Abstract
Post-traumatic stress disorder (PTSD) is an anxiety disorder that can occur after exposure to extreme traumatic experience such as war trauma, and is accompanied by fear, helplessness or horror. Exposure to trauma can result in immune dysregulation and influence susceptibility to infectious disease as well as vaccine efficacy. The aim of the study was to determine the relation of psychological stress and the immune response to influenza vaccination in combat-related PTSD patients (n = 28). Detection of anti-viral antibody titre was performed by inhibition of haemagglutination assay. Ex vivo tetramer staining of CD8+ T lymphocytes was used to monitor T cells specific for human leucocyte antigen (HLA)-A*0201-restricted influenza A haemagglutinin antigens before and after vaccination. Twenty patients showed a fourfold antibody titre increase to one or both influenza A viral strains, and 18 of them showed the same response for both influenza B viral strains. Ten of 15 healthy controls showed a fourfold rise in antibody titre to both influenza A viral strains and eight of them showed the same response for both influenza B viral strains. HLA-A*0201+ PTSD patients (n = 10) showed a significant increase of influenza-specific CD8 T cells after vaccination. Although those PTSD patients had a lower number of influenza-specific CD8+ T cells before vaccination compared to HLA-A*0201+ healthy controls (n = 6), there was no difference in influenza A antibody titre between PTSD patients and control subjects before vaccination. The generated humoral and cellular immune response in PTSD patients argues against the hypothesis that combat-related PTSD in war veterans might affect protection following influenza vaccination.
Keywords: cellular response, cytotoxic T cells, influenza vaccine, post-traumatic stress disorder
Introduction
Stress is known to affect immune function and to influence susceptibility to infectious disease [1,2]. Previous studies have reported that high levels of stress produce and maintain fewer protective antibodies against pathogens such as influenza, hepatitis B and pneumococcus [3–7]. Post-traumatic stress disorder (PTSD), according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), is defined as an anxiety disorder that can occur after exposure to extreme traumatic experience such as war trauma, and is accompanied by intense fear, helplessness or horror [8]. Those exposed to war trauma report a higher incidence of illness [9]. The published data now support the hypothesis that some of the biological dysfunctions can result from immune alterations associated with PTSD [10]. However, it is unknown whether combat-related PTSD may diminish vaccination efficacy and increase vulnerability to pathogens that give rise to infections.
The recent war in Croatia and Bosnia–Herzegovina affected not only soldiers, but also the general population. We previously studied immune reactivity in civilians (displaced people, refugees, detainees) and soldiers with PTSD (professional and enrolled) during or shortly after the war (reviewed in [11]). In general, fewer changes in immune and hormonal parameters were found in professional soldiers than in civilians or enrolled soldiers [12–14].
Influenza A viruses cause annual epidemics of acute respiratory infection which often result in significant morbidity and mortality in the human population. Since 1889 at least five pandemics have occurred, with Spanish influenza causing more than 20 million deaths worldwide in 1918 [15]. The possible threat for future influenza pandemics, either natural or man-made, in the globally interconnected 21st-century world should be high on the list of priorities for health authorities.
Antibody titre against haemagglutinin (HA) proteins provides a correlate of protection following vaccination, especially if inactivated influenza virus vaccine is used. However, seroconversion evaluates only the capacity of B cells to secrete anti-viral antibodies. The protective efficacy of the influenza vaccine in Croatia is similar to the efficacy reported worldwide (approximately 70%) [16]; the effect wanes to some degree in the elderly population [17]. Virus-specific CD8+ cytotoxic T lymphocytes (CTL) have been implicated as necessary for the clearance of the influenza virus during infection [18], and consequently are a valuable population to induce following influenza vaccination. We investigated whether combat-related PTSD in war veterans influenced protection following influenza vaccination.
Materials and methods
Subjects and study design
Twenty-eight (27 male and one female) combat-related chronic PTSD patients (mean age 39 years, range 30–55) selected randomly from the Croatian National Registry of PTSD patients were recruited for the study. The mean duration of their combat activity was 2·7 ± 1·9 (range 1–5) years. A mean of 9·2 ± 4·7 years had elapsed since they experienced combat traumas. Most of them (68%) were married and 71·8% had had secondary school education.
The structured clinical interview based on DSM-IV criteria [8] was used for the diagnosis of PTSD. The existence of current and lifetime symptoms was assessed using the Clinical Administered Post-traumatic Scale (CAPS) [19]. The inclusion criteria were current and chronic combat-related PTSD (CR-PTSD). The study was approved by Ethics Committees of both participating hospitals and all patients gave their informed consent. During the 12-month period prior to vaccination, they were treated mainly with selective serotonin reuptake inhibitors or tricyclic antidepressants, alone or in combination with other antidepressants, sedative hypnotics, anti-convulsants and anxiolytics. All participants had been free from any psychotropic or hormonal medication, drug or alcohol abuse for at least 1 month, and did not suffer from infectious, allergic or endocrine disorders. Additional exclusion criteria for PTSD patients were: (a) positive family history of psychosis; (b) history of schizophrenia, schizoaffective disorder or bipolar disorder; (c) serious concomitant medical condition (such as diabetes, hypertension and atherosclerosis); (d) history of seizures or misuse of alcohol or drugs; (e) clinically significant abnormalities in electrocardiogram or laboratory findings; and (f) risk of suicide. Eighty-one per cent of patients were smokers. The control group consisted of 15 healthy laboratory workers (mean age 32 years, range 23–54). The enrolment of the control group was approved by the Ethics Review Board of the Croatian Institute of Public Health, and all participants gave their informed consent.
During the 2003–04 winter, all subjects were immunized with the Agrippal® (Chiron, Italy) influenza vaccine, which contains influenza A (A/New Caledonia/H1N1-like, A/Moscow/H3N2-like) and B (B/Hong Kong/-like, B/Shangdong-like) purified surface antigens according to World Health Organization (WHO) recommendations [20]. The subjects had no known allergy to eggs, egg products or chicken protein, had not suffered from influenza-like disease within the previous 12 months or had a history of influenza vaccination. human leucocyte antigen (HLA)-A*0201+ status was analysed as described previously [21,22] and was confirmed in 10 male PTSD patients and six healthy male controls. Finally, peripheral blood mononuclear cells (PBMC) and sera were collected before and 14 days after vaccination.
PBMC were isolated from peripheral venous blood by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and stored in liquid nitrogen for later use. Cell freezing was performed following the manufacturer's instructions (Nalgene, NY, USA). All frozen cells were used within 6 months.
HLA-A*0201 tetramers
Phycoerythrin (PE)-labelled HLA class I tetramers (Proimmune, Oxford, UK) were loaded with two HLA-A*0201-restricted immunodominant New Caledonia virus haemagglutinin peptides, HA344−353 (GLFGAIAGFI) and HA541−549 (VLLVSLGAI) [23].
Immunofluorescence staining
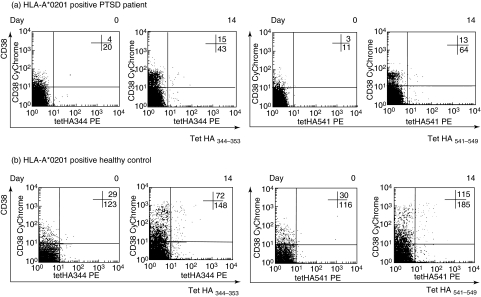
The following conjugated murine anti-human monoclonal antibodies (mAb) were used: CD3-APC, CD38-CyChrome (BD Biosciences, San Jose, CA, USA) and CD8-fluorescein isothiocyanate (FITC) (Serotec, Oxford, UK). For direct staining, thawed PBMC (2 × 106) were washed twice with 0·1% bovine serum albumin (BSA) in PBS (wash buffer), and stained first with HLA-tetramers at 4°C for 20 min in the dark and then for an additional 25 min with anti-CD3, anti-CD8 and anti-CD38 mAb. After incubation, cells were washed twice with wash buffer, resuspended in 0·5 ml of PBS with 1% fetal calf serum (FCS), and analysed immediately on a flow cytometer (FACSCalibur; BD Biosciences). Figure 1 shows representative dot-plots of HA344−353 and HA541−549 tetramer staining in HLA-A*0201+ PTSD patient and healthy control before and 14 days after vaccination. The negative control for tetramer analysis was PBMC from HLA-A*0201 negative vaccinated donors (n = 2). As positive control we used influenza-specific T cell lines, which we generated from PBMC of HLA-A*0201+ influenza-vaccinated donors. Briefly, PBMC were incubated in 12-well plates with 1 mM influenza peptide (HA344−353 or HA541−549) in RPMI-1640 supplemented with 10% human antibody serum (RPMI-AB) and antibiotics at 37°C and 5% CO2. After 1-h incubation, 2 × 106 cells/well from the same donor were added. RPMI-AB supplemented with 10 U/ml of human recombinant interleukin (IL)-2 (R&D Systems, Minneapolis, MN, USA) was changed on the third and seventh days of cell culture. Cells were harvested and analysed after 10 days' incubation.
Fig. 1.
Representative dot plots of haemagglutinin (HA)344−353 and HA541−549 tetramer staining. Recently activated (CD38 positive, upper right quadrant) and resting (CD38 negative, lower right quadrant) number of tetramer positive CD3+ CD8+ cells in peripheral blood in HLA-A*0201+ post-traumatic stress disorder (PTSD) patients (a) before and 14 days after vaccination. The x-axis represents staining with tetramers and the y axis represents staining with CD38; (b) a similar type of staining in healthy control subjects.
Flow cytometry analysis
Four-colour analyses were performed using dual-laser FACSCalibur flow cytometer and CellQuest software. Lymphocytes (gated from scatter graph based on size and density of the population) double-positive for CD3 and CD8 were designated CTL. For each sample 50 000 CD3+ CD8+ events were collected and analysed for co-expression of tetramer- and CD38-staining. To calculate the number of tetramer-positive cells per 5 × 104 CD3+ CD8+, the number of tetramer+/CD38+ cells determined by flow cytometry was divided by the number of cells gated by its co-expression of CD3 and CD8 antigens. This ratio was then normalized to 5 × 104 CD8+ T cells.
Antibody response
Serum samples from days 0 and 14 were tested simultaneously for all viral strains contained in the vaccine with strain-specific haemagglutination inhibition (HAI), as described previously [16]. The humoral response was assessed by calculating the following: geometric mean titres before and 14 days after vaccine administration; fold increases in the titre on day 14 after vaccination (i.e. geometric means of the ratio of the antibody titre after vaccination to the antibody titre on day 0); seroconversion rate (the percentage of subjects with a fourfold increase in HAI-antibody titres 14 days after vaccination as compared with baseline titres); seroprotection rate (the percentage of subjects with a titre of at least 1 : 40) before and 14 days after vaccination; and seroconversion factor (the ratio of the geometric mean titre before vaccination to the titre 14 days after vaccination) [24].
Statistical analysis
Because of the non-Gaussian distribution and small sample size, non-parametric statistical analysis was performed and medians were presented graphically. The number of antigen-specific T cells before and after vaccination was analysed by Wilcoxon's matched-pairs test. Comparison of the number of influenza-specific T cells in immunized individuals was performed by Mann–Whitney U-test. All summary statistics and analyses were performed by means of statistica version 6 (StatSoft, Inc., Tulsa, OK, USA). Control for multiple comparisons was made by the false discovery rate procedure [25] considering the proportion of erroneous rejections to the total number of rejections, by comparing individual P-values with 0·05x(i/m), where i is the ascending rank of particular P-value and m is the total number of comparisons (12 in this case). The values of P = 005x(i/m) were considered significant at the 0·05 level.
To test for possible impact of marital status and/or education on the vaccination response (both log-transformed antibody titres and frequency of tetramer positive events), differences were compared between married and unmarried/divorced or between subjects who had completed high school/faculty and those who had completed elementary school only. After homogeneity of variances (Levene's test) and linearity of covariance matrices (Box's M-test) were confirmed, data regarding four tested virus types were compared with the Hotelling T2 test [variant of multiple analyses of variance (manova) for comparing two groups]. The difference between tetramer positive events for each virus type on the day zero and 14 was calculated with the non-parametric Mann–Whitney U-test.
Results
Humoral immune response before and after vaccination in PTSD patients and healthy controls
There was no significant difference between the prevaccination titres to any viral strains between controls and PTSD patients (Table 1). The mean fold increase in titres did not differ significantly between the groups; the lower seroconversion rate in the response of healthy controls to A/Moscow/H3N2 (33·3) may have been due to the higher geometric mean antibody titres to this type of influenza A virus before vaccination (66·5) in comparison to the prevaccination titre in PTSD patients (42·2), resulting in a seroconversion factor increase (2·0) lower than recommended (2·5). On the other hand, the seroconversion rate to other vaccine strains was similar and ranged from 33·3% in healthy controls to up to 60·7% in PTSD patients, demonstrating at least a 2·5-fold increase in geometric mean titre. After vaccination, the seroprotection rates (defined by an HAI response of at least 1 : 40) were high and similar between PTSD patients and controls (60–100%) for all vaccine strains.
Table 1.
Strain-specific haemagglutination inhibition before and after influenza vaccination in post-traumatic stress disorder (PTSD) patients and healthy controls.*
| PTSD patients (n = 28) value (95% CI) | Healthy controls (n = 15) value (95% CI) | |
|---|---|---|
| Geometric mean titre | ||
| A/New Caledonia/H1N1 | ||
| Before vaccination | 5·9 (4·1–9·7) | 7·2 (3·6–17·1) |
| After vaccination | 41·1 (37·2–105·1) | 40·0 (20·2–136·5) |
| A/Moscow/H3N2 | ||
| Before vaccination | 42·2 (18·4–114·6) | 66·5 (44·9–133·8) |
| After vaccination | 119·3 (109·9–247) | 132·4 (106·9–195·8) |
| B/Shangdong/ | ||
| Before vaccination | 17·5 (15·8–30·7) | 13·8 (8·7–37·3) |
| After vaccination | 48·2 (44·9–76·6) | 38·2 (28·7–72·7) |
| B/Hong Kong/ | ||
| Before vaccination | 12·1 (9·2–25·4) | 12·0 (6·0–34·6) |
| After vaccination | 43·3 (40·8–73·8) | 36·5 (26·9–71·7) |
| Seroconversion rate (%) | ||
| A/New Caledonia/H1N1 | 60·7 (42·5–76·6) | 53·3 (30·3–75·4) |
| A/Moscow/H3N2 | 53·6 (35·9–70·6) | 33·3 (15·1–58·8) |
| B/Shangdong/ | 50·0 (32·7–67·5) | 33·3 (15·1–58·8) |
| B/Hong Kong/ | 57·1 (39·2–73·6) | 40·0 (19·9–64·6) |
| Seroconversion factor | ||
| A/New Caledonia/H1N1 | 7·0 (4·4–11·2) | 5·5 (2·8–10·7) |
| A/Moscow/H3N2 | 2·8 (2·0–3·9) | 2·0 (1·3–3·0) |
| B/Shangdong/ | 2·8 (1·9–3·9) | 2·8 (1·8–4·3) |
| B/Hong Kong/ | 3·6 (2·6–5·0) | 3·0 (1·9–5·0) |
| Seroprotection rate (%) | ||
| A/New Caledonia/H1N1 | ||
| Before vaccination | 10·7 | 13·0 |
| After vaccination | 67·9 | 60·0 |
| A/Moscow/H3N2 | ||
| Before vaccination | 75·0 | 93·3 |
| After vaccination | 100·0 | 100·0 |
| B/Shangdong/ | ||
| Before vaccination | 42·9 | 26·7 |
| After vaccination | 75·0 | 66·7 |
| B/Hong Kong/ | ||
| Before vaccination | 17·9 | 20·0 |
| After vaccination | 71·4 | 60·0 |
Values were compared following the Committee for Proprietary Medicinal Products (CPMP) guidelines [23]. Prevaccination blood samples were obtained at the time of vaccination, and postvaccination samples were obtained after 14 days. CI = confidence interval. The seroconversion factor is the ratio of the geometric mean titre before and after vaccination.
Cellular immune response to influenza vaccination in HLA-A*0201+ PTSD patients
Ex vivo tetramer staining of recently activated CD8+ T lymphocytes was used to monitor the T cell response specific for HLA-A*0201-restricted influenza A haemagglutinin antigens (A/New Caledonia/H1N1, HA344−353, HA541−549) before and 14 days after vaccination in HLA-A*0201+ subjects (Fig. 2). PTSD patients (n = 10) had a significantly lower number (P < 0·05) of HA344−353-specific CTL before vaccination in comparison to healthy controls (n = 6) (Fig. 2a).
Fig. 2.
Comparison of the number of influenza-specific T cells in immunized individuals. Number of recently activated CD38+ T cells specific for A/New Caledonia/H1N1 antigens haemagglutinin (HA)344−353 (a) and HA541−549 (b) in 50 000 CD3+ CD8+ T cells in HLA*A0201+ vaccinated post-traumatic stress disorder (PTSD) patients (filled triangles) and controls (filled circles) before and 14 days after vaccination. Median number of tetramer+ CD38+ in 50 000 CD8+ T cells is represented by black (PTSD patients) and dotted (controls) horizontal bars. The number of antigen-specific T cells before and after vaccination was analysed by Wilcoxon's matched pair test. Comparison of the number of influenza-specific T cells in immunized individuals was performed by Mann–Whitney U-test.
In at least three HLA-A*0201+ healthy controls an increase of one or both influenza-specific CTL was observed after vaccination. Six PTSD patients had increased numbers of at least one population (HA344−353 or HA541−549) of specific CTL (Fig. 2a,b). Although the magnitude of changes in the numbers of specific CTLs in PTSD patients was smaller than in controls, there was no difference in the number of HA541−549-specific CD8 T cells between PTSD patients and controls.
In three patients, the fourfold rise of influenza A antibody titre was accompanied by a two- to fourfold increase of influenza-specific T cells 14 days after vaccination. One of the six healthy controls showed the same response 14 days after vaccination.
Changes in either antibody titre or frequency of antigen-specific T cells were not recorded in a single patient. In one patient only the frequency of influenza-specific T cells increased, whereas three other patients showed only influenza A seroconversion.
The potential effect of current marital status or education on the influenza vaccination response was also evaluated. Comparisons of log-transformed antibody titres on days 0 and 14 were carried out among married (n = 16) and unmarried/divorced (n = 8), or among subjects who completed high school/faculty (n = 16) and those who completed only elementary school (n = 8). As the data regarding tetramer measures were available for a limited number of participants (n = 10), the influence of education was evaluated in five patients/group and of marital status in seven married versus three unmarried patients. No effect of patients' current marital status or education on the influenza vaccination response to any of the influenza strains present in the vaccine was observed (data not shown).
Discussion
A possible method to assess the effects of environmental exposures and a variety of other factors such as sex, genetic factors, age, psychological stress, nutrition and concomitant diseases on the immune system is to study the effects on vaccination responses [26]. In this study, we measured specific immune responses to influenza vaccination in immunized PTSD patients. Eighty-nine per cent of PTSD patients had no protective or baseline antibody titre for A/New Caledonia/H1N1 before vaccination. The Croatian Institute of Public Health, which is a WHO-appointed National Influenza Centre, monitors and identifies annually circulating influenza strains in Croatia. A/New Caledonia/H1N1 was identified and circulated in Croatia in the 2000/2001 and 2002/2003 seasons. This finding implies that these patients probably encountered A/New Caledonia/H1N1 antigen for the first time with vaccination. At the same time, most PTSD patients showed at least baseline antibody titres for three other components of the influenza vaccine, which indicated exposure to those antigens before vaccination. PTSD patients produced a significant antibody response after influenza vaccination, indicating that low prevaccination antibody titres are associated with higher humoral immune response induced by vaccine. A previous study showed that subjects vaccinated for the first time had higher response rates and antibody increase than those previously vaccinated [27].
Previous studies have demonstrated that chronic stress can impair the humoral immune response to influenza vaccination. The subjects tested were older adults (mean age 68 years) under long-term chronic stressors (carers of spouses with dementia) [3,5,6]. Contrary to these subjects, PTSD patients generated a humoral immune response after influenza vaccination. This difference could be explained by the age difference of PTSD patients in our study, their mean age being 39 years. Another explanation could be that psychoneuroimmune changes associated previously with PTSD do not influence the humoral immune response following vaccination. Previously published data have established that short-term stressors enhance natural immunity to defend the body in ‘fight-or-flight’ situations. At the same time, specific cellular immunity is suppressed while humoral immunity is preserved. However, chronic stress is associated with global suppression of the immune system, particularly specific immune functions [28]. One could also argue that so-called ‘flashbacks’ or episodes of relieving the stressor in our PTSD patients activated humoral immunity through the ‘fight or flight’ up-regulatory immune response [1]. The results are inconclusive regarding the impact of PTSD on cellular immune system. Both suppression [29] and enhancement [30] of cellular immunity in humans with a past history of PTSD have been reported. Different cellular responses in those PTSD patients and our group of PTSD patients may be explained by the subjects' age, type of traumatic event and time elapsed from exposure to trauma.
With the emergence of new tools, in particular major histocompatibility complex (MHC) class I tetramers loaded with CD8+ T cell viral antigens, it is now possible to study virus-specific CD8 populations in humans during different stages of viral infection and after vaccination. Published data on humans are inconsistent as to whether subunit vaccines are capable to elicit physiological CTL responses because inactivated viruses do not replicate, so antigen processing and presentation of CD8+ T cell-restrictive antigens are insufficient [31,32]. However, the only CD8 T cell epitope investigated so far is the M158−66 epitope contained in influenza matrix protein, which has been described as the immunodominant peptide in naturally infected HLA-A*0201+ donors [18]. Because the major aim of the vaccine is to elicit a specific immune response towards HA of viral strains contained in the vaccine, one could also argue that analysis of the CTL response should include analysis of HA-specific CTLs, as suggested recently by Gianfrani et al. [23]. Two antigens HA344−353 and HA541−549 in New Caledonia/H1N1-like strain are immunodominant epitopes contained in the vaccine and presented by HLA-A*0201 molecules [18]. A previous study reports that vaccinated humans who demonstrated neither an antibody- nor a cell-mediated response had a 25% risk of natural influenza infection, whereas humans who developed either an antibody response, a cell-mediated response or both had an approximately 15% risk of influenza infection [33]. Although PTSD patients had a lower number of HA344−353-specific CD8+ T cells compared to healthy controls, six of them responded by a significant increase in at least one monitored population of HA-specific CTL. The lack of enhanced HA-specific CD8+ T cell responses following vaccination in two controls could have been the result of the high pre-existing number of HA-specific CD8+ T cells in these subjects, which did not rise further upon immunization with inactivated influenza vaccine. A CTL primary response following influenza vaccination is detectable in blood after 6–14 days and reaches peak level on day 14 [34], so we presume that the time-point for blood collection was appropriate.
The magnitude of the influenza virus-specific CTL response depends on both HLA-A and -B phenotypes [35]. It is possible that in addition to the two epitopes examined, other epitopes, in particular those presented by HLA-B molecules, contribute to the overall pool of specific CD8 cells responsible for vaccine-induced protection against influenza infection.
It has been shown previously that education and marital status correlates with the severity of PTSD symptoms [36,37]. Furthermore, a recent study that investigated whether stressful life events and social support were related to antibody status following both thymus-dependent and thymus-independent vaccinations in healthy young adults showed strain-specific effects for the influenza vaccination [38]. A potential limitation of the present study was the relatively small sample size, so our finding that either current marital status or education showed no effect on the vaccination response to any of the influenza strains present in the vaccine should be interpreted with caution.
In conclusion, our findings suggest that most PTSD patients examined 9 years after war trauma have the ability to produce protective immunity 2 weeks following influenza vaccination. Because vaccination responses could be affected by the type of vaccine as well as the vaccination procedure, it would be of interest to determine the response of PTSD patients to other relevant vaccines, including those that elicit primarily cellular immune responses such as vaccines containing attenuated viruses, utilizing the recent advances in class I tetramer staining technology.
Acknowledgments
This study was supported by grants from the Croatian Ministry of Science, Education and Sports to A. Gagro (TP-01/0021–05, 0021004 and 108-1080229-0337) and D. Dekaris (0021003). We thank Dr Andelko Vidoviæ for help with statistical analysis and Professor Ana Marusic for critical reading of the manuscript.
References
- 1.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol. 1998;17:214–23. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 3.Vedhara K, Cox NK, Wilcock GK, et al. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353:627–31. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 4.Burns VE, Carroll D, Drayson M, Whitham M, Ring C. Life events, perceived stress and antibody response to influenza vaccination in young, healthy adults. J Psychosom Res. 2003;55:569–72. doi: 10.1016/s0022-3999(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 5.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. The influence of psychological stress on the immune response to vaccines. Ann NY Acad Sci. 1998;840:649–55. doi: 10.1111/j.1749-6632.1998.tb09603.x. [DOI] [PubMed] [Google Scholar]
- 7.Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000;62:804–7. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fourth. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 9.Lee KA, Vaillant GE, Torrey WC, Elder GH. A 50-year prospective study of the psychological sequelae of World War II combat. Am J Psychiatry. 1995;152:516–22. doi: 10.1176/ajp.152.4.516. [DOI] [PubMed] [Google Scholar]
- 10.Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in post-traumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 11.Sabioncello A, Gotovac K, Vidovic A, et al. The immune system under stress. Period Biol. 2004;106:317–23. [Google Scholar]
- 12.Dekaris D, Sabioncello A, Mazuran R, et al. Multiple changes of immunologic parameters in prisoners of war. Assessments after release from a camp in Manjac, Bosnia. JAMA. 1993;270:595–9. [PubMed] [Google Scholar]
- 13.Sabioncello A, Kocijan-Hercigonja D, Rabatic S, et al. Immune, endocrine, and psychological responses in civilians displaced by war. Psychosom Med. 2000;62:502–8. doi: 10.1097/00006842-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Gotovac K, Sabioncello A, Rabatic S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: lower quantity of GCR in patients with post-traumatic stress disorder (PTSD) Clin Exp Immunol. 2003;131:335–9. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox NJ, Tamblyn SE, Tam T. Influenza pandemic planning. Vaccine. 2003;21:1801–3. doi: 10.1016/s0264-410x(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 16.Draženovic V, Ilic A, Sim R, Božikov J, Mlinaric-Galinovic G. Immune response to influenza vaccine (A/New Caledonia/20/99 (H1N1)-like, A/Moscow/10/99 (H3N2)-like, and B/Bejing/184/93+B/Yamanashi/166/98-like) in elderly. Periodicum Biologorum. 2003;105:147–51. [Google Scholar]
- 17.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 18.Jameson J, Cruz J, Ennis FA. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol. 1998;72:8682–9. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weathers FW, Keane TM, Davidson JRT. Clinical-administered PTSD scale: a review of the first ten year research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO). Recommendations for Influenza Vaccine Composition for use in the 2001–02 and 2002–03 seasons. Available at: http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/index6.html and http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/index4.html (accessed 3 September 2004).
- 21.Grubic Z, Žunec R, Cecuk-Jelicic E, Kerhin-Brkljacic V, Kaštelan A. Polymorphism of HLA-A-B-DRB1-DQA1 and -DQB1 haplotypes in a Croatian population. Eur J Immunogenet. 2000;27:47–51. doi: 10.1046/j.1365-2370.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- 22.Cecuk-Jelicic E, Grubic Z, Žunec R, Labar B, Kerhin-Brkljacic V, Kaštelan A. Implication of molecular analysis of HLA-A*02 subtyping for unrelated bone-marrow donor selection. Bone Marrow Transplant. 1998;22:27–30. [PubMed] [Google Scholar]
- 23.Gianfrani C, Oseroff C, Sidney J, Chesnut RW, Sette A. Human memory CTL response specific for influenza A virus is broad and multispecfic. Hum Immunol. 2000;61:438–52. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 24.Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonisation of requirements for influenza vaccines. London: European Agency for the Evaluation of Medicinal Products; 1997. pp. 1–18. CPMP/BWP/214/96. [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 26.Van Loveren H, Van Amsterdam JG, Vandebriel RJ, et al. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Environ Health Perspect. 2001;109:757–64. doi: 10.1289/ehp.01109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine. 1996;14:1108–10. doi: 10.1016/0264-410x(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 28.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of post-traumatic stress disorder. Am J Psychiatry. 2001;158:484–6. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- 30.Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. Am J Psychiatry. 2003;160:1705–7. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- 31.Frank AL, Webster RG, Glezen WP, Cate TR. Immunogenicity of influenza A/USSR (H1N1) subunit vaccine in unprimed young adults. J Med Virol. 1981;7:135–42. doi: 10.1002/jmv.1890070207. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–46. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 33.Kilbourne ED, Cerini CP, Khan MW, Mitchell JW, Jr, Ogra PL. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin: I. Studies in human vaccinees. J Immunol. 1987;138:3010–3. [PubMed] [Google Scholar]
- 34.Ennis FA, Rook AH, Qi YH, et al. HLA restricted virus-specific cytotoxic T-lymphocyte responses to live and inactivated influenza vaccines. Lancet. 1981;2:887–91. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- 35.Boon AC, de Mutsert G, Graus YM, et al. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J Virol. 2002;76:582–90. doi: 10.1128/JVI.76.2.582-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riggs DS, Byrne CA, Weathers FW, Litz BT. The quality of the intimate relationships of male Vietnam veterans: problems associated with posttraumatic stress disorder. J Trauma Stress. 1998;11:87–101. doi: 10.1023/A:1024409200155. [DOI] [PubMed] [Google Scholar]
- 37.Kozaric-Kovacic D, Borovecki A. Prevalence of psychotic comorbidity in combat-related post-traumatic stress disorder. Mil Med. 2005;170:223–6. doi: 10.7205/milmed.170.3.223. [DOI] [PubMed] [Google Scholar]
- 38.Phillips AC, Burns VE, Carroll D, Ring C, Drayson M. The association between life events, social support, and antibody status following thymus-dependent and thymus-independent vaccinations in healthy young adults. Brain Behav Immun. 2005;19:325–33. doi: 10.1016/j.bbi.2004.10.004. [DOI] [PubMed] [Google Scholar]