Naylor MS, Stamp GWH, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. J Clin Invest. 1993;91:2194–206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassard L, Cohen-Solal JFG, Galinha A, et al. Modulation of tumor growth by inhibitory Fc{gamma} receptor expressed by human melanoma cells. J Clin Invest. 2002;110:1549–57. doi: 10.1172/JCI15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong A, Eck SL. EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2:320–6. doi: 10.4161/cbt.2.4.451. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher NJ, Eliopoulos AG, Agathangelo A, Oates J, Crocker J, Young LS. CD40 activation in epithelial ovarian carcinoma cells modulates growth, apoptosis, and cytokine secretion. Mol Pathol. 2002;55:110–20. doi: 10.1136/mp.55.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian Y, Zhao Z, Jiang Z, Li X. Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc Natl Acad Sci USA. 2002;99:9386–91. doi: 10.1073/pnas.142294499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 17.Jakobson E, Jonsson G, Bjorck P, Paulie S. Stimulation of CD40 in human bladder carcinoma cells inhibits anti-Fas/APO-1 (CD95)-induced apoptosis. Int J Cancer. 1998;77:849–53. doi: 10.1002/(sici)1097-0215(19980911)77:6<849::aid-ijc9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Biancone L, Cantaluppi V, Boccellino M, et al. Activation of CD40 favors the growth and vascularization of Kaposi's sarcoma. J Immunol. 1999;163:6201–8. [PubMed] [Google Scholar]
- 19.Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123:743–50. doi: 10.1530/rep.0.1230743. [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obata NH, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res. 1997;17:337–42. [PubMed] [Google Scholar]
- 23.Herrera CA, Xu L, Bucana CD, et al. Expression of metastasis-related genes in human epithelial ovarian tumors. Int J Oncol. 2002;20:5–13. [PubMed] [Google Scholar]