Abstract
Specific anti-polysaccharide antibody deficiency (SPAD) is an immune disorder. Diagnostic criteria have not yet been defined clearly. One hundred and seventy-six children evaluated for recurrent respiratory tract infections were analysed retrospectively. For each subject, specific anti-pneumococcal antibodies had been measured with two enzyme-linked immunosorbent assays (ELISAs), one overall assay (OA) using the 23-valent pneumococcal polysaccharide vaccine (23-PPSV) as detecting antigen and the other purified pneumococcal polysaccharide serotypes (serotype-specific assay, SSA) (serotypes 14, 19F and 23F). Antibody levels were measured before (n = 176) and after (n = 93) immunization with the 23-PPSV. Before immunization, low titres were found for 138 of 176 patients (78%) with OA, compared to 20 of 176 patients (11%) with the SSA. We found a significant correlation between OA and SSA results. After immunization, 88% (71 of 81) of the patients considered as responders in the OA test were also responders in the SSA; 93% (71 of 76) of the patients classified as responders according to the SSA were also responders in the OA. SPAD was diagnosed in 8% (seven of 93) of patients on the basis of the absence of response in both tests. Thus, we propose to use OA as a screening test for SPAD before 23-PPSV immunization. After immunization, SSA should be used only in case of a low response in OA. Only the absence of or a very low antibody response detected by both tests should be used as a diagnostic criterion for SPAD.
Keywords: anti-pneumococcal antibody, anti-polysaccharide antibody, pneumococcal polysaccharide vaccine, respiratory tract infection
Introduction
Specific anti-polysaccharide antibody deficiency (SPAD) is a well-reported immune disorder characterized by an abnormal response to polysaccharide antigens [1–5]. The condition is suspected in any child above 2 years of age who suffers from recurrent respiratory tract infections or in patients with unusually severe complications from infections under appropriate treatment (mastoiditis, empyema, etc.). Despite numerous studies, precise laboratory diagnostic criteria of SPAD are still unclear. Diagnosis is made in patients with normal B cell numbers and normal serum immunoglobulin (Ig) G levels who have an absent or diminished antibody response to immunization with the 23-valent pneumococcal polysaccharide vaccine (23-PPSV). Anti-pneumococcal antibodies can be determined by two different enzyme-linked immunosorbent assays (ELISAs). The serotype-specific assay (SSA) is a standardized tool that measures the concentration of IgG directed against specific pneumococcal serotypes [5,6]. Results are expressed as µg/ml. The overall assay (OA) is a non-standardized method of evaluation for antibodies recognizing the 23 serotypes included in the 23-PPSV. The method is considered as a screening test and results are expressed in arbitrary units (AU) [7].
The purpose of this study was to compare the value of these two assays for the evaluation of the ability of children with recurrent respiratory tract infections to produce anti-pneumococcal antibodies before and after immunization with 23-PPSV. Clinical and immunological data were analysed and correlated with anti-pneumococcal antibody concentration.
Materials and methods
Patients
The files of all children aged 17 months−18 years evaluated for recurrent upper or lower respiratory tract infections and/or pneumonia with empyema in three Belgian University Hospitals (Université Catholique de Louvain, Cliniques Universitaires de Mont-Godinne, Yvoir; Université Libre de Bruxelles, Hôpital Erasme, Brussels and Vrije Universiteit Brussel, Academische Ziekenhuis, Brussels) between January 2003 and June 2005 were analysed retrospectively. The decision to determine anti-pneumococcal antibody concentration as well as the evaluation of the immune response to the polysaccharide vaccine was made by the attending physician as part of the evaluation of humoral immunity. No informed consent was obtained. The indication of the test was discussed previously between all the investigators of the study to avoid unnecessary analysis. Patients should have at least one of the medical conditions described for the test to be ordered. Patients were classified into five different clinical categories: recurrent otitis media (≥ 3 episodes within 6 months or ≥ 4 episodes within 12 months), recurrent upper respiratory tract infections treated with antibiotics (≥ 4 or more episodes within 12 months), more than two episodes of sinusitis within 12 months defined by clinical history and abnormal X-ray, more than two X-rays indicating pneumonia or ≥ 1 complicated pneumonia. Patients with more than one relevant criterion were included in each corresponding category. Ear, nose and throat (ENT) interventions (adenoidectomy and ear tube placement) were recorded. Patients were included if the files contained complete medical history, they had normal age-specific serum IgG levels, and no previous immunization with any pneumococcal vaccine. One hundred and seventy-six patients fulfilled these criteria. Twenty-one patients were aged 18–24 months, 96 were aged 2–5, 47 were aged 6–10 years and 12 were aged over 10 years.
Serum IgA, IgG2, and IgG3 subclass levels were recorded in 126 patients, among whom 75 were vaccinated with the 23-PPSV. IgA and IgG subclass deficiency was diagnosed if IgA or one or two IgG subclass levels was 2 standard deviations (s.d.) below the geometric mean of the age-adjusted mean [5]. True IgA deficiency was defined as a serum IgA level < 0·7 mg/l [5].
Methods
Sera were pre-incubated with 50 µg of free Pneumococcus cell wall polysaccharide (Statens Serum Institute, Copenhagen, Denmark) and anti-pneumococcal IgG were then determined with two different assays.
Overall assay
Total IgG antibodies to the 23 vaccine serotypes were detected by ELISA, as described previously [8]. Briefly, polystyrene microtitre plates were coated overnight with 10 µg/ml of 23-valent pneumococcal vaccine diluted in phosphate-buffered saline (PBS). After washing and blocking the plates (PBS + 2% bovine serum albumin) diluted sera were incubated for 2 h at room temperature. After again washing the plates, peroxidase-labelled anti-human IgG (Dako, Glostrup, Denmark) was added at a 1/5000 dilution and incubated for 2 h at room temperature. The tetramethyl-benzidine substrate was finally added and the absorbance at 450 nm measured. They were converted to antigen-specific arbitrary units by reference to calibration curves based on serial dilution of a reference pooled serum. This pool was constituted by mixing 25 sera with high antibody titres. A level < 11 arbitrary units (AU)/ml was considered as a low antibody concentration.
Serotype-specific assay
The concentration of IgG directed against three different capsular polysaccharides of Streptococcus pneumoniae were measured by ELISA [9]. The serotypes 14, 19F and 23F were chosen because they are frequent in Belgium, but also because of their different immunogenicities, serotype 14 being highly immunogenic, serotype 23F poorly immunogenic and serotype 19F having intermediate immunogenicity [10–12]. An international reference serum (89-S, FDA) was used to generate standard curves. In order to determine a threshold level, serotype-specific IgG were determined with SSA in a control population of 100 healthy infants (6–12 months of age) not vaccinated against S. pneumoniae. A threshold level was defined as the upper 95% of their distribution. Those were 0·7 µg/ml for serotypes 14 and 19F and 0·4 µg/ml for serotype 23F, respectively. We considered a specific serotype IgG concentration as low when it was inferior to those values.
Criteria for a normal vaccine response
According to the decision of the attending physician, a subgroup of 93 patients with total anti-pneumococcal IgG concentration < 11 AU/ml was immunized with the 23–PPSV. The antibody response was determined 4–6 weeks later. The antibody response in the OA was considered normal when a twofold increase in the antibody level was observed and when the post-vaccination level was ≥ 11 AU/ml. We considered a response normal for the SSA when there was at least a twofold increase in the specific antibody concentration and when the post-vaccination level reached the threshold value for at least one pneumococcal serotype.
We defined four subpopulations of patients based on their IgG response to 23-PPSV; group I: responders in both assays, group II: responders in the OA only, group III: responders in the SSA only, and group IV: with no response in any assay.
Six children with a low response to 23-PPSV were then vaccinated with heptavalent pneumococcal conjugate vaccine (Prevenar®-PCV-7).
Statistical analysis
Antibody concentrations were presented as geometric means and geometric s.d. or ranges and were compared between groups by Wilcoxon's rank sum test. The χ2 test was used for comparing categorical variables and Spearman's rank coefficient to assess correlation between numerical or ordinal variables. All tests were two-tailed and were performed using SPSS statistical software (SPSS Inc., Chicago, IL, USA), with P-values < 0·05/26 = 0·002 considered significant, according to Bonferroni criteria (26 is the number of tests performed).
Results
Pre-immunization anti-pneumococcal antibody concentration
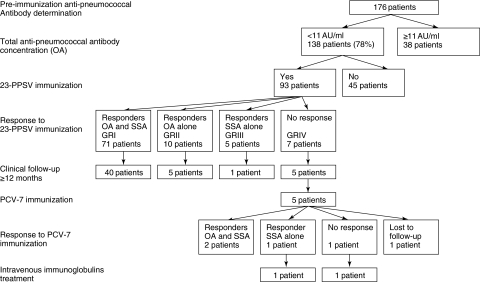
Pre-immunization anti-pneumococcal antibody concentration was measured in 176 patients (median age: 48 months) (Fig. 1). The mean anti-pneumococcal antibody levels were correlated positively to the patient's age (Table 1). The threshold value was reached more frequently for serotypes 14 and 19F [102 of 176 (58%) and 125 of 176 (71%), respectively] than for serotype 23F [83 of 176 (47%) patients].
Fig. 1.
Patient flowchart according to anti-pneumococcal antibody determination and response to immunization.
Table 1.
Pre-immunization anti-pneumococcal antibody concentrations in different age groups according to the two assays.
| Age groups | |||||
|---|---|---|---|---|---|
| < 2 years | 2–5 years | 6–10 years | > 10 years | ||
| n = 21 | n = 96 | n = 47 | n = 12 | Correlation | |
| Serotype 14 | 0·63* | 0·94 | 1·84 | 1·62 | r = 0·305 |
| 0·12–11·92 | 0·04–63·67 | 0·17–27·17 | 0·11–38·30 | P < 0·001 | |
| Serotype 19F | 0·64 | 1·33 | 1·47 | 2·92 | r = 0·185 |
| 0·12–7·06 | 0·09–13·73 | 0·16–71·3 | 0·32–38·4 | P = 0·014 | |
| Serotype 23F | 0·24 | 0·42 | 0·63 | 0·96 | r = 0·264 |
| 0·04–2·60 | 0·04–9·48 | 0·09–22·04 | 0·04–25 | P < 0·001 | |
| Overall assay | 3 | 5 | 9 | 12 | r = 0·504 |
| < 3–9 | < 3–120 | < 3–95 | 3–62 | P < 0·001 | |
Pre-immunization anti-pneumococcal antibody concentrations were correlated significantly with the patient's ages for all serotypes and for the overall assay.
Values are geometric means and ranges in µg/ml for each serotype for the serotype-specific assay (SSA) and in AU/ml for the overall assay (OA).
Response assayed by OA was low (< 11 AU/ml) in 138 patients (78%), but only 20 patients (11%) had serotype-specific antibodies below the threshold for the three serotypes. The SSA test was more sensitive than the OA test in detecting low levels of anti-pneumococcal antibodies. The mean serotype-specific antibody concentrations were significantly lower in sera from patients with low OA results than in those with high OA results (Table 2). Analysis also showed that 37 of 38 (97%) and 32 of 38 (84%) of the sera with total IgG to Pneumococcus above 11 AU/ml had at least one or two serotype-specific antibody levels equal or above the threshold value. Except for age distribution, there was no significant difference from a clinical viewpoint between patients with low or high total anti-pneumococcal antibodies (Table 2).
Table 2.
Comparison of serotype specific anti-pneumococcal antibody concentration, clinical and immunological data according to pre-immunization total anti-pneumococcal antibody concentration.
| Pre-immunization total anti-pneumococcal antibody concentration | |||
|---|---|---|---|
| < 11 AU/ml | ≥ 11 AU/ml | P-value | |
| Serotype concentrations | |||
| Serotype 14 | 0·87 (4) | 2·78 (4) | < 0·001 |
| Serotype 19F | 1·06 (3) | 2·99 (3) | < 0·001 |
| Serotype 23F | 0·39 (3) | 0·86 (3) | 0·001 |
| Clinical categories | |||
| Number of patients | 138 | 38 | |
| Median age, months (range) | 43 (17–204) | 66 (25–173) | < 0·001 |
| Otitis media | 83 (60) | 15 (39) | n.s. |
| Sinusitis | 16 (12) | 10 (26) | n.s. |
| URTI | 83 (60) | 17 (45) | n.s. |
| Pneumonia | 47 (34) | 9 (24) | n.s. |
| Ear tube placement | 44 (32) | 7 (18) | n.s. |
| Adenoidectomy | 57 (41) | 11 (29) | n.s. |
| Immunological categories | |||
| Number of patient | 97 | 29 | |
| IgA deficiency | 20 (21) | 7 (24) | n.s. |
| Ig 2 deficiency | 2 (2) | 1 (3) | n.s. |
| IgG3 deficiency | 10 (10) | 6 (21) | n.s. |
| IgA and/or IgG subclass deficiency | 27 (28) | 12 (41) | n.s. |
Specific serotype antibody concentrations are expressed as geometric means in µg/ml and standard deviation in brackets. Others values are expressed as numbers of patients and percentages in brackets. URTI = upper respiratory tract infection; n.s. = non-significant.
Response to the 23-PPSV immunization
Ninety-three patients (median age 53 months) with pre-immunization IgG levels < 11 AU/ml were immunized with 23-PPSV (Fig. 1). Eighteen patients were aged 18–24 months, 53 were aged 2–5 years, 19 were aged 6–10 years and three were aged over 10 years.
Analysis of the antibody responses 1 month after vaccine administration indicated that 81 of 93 (87%) and 76 of 93 (82%) of the children produced anti-pneumococcal antibodies detected by the OA and by SSA, respectively. There was no significant correlation between age group and proportion of responders in either of the two assays. There was a trend towards a lower proportion of patients with pre-immunization serotype-specific antibody concentration below the threshold who had a normal response after vaccination for serotype 23F (23 of 53 = 43%) compared to serotype 14 (29 of 46 = 63%) and serotype 19F (23 of 35 = 66%) (P ≤ 0·05) without reaching a formal statistically significant level. A normal response was detected for 71 of 93 patients (76%) by both assays (group I; median age: 43 months), for 10 of 93 patients (11%) by OA only (group II; median age: 36 months) and for five of 93 patients (5%) by SSA only (group III; median age: 27 months). An absence of response to the vaccine was detected in seven of 93 (8%) of the patients, regardless of the assay used (group IV; median age: 35 months). Post-immunization total IgG levels were significantly higher in the sera from children who had responded to at least one serotype (groups I and III) than in the sera from those who did not respond to any serotype (groups II and IV) (geometric means 42·3 AU/ml versus 15·7 AU/ml; P < 0·001). Post-immunization serotype-specific IgG concentrations were significantly higher in sera from children who showed a positive response in the OA (groups I and II) than in sera from children who showed no response in the OA (groups III and IV) (Table 3).
Table 3.
Post-immunization serotype-specific antibody concentrations: comparison between responders and non responders according to the OA results.
| Responders* (groups I and II; n = 81) | Non-responders (groups III and IV; n = 12) | P-value | |
|---|---|---|---|
| Serotype 14 | 3·40 (4) | 0·95 (3) | 0·006 |
| Serotype 19F | 2·89 (4) | 0·72 (2) | < 0·001 |
| Serotype 23F | 0·94 (4) | 0·20 (2) | 0·001 |
Results are expressed as geometric means in µg/ml and standard deviation in brackets.
Responders = ratio ≥2 and post-immunization level ≥11 AU/ml.
Immunological data
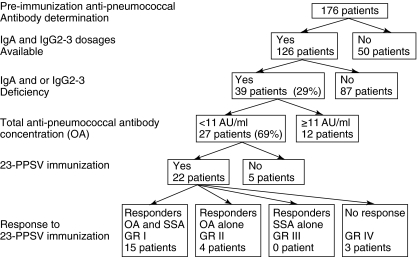
All patients had normal total serum IgG. IgA, IgG2 and IgG3 dosages were available, respectively, for 171, 155 and 127 patients. Both IgA and IgG subclasses (IgG2 and IgG3) dosages were available for 126 patients, among whom 75 were vaccinated (Fig. 2), 27 (21%) had an IgA deficiency, three (2%) had an IgG2 deficiency and 16 (13%) had an IgG3 deficiency (Table 2). Of the 27 patients with IgA deficiency, only one had a true IgA deficiency, five had an IgA and IgG3 deficiency and one had an IgA deficiency combined with an IgG2 and IgG3 deficiency. One patient had combined IgG2 and IgG3 deficiency. Overall, an IgA and/or IgG subclass deficiency was found in 39 of 126 patients (29%). No difference in the proportion of patients with low pre-immunization total anti-pneumococcal antibodies was observed between patients with or without an IgA and/or IgG subclass deficiency. Twenty-two of the 75 patients vaccinated (29%) had an IgA and/or IgG subclass deficiency; three belonged to group IV and 19 patients belonged to groups I and II (Fig. 2).
Fig. 2.
Patient flowchart according to immunological data.
Clinical follow-up of the vaccinated children
The files of 51 of the 93 vaccinated patients (group I: 40 patients, group II: five patients, group III: one patient and group IV: five patients) were analysed up to 12 months after immunization with the 23-PPSV (Fig. 1).
For the other 42 patients, sufficient follow-up data were not available. None of the 40 patients from group I and none, except one patient from group II, required substitutive immunoglobulins or immunization with the conjugated vaccine PCV-7, indicating that these children did not have any immune deficiency. The only child from group II finally requiring substitutive immunotherapy was a 7-year-old boy suffering from recurrent pneumonia and sinusitis. IgG3 deficiency was associated with the absence of antibody response to the three individual pneumococcal serotypes. Despite a normal antibody response recorded after the administration of PCV-7, recurrent sinusitis persisted and intravenous immunoglobulins had to be administered for 1 year. This treatment resulted in clinical improvement and there was no recurrence of infection; no decrease in immunoglobulin levels was observed after the infusion was stopped, so that the child could probably be classified as suffering from a transient SPAD. The only patient included in group III available for follow-up had a good clinical evolution, thus invalidating the diagnosis of SPAD. Five patients from group IV, and therefore classified as SPAD, were followed. They were immunized with PCV-7 and their antibody response was evaluated 4 weeks after immunization (Fig. 1). One patient had a normal antibody response to the three serotypes and another was lost to follow-up after this second vaccination. The third patient responded to both assays and improved significantly from a clinical viewpoint, as no recurrent infections were recorded. The last two SPAD patients required intravenous immunoglobulin treatment. The first was a 9-year-old boy with a family history of IgA deficiency (< 0·7 mg/l). He suffered from recurrent upper and lower respiratory tract infections, of which one was pneumococcal pneumonia with empyema. The absence of anti-PSS antibodies was associated with an IgA deficiency. Anti-pneumococcal antibodies were detected only after immunization with PCV-7, and response was limited to serotypes 14 and 19F without any increase of the OA response. In spite of this antibody response, no clinical improvement was noted and intravenous immunoglobulins were administered for 1 year, leading to clinical improvement. Three months after the discontinuation of immunoglobulin therapy he developed bacterial pneumonia associated with decreased IgG, IgG subclasses, IgA and IgM levels, suggesting a more severe immunodeficiency. This child could be considered to have suffered from SPAD prior to the development of a common variable immnunodeficiency. The second patient from group IV requiring immunoglobulin therapy was a 17-year-old girl with recurrent otitis media and therapy-refractory recurrent rhinosinusitis with S. pneumoniae isolates. No increase in antibody levels was noted after immunization with the PCV-7. In addition to the absence of anti-pneumococcal antibodies she had low IgA, IgM and IgG4 concentrations, but her antibody response to protein antigens was maintained. She was treated successfully with intravenous immunoglobulins during 1 year. Three months after the treatment was stopped, both the total and serotype specific anti-pneumococcal antibody dropped. IgA and IgM levels were persistently low, but there was no recurrence of infection. The decision to start the treatment again will depend upon the recurrence of infection.
Discussion
SPAD, reported more often as ‘antibody deficiency with normal immunoglobulin concentrations’, is recognized as a form of primary immunodeficiency disease [1–5]. The characteristic defect is a failure to mount an antibody response to polysaccharide antigens. While most subjects with a diagnosis of SPAD are free of symptoms, some may suffer from recurrent sinusitis and/or pulmonary infections [1,2,11]. The defect may be isolated or associated with other immune deficiency syndromes. Sometimes it might be the first immune defect identified in patients who will suffer further from common variable immunodeficiency [13]. This defect might be transient or persistent, and in some patients it may be overcome by the administration of pneumococcal conjugate vaccine. The 23-PPSV is often used as a prototype to evaluate the antibody response to polysaccharides. Indeed, the antibody titres may be measured easily in serum, both before and after the immunization, and only the absence of antibody response after the immunization defines SPAD [1–3,5].
Two ELISAs are available to measure anti-pneumococcal antibodies: SSA and OA. For both tests, the specificity is increased by removing antibodies to pneumococcal cell wall polysaccharides by pre-incubation with C-polysaccharides. It can be further increased by performing a second absorption of the sera with an irrelevant pneumococcal capsular polysaccharide, namely with serotype 22F, to remove antibodies to some common epitopes of the pneumococcal capsules [7,14].
SSA has been developed to evaluate the immunogenicity of new pneumococcal conjugate vaccines [6]. A consensus methodology is available, as well as international standards. Numerous attempts have been made to assign criteria for a normal response and a protective IgG level that should be obtained after a pneumococcal vaccination. Based on conjugate pneumococcal vaccine efficacy studies, WHO has proposed a protective threshold of 0·35 µg/ml for each serotype using the serotype assay with C-polysaccharide alone as adsorbent or 0·2 µg/ml if using both C-polysaccharide and serotype 22F as adsorbents [6]. However, when the serotype assay is used to assess the ability of an individual to mount an antibody response against polysaccharide antigens, the number of pneumococcal serotypes to which he or she should be able to respond after the administration of the 23 PPSV is not defined clearly, nor is the antibody level that should be reached. The antibody response may vary according to the immunogenicity of each serotype, so that choosing the adequate serotypes to be tested is somewhat delicate. Serotype 3 is not generally used in these tests, as it is so strongly immunogenic that its antibody levels and responses are not considered as representative of the ability to produce anti-pneumococcal antibodies. In contrast, serotype 23F is known to be weakly immunogenic [11,12], as confirmed in this study, and although anti-23F antibody determination might be interesting to measure or detect minor defects, very few data on this subject are available in the literature. It is possible, but not certain, that the lack of response to some serotypes is not associated as tightly with recurrent infections as the lack of response to others. OA is considered as a simple screening test for the distinction between normal or abnormal antibody response after immunization with 23-PPSV [7]. Unfortunately, as this test is not standardized, normal response criteria vary from one laboratory to another and no protective thresholds are defined.
In this study, we have evaluated the humoral immunity of children with recurrent respiratory tract infections and we have compared the capacity of OA and SSA tests to evaluate the antibody responses of children to polysaccharide antigens. An IgA and/or IgG subclass deficiency was found in 29% of the children suffering from recurrent respiratory tract infections, which is in agreement with rates between 25% and 65% reported in the literature [15,16]. Similarly, as in our study, SPAD has been reported to be in some cases associated with another humoral deficiency, such as IgA, Ig G2 or IgG3 deficiency [16].
With both assays, we found an association between the patient's age and pre-immunization anti-pneumococcal antibody titres reflecting natural colonization and/or infections, but age had no effect on the response to vaccination, even in patients below 24 months of age. Other studies have demonstrated that serotype-specific anti-pneumococcal antibody levels declined during the first 6 months of life due to the loss of maternal antibodies and then increased, particularly between 12 and 18 months, in relation to a high pneumococcal carriage rate [17,18].
SPAD was diagnosed in 7% of the children included in this study, confirming studies performed by other groups [1–3,16]. SPAD diagnosis was based here on an absence of pneumococcal antibody response detected both by the OA test and by the SSA test using three different serotypes. The use of three different serotypes to detect the antibody response and the criteria used in this study for the definition of SPAD appear to be satisfactory, as the clinical follow-up of the children included has demonstrated a favourable clinical evolution in all except one of the children classified as having no SPAD.
Comparisons between the two ELISA methods, including pre-absorption with cell wall polysaccharide to evaluate the anti-polysaccharide antibody responses, have been reported only occasionally in paediatric patients. Using OA, a good antibody response to PCV-7 was observed in only one of five children with SPAD [19]. Five out five showed a fourfold increase to at least four of seven serotypes in the SSA [19]. More recently, a study has compared total anti-pneumococcal IgG, IgG1 and IgG2 antibody titres and serotype-specific antibody concentrations in children with a history of recurrent infection who had received two doses of PCV-7, followed by one dose of 23-PPSV [20]. Results suggested that OA is a useful and simple screening test to evaluate the response to polysaccharide antigens and that serotype-specific IgG determination provided additional information with regard to protection against specific pneumococcal strains. As SSA is more expensive and time-consuming than OA, we propose the sequential use of these tests.
We suggest using the OA to screen patients requiring a detailed humoral immune investigation, including response to polysaccharide antigens. We showed a good correlation between the total anti-pneumococcal antibody concentration and serotype-specific antibody concentrations and propose to test antibody responses to 23-PPSV with the OA in patients with low pre-immunization total anti-pneumococcocal antibody levels. SSA should be performed only if no significant antibody response is detected with the OA test after vaccination. Only the absence of, or very low, response when using all the tests may be used as diagnostic criteria for SPAD. Further studies are needed to validate this proposed protocol in children vaccinated previously with the PCV-7. Uddin et al. suggested that an ELISA detecting IgG1 antibodies to 23-PPSV remains a useful screening test to detect children with abnormal humoral immunity, even after previous immunization with PCV-7 [20].
We conclude that the sequential determination of anti-polysaccharide antibody levels is a time- and cost-saving practice to select the few children with SPAD among those presenting with recurrent respiratory tract infections. It appears that low pre-immunization anti-pneumococcal antibody concentrations are related most often to the young patient's age, and not to an inability to produce anti-polysaccharide antibodies. Indeed, the majority of our patients with recurrent respiratory tract infections associated with a low pre-immunization anti-pneumococcal antibody levels developed an adequate antibody response after the administration of the 23-valent vaccine.
Acknowledgments
We thank Dr Anne Malfroot and Dr Iris De Schutter for providing clinical data, Alain Crusiaux and Christine Servais for performing the ELISA assays and C. Farber for critical reading of the manuscript.
References
- 1.Rijkers GT, Sanders LA, Zegers BJ. Anti-capsular polysaccharide antibody deficiency states. Immunodefiency. 1993;5:1–21. [PubMed] [Google Scholar]
- 2.Sorensen RU, Moore C. Antibody deficiency syndromes. Pediatr Clin North Am. 2000;47:1225–52. doi: 10.1016/s0031-3955(05)70269-8. [DOI] [PubMed] [Google Scholar]
- 3.Wassaerman RL, Sorensen RU. Evaluating children with respiratory tract infections: the role of immunisation with bacterial polysaccharide vaccine. Pediatr Infect Dis J. 1999;18:157–63. doi: 10.1097/00006454-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Notarangelo L, Casanova JL, Conley MH, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee meeting in Budapest, 2005. J Allergy Clin Immunol. 2006;117:883–96. doi: 10.1016/j.jaci.2005.12.1347. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla FA, Bernstein IL, Khan DA et al. for the American Academy of Allergy Asthma and Immunology, American College of Allergy, Asthma, Immunology, Joint Council of Allergy Asthma and Immunology. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl. 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Pneumococcal conjugate vaccines. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Tech Rep Ser. 2005;927:64–98. Annex 2. [Google Scholar]
- 7.Balmer P, Cant AJ, Borrow R. Anti-pneumococcal antibody titre measurement-what useful information does it yield? J Clin Pathol. 2007;60:345–50. doi: 10.1136/jcp.2006.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascart-Lemone F, Gerard M, Libin M, et al. Differential effect of human immunodeficiency virus infection on the Ig A and IgG antibody responses to pneumococcal vaccine. J Infect Dis. 1995;172:1253–60. doi: 10.1093/infdis/172.5.1253. [DOI] [PubMed] [Google Scholar]
- 9.Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–19. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergison A, Tuerlinckx D, Verhaegen J, Malfroot A. Invasive pneumococcal disease epidemiology in Belgian children: passive surveillance is not enough. Pediatrics. 2006;118:e801–9. doi: 10.1542/peds.2005-3195. [DOI] [PubMed] [Google Scholar]
- 11.Akikusa JD, Kemp AS. Clinical correlates of response to pneumococcal immunization. J Paediatr Child Health. 2001;37:382–7. doi: 10.1046/j.1440-1754.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhooge IJ, van Kempen MJP, Sanders LAM, Rijkers GT. Deficient Ig A and Ig G2 anti-pneumococcal antibody levels and response to vaccination in otitis prone children. Int J Pediatr Otorhinolaryngol. 2002;17:133–41. doi: 10.1016/s0165-5876(02)00068-x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Herz W, McGeady SJ. Antibody response in common variable immunodeficiency. Ann Allergy Asthma Immunol. 2003;90:244–7. doi: 10.1016/S1081-1206(10)62149-7. [DOI] [PubMed] [Google Scholar]
- 14.Musher DM, Luchi MJ, Watson DA, Hamilton R, Baughn RE. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG response by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–35. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 15.Finocchi A, Angelini F, Chini L, et al. Evaluation of the relevance of humoral immunodeficiencies in a pediatric population affected by recurrent infections. Pediatr Allergy Immunol. 2002;23:443–7. doi: 10.1034/j.1399-3038.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 16.Bossuyt X, Moens L, Van Hoeyveld E, et al. Coexistence of (partial) immune defects and risk of recurrent respiratory infections. Clin Chem. 2007;53:124–30. doi: 10.1373/clinchem.2007.075861. [DOI] [PubMed] [Google Scholar]
- 17.Soininen A, Pursiainen H, Kilpi T, Käyhty H. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J Infect Dis. 2001;184:569–76. doi: 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- 18.Brüssow H, Baensch M, Sidoti J. Seroprevalence of immunoglobulin M (IgM) and IgG antibodies to polysaccharides of Streptococcus pneumoniae in different age groups of Ecuadorian and German children. J Clin Microbiol. 1992;30:2765–71. doi: 10.1128/jcm.30.11.2765-2771.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakshman R, Gennery AR, Arkwright PD, et al. Assessing immune responses to pneumococcal vaccines. Arch Dis Child. 2003;88:648–9. doi: 10.1136/adc.88.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uddin S, Borrow R, Haeney MR, et al. Total and serotype-specific pneumococcal antibody titres in children with normal and abnormal humoral immunity. Vaccine. 2006;24:5637–44. doi: 10.1016/j.vaccine.2006.03.088. [DOI] [PubMed] [Google Scholar]