Abstract
Interferon-inducible protein-10 (IP-10)/CXCL10, which is a ligand for CXC chemokine receptor 3 (CXCR3), is known to be involved in the pathogenesis of pulmonary sarcoidosis. However, the roles of monokine induced by interferon γ (Mig)/CXCL9 and interferon-inducible T cell α chemoattractant (I-TAC)/CXCL11, which are also CXCR3 ligands, remain unclear. Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in both bronchoalveolar lavage fluid (BALF) and serum in patients with pulmonary sarcoidosis were measured by enzyme-linked immunosorbent assay (ELISA). The expression of these chemokines in alveolar macrophages was examined using ELISA, quantitative real-time polymerase chain reaction and immunostaining. In BALF, Mig/CXCL9 and IP-10/CXCL10 were significantly elevated in stage II sarcoidosis as compared with the levels in healthy volunteers. In serum, Mig/CXCL9 and I-TAC/CXCL11 were increased in stage II of the disease. The levels of all CXCR3 ligands in BALF were correlated with the numbers of both total and CD4+ lymphocytes. Alveolar macrophages were stained positive for all CXCR3 ligands and produced increased amounts of these chemokines. Positive staining of the three chemokines was also observed in the epithelioid and giant cells in the sarcoid lungs. These findings suggest that Mig/CXCL9 and I-TAC/CXCL11 as well as IP-10/CXCL10 play important roles in the accumulation of Th1 lymphocytes in sarcoid lungs.
Keywords: alveolar macrophages, CXCL9, CXCL10, CXCL11, sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause that mainly affects the lung and lymphatic system of the body [1–4]. Although spontaneous remission occurs in nearly two-thirds of patients, chronic and progressive courses are observed in 10–30% of patients [1,2]. The sarcoid granuloma is characterized by non-caseating epitheloid cells with the dominant accumulation of CD4+ T cells and macrophages [1–4].
CD4+ T lymphocytes are divided into two subgroups, T helper (Th) 1 and Th2 cells, on the basis of their cytokine production [5]. Based on the Th1/Th2 paradigm, sarcoidosis is considered to be a typical Th1-dominant disease, as T lymphocytes in bronchoalveolar lavage fluid (BALF) and lymph nodes from sarcoidosis preferentially produce interferon (IFN)-γ, interleukin (IL)-2 and tumour necrosis factor (TNF)-α or β[6–8]. Recently, the chemokine receptors expressed specifically on Th1 or Th2 cells were identified, and it was reported that Th1 cells are characterized by the expression of CCR5 or CXCR3, and Th2 cells by CCR4 expression [9]. BAL lymphocytes in sarcoidosis have been reported to be highly positive for CCR5 and CXCR3 [10–12]. In particular, CXCR3 is expressed on more than 97% (88·6–99·2%) of BAL CD4+ T cells in sarcoidosis, while CCR5 is expressed on 50·5% (30·5–81·2%) [12]. Therefore, the analysis of CXCR3 ligands in sarcoidosis is of importance to clarify the mechanisms of accumulation of Th1 cells into the sarcoid lungs. Interferon-inducible protein-10 (IP-10)/CXCL10, which is one of the CXCR3 ligands, was reported to be elevated in BALF of patients with sarcoidosis and to play crucial roles in the Th1-immune response in the sarcoid lungs [11,13]. Alveolar macrophages and epithelioid cells are known to be the main producers of IP-10/CXCL10 [13]. However, the roles of other CXCR3 ligands, monokine induced by interferon γ (Mig)/CXCL9 and interferon-inducible T cell α-chemoattractant (I-TAC)/CXCL11, in the pathogenesis of sarcoidosis, still remain unclear. We therefore examined the levels of Mig/CXCL9 and I-TAC/CXCL11 as well as IP-10/CXCL10 in BALF and serum in patients with different stages of sarcoidosis and the expressions of these chemokines in alveolar macrophages.
Methods
Subjects
Studies were performed in 44 patients with sarcoidosis and nine healthy volunteers (HV) (Table 1). Sarcoidosis was diagnosed according to previously described clinical and histological criteria [1]. Based on chest radiography, patients were subdivided into four stages (stage 0: n = 10, stage I; n = 18, stage II: n = 12, stages III and IV: n = 4). Ten patients with normal chest X-ray findings (stage 0) had positive biopsy findings of the lung or skin and uveitis. None of the patients were receiving corticosteroid therapy at the time of the investigation. None of the HV showed any abnormalities on physical examinations, chest X-ray and lung function tests. The study was approved by the ethics committee of the University of Tokushima and written informed consent was obtained from the subjects.
Table 1.
Characteristics of study population.
| Bronchoalveolar lavage | ||||||||
|---|---|---|---|---|---|---|---|---|
| % Total cells | ||||||||
| Group | Male/female | Age (years) | Total cells ( × 105/ml) | AM | Ly | Neut | Eo | CD4+/CD8+ |
| HV | 8/1 | 23·7 ± 2·1 | 1·6 ± 0·2 | 87·9 ± 2·7 | 9·8 ± 2·0 | 1·8 ± 1·3 | 0·4 ± 0·1 | n.d. |
| Sar | 18/26 | 49·1 ± 13·3 | 2·4 ± 0·3* | 71·0 ± 2·3† | 27·1 ± 2·2† | 1·1 ± 0·4 | 0·7 ± 0·3 | 5·25 ± 0·5 |
AM = alveolar macrophages; Eo = eosinophils; HV = healthy volunteers; Ly = lymphocytes; Neut = neutrophils; n.d. = not determined; Sar = sarcoidosis.
Data are shown as mean ± standard error of the mean.
P < 0·05, compared with the group of healthy volunteers.
P < 0·001, compared with the group of healthy volunteers.
Bronchoalveolar lavage
BAL was performed as described previously [14,15]. Briefly, a flexible fibreoptic bronchoscope (Model 1T20; Olympus Co., Tokyo, Japan) was wedged into a segmental or subsegmental bronchus of the middle lobe or lingula, and lavage was performed with a total volume of 150 ml of sterile 0·9% saline warmed at 37°C in three 50-ml aliquots. The fluid recovered was passed through the sterile gauze and the supernatants were stored at −80°C until examination. The total number of cells was counted using the trypan-blue dye exclusion test. Differential counts on 400 cells were carried out on smears of sedimented cells stained with Diff-Quik stain (Baxter Dade AG, Duedingen, Switzerland) [14,15]. Blood samples were collected at the time of diagnosis.
Measurement of CXCR3 ligands by enzyme-linked immunosorbent assay (ELISA)
The frozen BALF was thawed quickly and used to examine the concentration of three CXCR3 ligands by ELISA (R&D System, Minneapolis, MN, USA) [15,16]. The minimal detectable level of Mig/CXCL9 was 3·84 pg/ml, and those of IP-10/CXCL10 and I-TAC/CXCL11 were 1·67 pg/ml.
Harvesting of the supernatant of alveolar macrophages
BAL cells were added to a 96-well flat-bottomed plate at 1 × 105 of alveolar macrophages per well in triplicate cultures [15]. These cells were washed with cold phosphate-buffered saline (PBS) to remove the non-adherent cells. The purity of alveolar macrophages at this point was more than 90%. More than 93% were viable as judged by the trypan blue dye exclusion test. Cells were subsequently cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA). Their supernatants were harvested after culture for 24 and 48 h.
Preparation of total RNA of alveolar macrophages
Total RNA was isolated from alveolar macrophages using Isogen (Wako KK, Kyoto, Japan) as described previously [15]. Briefly, 1 ml of Isogen was added to the culture of alveolar macrophages in a 10-cm dish, and then the cells were harvested into an Eppendorf tube using a cell-scraper (Sumitomo Bakelite Co., Ltd, Tokyo, Japan). Total RNA was extracted according to the manufacturer's instructions [15].
Real-time quantitative polymerase chain reaction (PCR) analysis for CXCR3 ligands
Ten nanograms of total RNA were used for RT–PCR using a one-step real-time PCR kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative PCR was performed using an ABI prism 7700 Sequence detector (Applied Biosystems) [15]. The primers and probes for human CXCR3 ligands were designed based on published sequence data [17–19], and were as follows: Mig/CXCL9 forward primer, 5′-CCAAGGGACTATCCACCTACAATC-3′; Mig/CXCL9 reverse primer, 5′-GGTTTAGACATGTTTGAACTCCATTC-3′; Mig/CXCL9 TaqMan probe, 5′-FAM-CCTTAAACAATTTGCCCCAAGCCCTTC-TAMRA-3′: IP-10/CXCL10 forward primer, 5′-CTGACTCTAAGTGGCATTCAAGGA-3′; IP-10/CXCL10 reverse primer, 5′-CAATGATCTCAACACGTGGACAA-3′; IP-10/CXCL10 TaqMan probe, 5′-FAM-AGAACCGTACGCTGTACCTGCATCAGCA-TAMRA-3′: I-TAC/CXCL11 forward primer, 5′-GGGTACATTATGGAGGCTTTCTCA-3′; I-TAC/CXCL11 reverse primer, 5′-GAGGACGCTGTCTTTGCATAGG-3′; and I-TAC/CXCL11 TaqMan probe, 5′-FAM-TCTGCCACTTTCACTGCTTTTACCCCA-TAMRA-3′. Each RT–PCR reaction was performed in duplicate wells using a TaqMan one-step RT–PCR Master Mix Reagents Kit (Applied Biosystems). The RT reaction was performed at 48°C for 30 min and 95°C for 10 min. PCR was performed for 40 cycles at 95°C for 15 s and 60°C for 1 min. Real-time RT–PCR for β-actin was also performed as a control using TaqMan β-actin Control Reagents (Applied Biosystems) under the same conditions as for the three CXCR3 ligands. The relative expression level of each CXCR3 ligand mRNA was calculated as the value of CXCR3 ligand mRNA/the value of β-actin mRNA [15].
Immunofluorescence staining
Immmunofluorescence staining was performed as described previously [20]. BAL cells were cytocentrifuged onto glass slides and fixed in cold acetone for 10 min, and were stained with rabbit anti-human Mig/CXCL9, IP-10/CXCL10 or I-TAC/CXCL11 polyclonal antibodies (PeproTech EC Ltd, London, UK) and Alexa fluor 488-conjugated goat anti-rabbit IgG (H + L) antibody (Molecular Probes, Eugene, OR, USA). These slides were mounted with Vectashield mounting medium containing 4′, 6 diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA), and visualized using a fluorescence microscope (Olympus BX61; Olympus Optical Co. Ltd, Tokyo, Japan).
Immunohistochemistry
Fresh frozen lung tissues were obtained from patients with sarcoidosis by lung biopsy at video-assisted thoracic surgery. Fragments of the tissues were covered with Tissue-Tek optimal cutting temperature (OCT) compound (Ames, Elkhart, IN, USA), snap-frozen in liquid nitrogen, and stored at −80°C until analysed. Six-micron sections were dried and fixed in cold acetone for 10 min. Staining was performed using the RTU Vectastain Universal Quick Kit (Vector Laboratories) [21,22]. The sections were incubated in 3% H2O2 in methanol for 30 min to inhibit endogenous peroxidase, and incubated in blocking serum for 10 min. The slides were then incubated overnight with rabbit anti-human Mig/CXCL9, IP-10/CXCL10 or I-TAC/CXCL11 polyclonal antibodies (PeproTech) at 4°C. After washing, sections were incubated in prediluted biotinylated panspecific universal secondary antibody for 10 min, followed by incubation in ready-to-use streptavidin/peroxidase complex reagent for 5 min. Sections were developed with a diaminobenzidine substrate kit (Vector Laboratories, Inc.) and counterstained with Mayer's haematoxylin (Muto Pure Chemicals Co., Ltd, Tokyo, Japan).
Statistical analysis
Comparisons among multiple groups were analysed using the one-way analysis of variance (anova) with Newman–Keuls post-hoc correction (GraphPad Prism, version 3·0). Statistical analysis between two groups was performed using the unpaired two-tailed Student's t-test. Correlation coefficients were determined using the Pearson's linear regression analysis in StatView software. Differences were considered significant when P-values were less than 0·05.
Results
Characteristics of BALF of patients with sarcoidosis
The characteristics of the BALF of patients with sarcoidosis and BALF of healthy volunteers (HV) are shown in Table 1. The total number of cells in the BALF of patients with sarcoidosis was significantly higher than that in the BALF of HV (P < 0·05). The percentage of lymphocytes was increased significantly in sarcoidosis patients compared with HV (P < 0·001).
Concentrations of CXCR3 ligands, Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in BALF and serum of patients with sarcoidosis
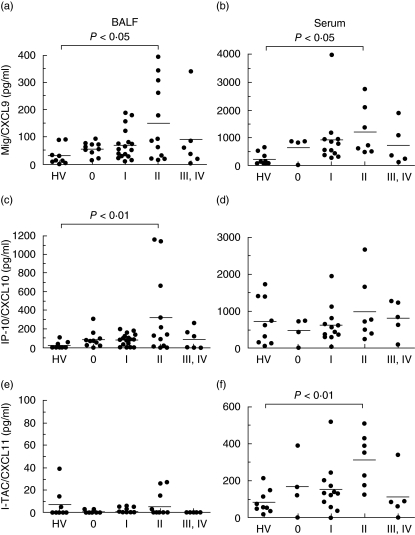
We examined the levels of Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in both the BALF and serum of patients with sarcoidosis using ELISA. The results are shown in Fig. 1. In BALF, Mig/CXCL9 and IP-10/CXCL10 tended to be higher in patients at all stages of sarcoidosis, and were significantly elevated in stage II sarcoidosis compared with that of HV (P < 0·05). The level of I-TAC/CXCL11 in BALF was too low to detect in most samples, although in some samples from stage II sarcoidosis and HV I-TAC/CXCL11 was present at a detectable level. In serum, Mig/CXCL9 and I-TAC/CXCL11 tended to be higher in patients at all stages of sarcoidosis, and were elevated significantly in stage II sarcoidosis compared with HV (Mig/CXCL9: P < 0·05, I-TAC/CXCL11: P < 0·01). However, there was no difference in serum IP-10/CXCL10 among all stages of sarcoidosis and HV.
Fig. 1.
Concentrations of monokine induced by interferon γ(Mig)/CXCL9, interferon-inducible protein-10 (IP-10)/CXCL10 and interferon-inducible T cell α-chemoattractant (I-TAC)/CXCL11 in both bronchoalveolar lavage fluid (BALF) and serum of patients with sarcoidosis and healthy subjects. The BALF harvested from healthy volunteers (HV) or patients with various stages of sarcoidosis were used for the measurement of CXCR3 ligands by enzyme-linked immunosorbent assay (ELISA). Each closed circle indicates an individual specimen. A value of 0 (pg/ml) was given to all samples that read out below the level of detection in the ELISA.
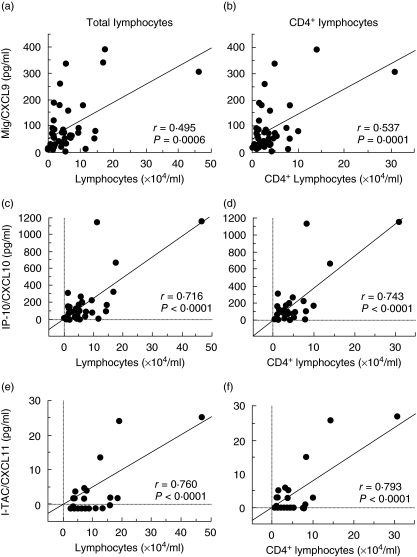
Relationship between the level of each CXCR3 ligand (Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11) and the number of lymphocytes in BALF of patients with sarcoidosis
We examined the correlations between the level of each CXCR3 ligand and the number of lymphocytes in the BALF of patients with sarcoidosis. As shown in Fig. 2, there was a positive correlation between the concentration of Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 and the number of lymphocytes (Mig/CXCL9: r = 0·495, P = 0·0006; IP-10/CXCL10: r = 0·716, P < 0·0001; I-TAC/CXCL11: r = 0·760, P < 0·001). When we analysed the correlation between the number of CD4+ lymphocytes and the level of each CXCR3 ligand in BALF, higher correlations than those between total lymphocytes and each CXCR3 ligand were observed (Mig/CXCL9: r = 0·537, P = 0·0001; IP-10/CXCL10: r = 0·743, P < 0·0001; I-TAC/CXCL11: r = 0·793, P < 0·0001).
Fig. 2.
Correlation between the level of each CXCR3 ligand and the number of total or CD4+ lymphocytes in bronchoalveolar lavage fluid (BALF). The correlations between the level of each CXCR3 ligand and the number of lymphocytes (a, c, e) or CD4+ lymphocytes (b, d, f) in the BALF of patients with sarcoidosis was examined.
Relationship among the levels of Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in patients with sarcoidosis
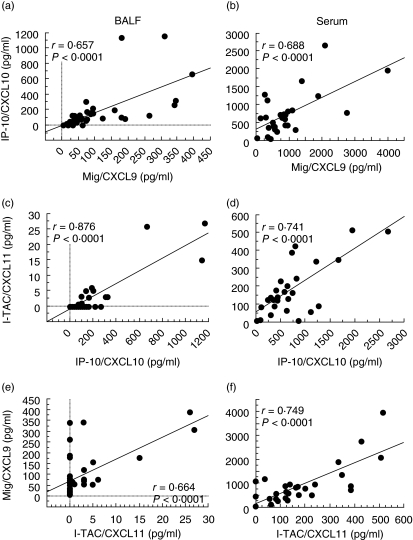
We next examined the correlations between the levels of each CXCR3 ligand in BALF and those in serum in patients with sarcoidosis. There were significant correlations between the BALF and serum levels of each CXCR3 ligand (Mig/CXCL9: r = 0·569, P = 0·002; IP-10/CXCL10: r = 0·639, P = 0·0003; I-TAC/CXCL11: r = 0·501, P = 0·0082).
Furthermore, high correlations among the levels of the three CXCR3 ligands in both BALF and serum were observed (Mig/CXCL9 and IP-10/CXCL10: BALF; r = 0·657, P < 0·0001, serum; r = 0·688, P < 0·0001; IP-10/CXCL10 and I-TAC/CXCL11: BALF; r = 0·876, P < 0·0001, serum; r = 0·741, P < 0·0001; I-TAC/CXCL11 and Mig/CXCL9: BALF; r = 0·664, P < 0·0001, serum; r = 0·749, P < 0·0001) (Fig. 3).
Fig. 3.
Correlation among the levels of three CXCR3 ligands in bronchoalveolar lavage fluid (BALF) and serum. The correlations between the levels of each CXCR3 ligand in BALF (a, c, e) and those in serum (b, d, f) in patients with sarcoidosis.
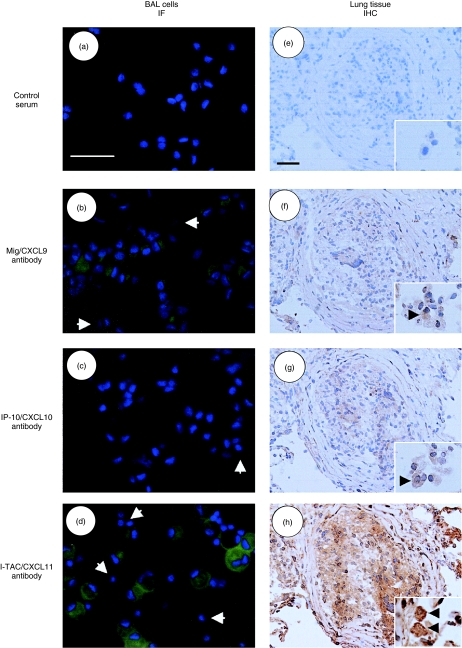
Immunostaining of Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in BAL cells and lung tissue in sarcoidosis
To analyse which cells produce Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 in the sarcoid lungs, we performed the immunostaining of both BAL cells and lung tissues. Immunofluorescent staining of BAL cells showed that alveolar macrophages, but not lymphocytes, were positive for all CXCR3 ligands (Fig. 4). In particular, I-TAC/CXCL11 was stained strongly when compared with Mig/CXCL9 and IP-10/CXCL10. All CXCR3 ligands were also stained in epithelioid and giant cells in sarcoid granuloma in immunohistochemical analysis. Again, macrophages in the alveolar lumens were positive for all three CXCR3 ligands.
Fig. 4.
Immunostaining of monokine induced by interferon γ (Mig)/CXCL9, interferon-inducible protein-10 (IP-10)/CXCL10 and interferon-inducible T cell α-chemoattractant (I-TAC)/CXCL11 in bronchoalveolar lavage (BA) cells and sarcoid lungs. BAL cells were cytocentrifuged on glass slides and fixed. The slides were incubated with control rabbit serum (a), anti-Mig/CXCL9 (b), IP-10/CXCL10 (c) or I-TAC/CXCL11 antibody (d), and stained with Alexa fluor 488-conjugated goat anti-rabbit IgG (H + l) antibody (green fluorescence) and 4′,6 diamidino-2-phenylindole (DAPI; by which nuclei were counterstained) (blue). Immunostaining was visualized using a fluorescence microscope (original magnifications: × 400). Arrows point to the lymphocytes which are negative for CXCR3 ligands. Freshly frozen lung sections derived from three patients with stage II of sarcoidosis were also stained with control rabbit serum (e), anti-Mig/CXCL9 (f), IP-10/CXCL10 (g) or I-TAC/CXCL11 antibody (h) and RTU Vectastain Universal Quick Kit, and developed with diaminobenzidine (DAB) substrate (original magnifications: × 200). The insets in e, f, g and h show the staining of macrophages in the alveolar lumen (original magnifications: × 400). Scale bars = 50 µm.
Increased production of Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11 by alveolar macrophages from patients with sarcoidosis
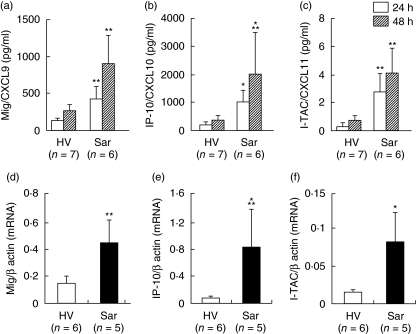
Immunostaining showed that alveolar macrophages are one of the crucial producers of CXCR3 ligands. We therefore examined the production of CXCR3 ligands by alveolar macrophages harvested from HV and patients with sarcoidosis. Alveolar macrophages harvested from patients with sarcoidosis had the ability to spontaneously produce a higher amount of all CXCR3 ligands compared with those from HV (Fig. 5). Alveolar macrophages from patients with sarcoidosis also expressed higher amounts of mRNAs of all CXCR3 ligands than those from HV (Fig. 5).
Fig. 5.
Expression of monokine induced by interferon γ (Mig)/CXCL9, interferon-inducible protein-10 (IP-10)/CXCL10 and interferon-inducible T cell α-chemoattractant (I-TAC)/CXCL11 by alveolar macrophages harvested from patients with sarcoidosis. Alveolar macrophages were harvested from healthy volunteers (HV) or patients with sarcoidosis, and cultured without stimulation for 24 or 48 h. The supernatants were harvested, and the concentrations of Mig/CXCL9 (a), IP-10/CXCL10 (b) and I-TAC/CXCL11 (c) were measured with enzyme-linked immunosorbent assay (ELISA). Data are shown as mean ± standard deviation (s.d.). Total RNA was also extracted from alveolar macrophages in HV or patients with sarcoidosis. The real-time quantitative reverse transcription–polymerase chain reactions (RT–PCRs) for Mig/CXCL9 (d), IP-10/CXCL10 (e) and I-TAC/CXCL11 (f) were performed as described in Methods. Data are shown as mean ± s.d. of relative expression compared to β-actin. *P < 0·005 versus HV groups, **P < 0·01 versus HV groups, ***P < 0·05 versus HV groups.
Discussion
In the present study, we examined the levels of Mig/CXCL9 and I-TAC/CXCL11 as well as IP-10/CXCL10 in BALF and serum in patients with different stages of sarcoidosis. IP-10/CXCL10, which is one of the CXCR3 ligands, has been reported to play crucial roles in Th1-immune responses in sarcoid lungs [11,13]. However, three ligands for CXCR3 including Mig/CXCL9, IP-10/CXCL10 and I-TAC/CXCL11, have been identified [9]. In fact, co-expression of these chemokines in the lungs has been reported in mouse [23,24] as well as in humans [25]. In addition, there are some differences in the levels and time-courses of expression of these CXCR3 ligands [23–25]. These findings prompted us to study the expressions of Mig/CXCL9 and I-TAC/CXCL11 in sarcoidosis. We observed the same levels of Mig/CXCL9 as of IP-10/CXCL10 in BALF, whereas the concentration of I-TAC/CXCL11 was below the detectable level in most cases. More recently, Pignatti et al. studied the BAL fluid of eight patients with sarcoidosis and was not able to detect the expression of I-TAC/CXCL11 [26]. Our results seem to be consistent with their report.
On the other hand, the concentration of I-TAC/CXCL11 in serum was detectable but low, being average one-tenth of the levels of Mig/CXCL9 and IP-10/CXCL10. Interestingly, no significant difference was found among the concentrations at the various stages of sarcoidosis and HV for serum IP-10/CXCL10, although Mig/CXCL9 and I-TAC/CXCL11 were elevated at stage II of sarcoidosis. The reason for this is not clear, but IP-10/CXCL10 is likely to increase at various conditions in the host. In particular, the differences in sex and age between HV and patients with sarcoidosis might affect these results, because we found some HV with a high level of IP-10/CXCL10 in the serum.
There is little information regarding patients with the different stages of sarcoidosis. Capelli et al. reported that the highest level of macrophage inflammatory protein (MIP)-1α at stage III and MIP-1β at stage II of sarcoidosis were observed [10]. Shigehara et al. reported that IL-18 was high at stages I and II [27]. In the present study, Mig/CXCL9 and IP-10/CXCL10 in BALF were highest at stage II, suggesting that these CXCR3 ligands were involved in the active phase of granulomatous responses in the lungs. Recently, Takeuchi et al. reported that serum levels of two CXCR3 ligands, Mig/CXCL9 and IP-10/CXCL10, correlated with disease activity of ocular sarcoidosis [28]. They showed that there was no significant difference in the levels of Mig/CXCL9 and IP-10/CXCL10 between in patients with ocular sarcoidosis with bilateral hilar lymphadenopathy (BHL) and in those without BHL. Considering these findings, our data revealed that the patients with stage II, but not stage I, of pulmonary sarcoidosis showed the elevated levels of serum Mig/CXCL9, suggests that pulmonary infiltrations might be related with the elevation of serum Mig/CXCL9. Further analysis, including ocular sarcoidosis, would be useful to clarify the role of CXCR3 ligands in sarcoidosis.
Alveolar macrophages appeared to be the main producers of CXCR3 ligands in the sarcoid lungs as alveolar macrophages harvested from BAL expressed high levels of CXCR3 ligands, and were stained by immunostaining using the three anti-CXCR3 antibodies. The epithelioid cells and giant cells also appeared to produce these CXCR3 ligands because positive staining of these cells was observed in immunohistochemistry, as in the cases of IL-12, IL-18 [29] and thioredoxin [30]. As these CXCR3 ligands are expressed in other cells, such as endothelial cells [31] and bronchial epithelial cells [32], further studies are required to explore the expression of CXCR3 ligands in sarcoid lungs.
The level of I-TAC/CXCL11 was ∼10-fold lower than those of Mig/CXCL9 and IP-10/CXCL10 in both BALF and serum, and 100–1000-fold lower in the supernatants of alveolar macrophages. However, we cannot rule out the possibility that I-TAC/CXCL11 may play a role in the accumulation of Th1 cells in the sarcoid lungs as I-TAC/CXCL11 has ∼100-fold higher affinity to CXCR3 and twice-higher chemotactic activity towards stable CXCR3 transfectants when compared with the other CXCR3 ligands [19]. Furthermore, the strongest staining was observed when anti-I-TAC/CXCL11 antibody was used for immunostaining. The forward study would be required to clarify this point.
It has been reported that several Th1-related cytokines and chemokines such as IL-12, IFN-γ, macrophage inflammatory protein (MIP)-1α, MIP-1β and CXCL16 were elevated in BALF of patients with sarcoidosis [7,10,29,33]. In the present study, the levels of CXCR3 ligands in BALF were correlated positively with the number of CD4+ lymphocytes. These data suggest that CXCR3 ligands, in addition to other Th1-related factors, act as important chemotactic factors for CD4+ Th1 cells in sarcoid lungs as most CD4+ cells (more than 90%) in BAL cells in patients with sarcoidosis were reported to express CXCR3 [12].
In summary, the results of the present study suggest that Mig/CXCL9 and I-TAC/CXCL11, in addition to IP-10/CXCL10, which are produced from alveolar macrophages, epithelioid and giant cells, play a role in the accumulation of Th1 cells into sarcoid lungs.
Acknowledgments
The authors thank Ms Tomoko Oka for her technical assistance. This work was supported by a grant-in-aid for scientific research by the Ministry of Education, Science, Sports and Culture, Japan.
References
- 1.American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders. Statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Costabel U. Sarcoidosis: clinical update. Eur Respir J. 2001;18:s56–68. [PubMed] [Google Scholar]
- 3.Baughman RP, Lower EE, du Bois R. Sarcoidosis. Lancet. 2003;361:1111–18. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 4.Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60:565–82. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–24. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahlstrom J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–21. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 7.Prasse A, Georges CG, Biller H, et al. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol. 2000;122:241–8. doi: 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergeron A, Bonay M, Kambouchner M, et al. Cytokine patterns in tuberculous and sarcoid granulomas: correlation with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–43. [PubMed] [Google Scholar]
- 9.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelli A, di Stefano A, Lusuards M, Gnemmi I, Donner CF. Increased macrophage inflammatory protein-1α and macrophage inflammatory protein-1β levels in bronchoalveolar lavage fluid of patients affected by different stages of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2002;165:236–41. doi: 10.1164/ajrccm.165.2.2106084. [DOI] [PubMed] [Google Scholar]
- 11.Agostini C, Cassatella M, Zambello R, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–20. [PubMed] [Google Scholar]
- 12.Katchar K, Eklund A, Grunewald J. Expression of Th1 markers by lung accumulated T cells in pulmonary sarcoidosis. J Intern Med. 2003;254:564–71. doi: 10.1111/j.1365-2796.2003.01230.x. [DOI] [PubMed] [Google Scholar]
- 13.Miotto D, Christodoulopoulos P, Olivenstein R, et al. Expression of IFN-γ-inducible protein; monocyte chemotactic proteins 1, 3, and 4; and eotaxin in TH1- and TH2-mediated lung disease. J Allergy Clin Immunol. 2001;107:664–70. doi: 10.1067/mai.2001.113524. [DOI] [PubMed] [Google Scholar]
- 14.Tani K, Ogushi F, Huang L, Kawano T, Tada H, Hariguchi N, Sone S. CD13/aminopeptidase N, a novel chemoattractant for T lymphocytes in pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000;161:1636–42. doi: 10.1164/ajrccm.161.5.9902008. [DOI] [PubMed] [Google Scholar]
- 15.Manabe K, Nishioka Y, Kishi J, et al. Elevation of macrophage-derived chemokine in eosinophilic pneumonia: a role of alveolar macrophages. J Med Invest. 2005;52:85–92. doi: 10.2152/jmi.52.85. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka Y, Yano S, Fujiki F, et al. Combined therapy of multidrug-resistant human lung cancer with anti-P-glycoprotein antibody and monocyte chemoattractant protein-1 gene transduction: the possibility of immunological overcoming of multidrug resistance. Int J Cancer. 1997;71:170–7. doi: 10.1002/(sici)1097-0215(19970410)71:2<170::aid-ijc8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Farber JM. A macrophage mRNA selectively induced by γ-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87:5238–42. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luster AD, Unkeless JC, Ravetch JV. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 19.Cole KE, Strick CA, Paradis TJ, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto H, Yano S, Zhang H, et al. Activity of a new vascular targeting agent, ZD6126, in pulmonary metastases by human lung adenocarcinoma in nude mice. Cancer Res. 2002;62:3711–5. [PubMed] [Google Scholar]
- 21.Zhao DX-M, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-γ-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell α chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J Immunol. 2002;169:1556–60. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- 22.Nishioka Y, Nishimura N, Suzuki Y, Sone S. Human monocyte-derived and CD83+ blood dendritic cells enhance NK cell-mediated cytotoxicity. Eur J Immunol. 2001;31:2633–41. doi: 10.1002/1521-4141(200109)31:9<2633::aid-immu2633>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt GC, Corrion LA, Olkiewicz KM, et al. Blockade of CXCR3 receptor : ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173:2050–9. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 24.Nance S, Cross R, Fitzpatrick E. Chemokine production during hypersensitivity pneumonitis. Eur J Immunol. 2004;34:677–85. doi: 10.1002/eji.200324634. [DOI] [PubMed] [Google Scholar]
- 25.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–49. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 26.Pignatti P, Brunetti G, Moretto D, et al. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. Am J Respir Crit Care Med. 2006;173:310–7. doi: 10.1164/rccm.200502-244OC. [DOI] [PubMed] [Google Scholar]
- 27.Shigehara K, Shijubo N, Ohmichi M, et al. Increased levels of interleukin-18 in patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000;162:1979–82. doi: 10.1164/ajrccm.162.5.9911113. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi M, Oh IK, Suzuki J, et al. Elevated serum levels of CXCL9/monokine induced by interferon-γ and CXCL10/interferon-γ-inducible protein-10 in ocular sarcoidosis. Invest Ophthalmol Vis Sci. 2006;47:1063–8. doi: 10.1167/iovs.05-0966. [DOI] [PubMed] [Google Scholar]
- 29.Shigehara K, Shijubo N, Ohmichi M, et al. IL-12 and IL-18 are increased and stimulate IFN-γ production in sarcoid lungs. J Immunol. 2001;166:642–9. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 30.Koura T, Gon Y, Hashimoto S, et al. Expression of thioredoxin in granulomas of sarcoidosis: possible role in the development of T lymphocyte activation. Thorax. 2000;55:755–61. doi: 10.1136/thorax.55.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx N, Mach F, Sauty A, et al. Peroxisome proliferator-activated receptor-γ activators inhibit IFN-γ-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–8. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauty A, Dziejman M, Taha RA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–58. [PubMed] [Google Scholar]
- 33.Agostini C, Cabrelle A, Calabrese F, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–8. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]