Abstract
The role of the liver in the initiation and maintenance of tolerance is a critical immune function that involves multiple lineages of immune cells. Included within these populations are liver dendritic cells (DCs). Although there has been significant work on the phenotypic and functional roles of splenic and bone marrow dendritic cells, as well as their subsets, comparable studies in liver have often been difficult. To address this issue we have isolated, from C57BL/6 mice, relatively pure populations of DCs and compared phenotype and function to the data from spleen using flow cytometry, cell sorter assisted purification and culture, morphology by cytospin and May–Giemsa staining, cell cycle progression, antigen uptake, cytokine production and allo-activation potential. natural killer (NK)1·1–CD11c+ liver DC subsets (conventional DCs, T cell receptor (TcR)β–NK1·1–CD11c+B220– and plasmacytoid DCs, TcRβ–NK1·1–CD11c+B220+) efficiently endocytose dextran and produce significant levels of tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-12 p40 in response to Toll-like receptor (TLR) ligands, with responses higher than splenic DCs. There is also a differential capability of hepatic DCs to respond to innate signals. Indeed, CD11c+ hepatic DCs have a greater capacity to respond to innate stimulation but are less capable of inducing CpG activated-allogeneic T cells. These data suggest that hepatic dendritic cells function as a critical bridge between innate and adaptive immunity and are capable of inducing stronger innate responses with a lower capacity for allo-stimulation than splenic dendritic cells. These properties of liver dendritic cells contribute to their unique role in the induction of tolerance.
Keywords: allo-stimulation, hepatic cDC, innate immune response, pDC
Introduction
The liver is responsible for filtering blood which contains both self-antigens and environmentally derived antigens ingested orally [1], and initiates preferentially tolerance to a large quantity of pathogens it encounters every day; these functions are attributable to the liver as a lymphoid organ, including sinusoidal endothelial cells, Kupffer cells and dendritic cells (DCs) [2–5]. Multiple DC subsets have been identified in both mice and humans and work has focused on understanding the mechanisms by which DC subsets in liver distinguish antigens to deliver tolerogenic signals compared with those that require activation of innate and adaptive immunity, or both. Unlike splenic DCs that are considerably potent in phagocytosis and express low levels of co-stimulatory molecules, progress in understanding hepatic DCs has been slow, due primarily to their scarcity and the technical difficulties associated with isolation of these DCs [6,7]. To address these issues further, we performed a comprehensive phenotypic and functional comparison of hepatic and splenic DCs and their subsets under normal steady state and after Toll-like receptor (TLR) ligand stimulation. Our results imply that hepatic DCs serve to bridge the transition from innate and adaptive immunity and induce better innate responses but less allo-stimulation than splenic DCs. We submit that these populations contribute to their unique properties in the induction of tolerance.
Materials and methods
Mice
C57BL/6 J (H-2b, B6) and BALB/CJ (H-2d) female mice (8–12 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME, USA), maintained under pathogen-free conditions and studied at 8–12 weeks of age. The data showed no difference between female and male mice.
Isolation of liver and spleen mononuclear cells
Livers were first perfused with phosphate-buffered saline containing 0·2% bovine serum albumin (PBS/0·2% BSA), passed through a nylon mesh, and resuspended in PBS/0·2% BSA. Hepatocytes were removed as pellets after centrifugation for 1 min at 100 g and washed with PBS/0·2% BSA twice at 500 g for 5-min intervals. Spleen cells were disrupted between two glass slides and resuspended in PBS/0·2% BSA. Lymphocytes from suspended liver and spleen cells were then isolated using Histopaque-1077 (Sigma Chemical Co., St Louis, MO, USA). After centrifugation, cells were washed with PBS/0·2% BSA and the viability of cells confirmed using trypan blue dye exclusion.
Flow cytometry staining and analysis
Briefly, an aliquot (1–5 × 105) of freshly isolated lymphocytes was resuspended in staining buffer (0·5% BSA, 0·05% sodium azide in PBS) and preincubated with FcR blocking reagent (except for CD16/32 staining) (Biolegend, San Diego, CA, USA) for 15 min at 4°C. Immunofluorescent labelling was performed as described previously [8]. The frequency of cells expressing individual and/or sets of cell surface markers and the mean fluorescence intensity (MFI) of expression of such markers was determined by analysing a minimum of 50 000 cells using CellQuestPro software (BDBiosciences, San Jose, CA, USA). The following unconjugatedor directly conjugated monoclonal antibodies (mAbs) were used: purified anti-CD16/CD32 (FcII/IIIR, 93) (Biolegend); fluorescein isothiocyanate (FITC)-labelled T cell receptor (TcR)β (H57-597), Sca-1 (E13-161·7), CD4 (GK1·5), TLR2 (CD282, 6C2) from BD PharMingen (San Jose, CA, USA); phycoerythrin (PE)-labelled TcRβ (H57-597), natural killer (NK)1·1 (PK136), TLR4 (MTS510) (eBioscience), B7-DC (TY25) and CD19 (6D5) (Biolegend); PE/Cy5 anti-CD11c (N418) (eBioscience); allophycocyanin-labelled anti-CD19 (MB19-1) and CD11c (HL3) (BD PharMingen); allophycocyacin (APC)/Cy7-labelled anti-B220(CD45R, RA3–6B2) (BD PharMingen); biotin-labelled anti-CD4 (GK1·4), CD8 (53–6·7), CD11b (Mac-1, M1/70), CD19 (1D3), CD16/32 (FcIII/IIR, 2·4G2), CD80 (B7·1, 16–10A1), CD86 (B7·2, GL1), CD117 (c-kit, 2B8), TcRβ-chain (H28-710), H-2Kb[major histocompatibility complex (MHC) class I, AF6-88·5], CD45RA (14·8) (BD PharMingen) and NK1·1 (P36) (e-Bioscience). Biotinylated antibodies were stained secondarily with streptavidin–PE/Cy5. All isotype controls were obtained from BD PharMingen.
Cell sorter assisted purification and culture of liver and splenic DC
Briefly, splenic cells were isolated and incubated with a predetermined optimum dilution of rat mAbs against CD3, followed by sheep anti-rat IgG-conjugated magnetic beads (Dynabeads; Invitrogen, Carlsbad, CA, USA). After CD3 cell depletion, the residual splenic cells or hepatic non-parenchymal cells were incubated with a predetermined optimal amount of anti-CD16/CD32 at 4°C for 5 min to block the non-specific FcR, and then stained with the following mAbs, FITC anti-TcRβ, PE anti-NK1·1, PE/Cy5 anti-CD11c, APC anti-CD19 and PE/Cy7 anti-B220, for sorting. After washing twice, cells were sorted using a 10-parameter MoFlo cell sorter (Cytomation, Fort Collins, CO, USA) into two groups: NK1·1–,TcRβ–, B220+, CD11c+ (pDC) and NK1·1–, TcRβ–, B220–, CD11c+ (cDC). The purity of sorted cells, based on the above phenotypic expression of cell surface markers, was always > 97%. Aliquots of sorted DC were cultured in 200 µl of complete RPMI-1640 medium (Invitrogen) containing 10% fetal calf serum (FCS), 20 mM HEPES, 2-mercaptoethanol (2-ME), penicillin and streptomycin in U-bottomed 96-well plates.
Morphology by cytospin and May–Giemsa staining
Freshly sorted cDCs and pDCs from liver non-parenchymal cells and splenocytes were cytospun onto glass slides (5 min at 230 g) and fixed in methanol for 15 min at room temperature (RT), washed and stained with Giemsa (Sigma) and photographed.
Detection of cell cycle progression
Cell cycle progression was detected using the bromodeoxyuridine (BrdU) flow kit (BD Pharmingen). Two hours after intravenous (i.v.) injection of BrdU (1 mg/ml PBS), spleen or liver lymphocytes were first stained with PE-labelled anti-TcRβ, CD19 and anti-NK1·1, APC-labelled anti-CD11c+, and APC/Cy7-labelled anti-B220. Cells were fixed with Cytofix/Cytoperm buffer, and permeabilized with Cytoperm Plus buffer. Cells were then incubated again with Cytofix/Cytoperm buffer (BD Pharmingen), followed by treatment with DNase to expose BrdU epitopes and stained with FITC-labelled anti-BrdU and 7-amino-actinomycin D (7-AAD) dye (BrdU flow kit; BD Pharmingen). Finally, cell cycle progression was analysed by five-colour FACScan (BD PharMingen) that had been upgraded by Cytek Development (Fremont, CA, USA).
Antigen uptake
Mice were anaesthetized and injected i.v. with 2·5 mg of FITC-labelled dextran (Sigma) in 100 µl of PBS per mouse. Dendritic cells from mice injected with FITC–dextran were analysed at various time-points for FITC-positive expression by flow cytometry. The fraction of FITC-positive cells was calculated.
Cytokine production
To induce cytokine production, aliquots of 1 × 105 liver or splenic DCs were cultured in vitro with the following stimuli: 10 µg/ml peptidoglycan (PGN) from Staphylococcus aureus, 50 µg/ml polyinosinic–polycytidylnic acid [poly(I:C)], 10 µg/ml lipopolysaccharide (LPS) from Salmonella minnesota serotype Re595, 1 µg/ml flagellin, 1 µg/ml FSL-1 (Pam2CGDPKHPKSF), 300 µg/ml Loxorobine, 1 µg/ml R848 or 2 µM CpG ODN 1826 (InvivoGen, San Diego, CA, USA), in complete RPMI-1640 for 48 h at 37°C in 5% CO2. An equal volume of media containing the appropriate TLR ligands was added to duplicate wells of DC cultures. In addition, a baseline control containing media only was included to assess any inadvertent activation of DCs caused by the isolation method used. Supernatants were analysed for interleukin (IL)-6, IL-10, monocyte chemoattractant protein (MCP)-1, interferon (IFN)-γ, tumour necrosis factor (TNF)-α and IL-12 p70 using a cytometric bead array kit (CBA kit; BD Biosciences). The level of IL-12 p40 was measured by enzyme-linked immunosorbent assay (ELISA) (R&D, Minneapolis, MN, USA).
Assessment of allo-activation potential by DCs
Freshly sorted DC subsets from B6 (H-2b) mice were co-cultured with CD4+ T cells isolated from BALB/C (H-2d) mice. Briefly, a lymphocyte suspension derived from a pool of lymph nodes (LN) and spleen was overlaid onto Histopaque-1·077 and centrifuged for 20 min at 750 g. Low-density lymphocytes were collected from the interface, washed with PBS and resuspended in complete medium. CD4+ T cells were purified from the low-density lymphocytes by positive selection with CD4+ MicroBeads and MiniMacs (Miltenyi Biotec). Aliquots of 1 × 105 purified CD4+ T cells were co-cultured in duplicate with 2 × 104 DCs in 200 µl of complete medium with or without 2 µM CpG 1826 for 4 days in U-bottomed 96-well plates. [3H]-Thymidine (1 µCi/well) was added during the last 18 h of culture and cells were harvested using a Skatron (Molecular Devices, Sunnyvale, CA, USA). The levels of 3-Tdr incorporated by the T cells was measured in a liquid scintillation counter (PerkinElmer Life Sciences, Boston, MA, USA) and the mean uptake of [3H]-thymidine deoxyribose was determined.
Statistics
The significance of differences between means was determined using the unpaired Student's t-test.
Results
Classification of hepatic and splenic conventional DC (cDC) and plasmacytoid DC (pDC) subsets
Several studies of hepatic DCs have used CD11c and B220 as cell surface markers to identify cDC and pDC subsets [8–10]. However, these are also expressed to a variable extent on NK and B cells, respectively. In addition, contradictory results in terms of the function and phenotype of these DCs have been presented by different groups using various additional surface markers to identify these DC subsets. In order to characterize these two populations more carefully, liver and spleen lymphoid cells were stained with the following surface markers: NK1·1, TcRβ, CD11c and B220. Neither T (TcRβ+ NK1·1–) nor NK T (TcRβ+ NK1·1+) cells expressed CD11c (Fig. 1a). CD11c was expressed on NK cells (TcRβ–NK1·1+) from both the liver and spleen. In order to eliminate NK cell contamination, a TcRβ–NK1·1– population was gated to sort for either cDC or pDC based on B220 and CD11c expression. While a higher frequency (85·6%) of spleen cells were identified as cDCs (TcRβ–, NK1·1–, B220–, CD11c+) than among liver lymphoid cells (55·8%), there were fewer pDCs (TcRβ–, NK1·1–, B220+, CD11c+) identified in spleen (14·3%) than in the liver (44·1%) (Fig. 1b). The ratio of cDC to pDC in the liver (1·27 ± 0·14) was found to be about five times lower than the ratio in the spleen (6·01 ± 0·46) (Fig. 1c, P < 0·001).
Fig. 1.
Identification of plasmacytoid dendritic cells (pDC) and conventional (cDC) subsets in liver and spleen. Freshly isolated murine liver non-parenchymal mononuclear cells and splenocytes were surface-stained with anti-T cell receptor (TcR)β, anti-natural killer (NK)1·1, anti-B220 and anti-CD11c monoclonal antibody. (a) Four populations were separated based on TcRβ and NK1·1 expression. CD11c+ was also expressed on NK1·1+ cells. Therefore, CD11c+ DCs were gated from the TcRβ and NK1·1 double negative population. (b) pDC and cDC from spleen and liver were gated from TcRβ–NK1·1–CD11c+ population with labelled anti-B220. Percentages from a representative experiment of pDC (TcRβ–NK1·1–CD11c+B220+) and cDC (TcRβ–NK1·1–CD11c+B220–) are shown. (c) Ratio of cDC to pDC in spleen and liver.
Comparison of liver and splenic DC subsets surface phenotype and morphology
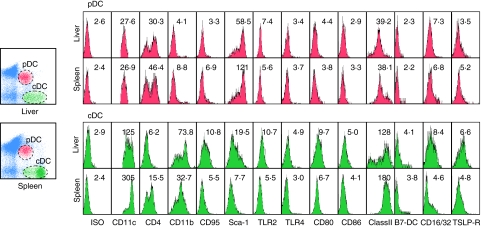
DC subsets from normal liver and spleen lymphoid cells were stained with antibodies against a panel of monoclonal antibodies which included CD11c, CD4, CD11b, CD95, Sca-1, TLR2, TLR4, CD80, CD86, MHC class II, B7-DC, CD16/32, and thymic stromal lymphopoietin receptor (TSLP-R). Controls consisted of similarly conjugated isotype antibodies. A markedly different cell surface marker profile between liver and spleen cDC from the same cohort of mice is shown in Fig. 2. Thus, while cDCs from the liver consisted of both a lower and higher MFI of MHC class II and CD11b-expressing cells, the splenic cDCs expressed a generally high MFI of both these molecules. Hepatic cDCs also expressed higher MFI of the T cell co-stimulatory molecules, CD80 (9·59 ± 0·21) and CD86 (5·14 ± 0·18), than spleen cDCs (6·67 ± 0·72 and 4·12 ± 0·12, respectively). In addition, Sca-1**, CD16/32***, B7-DC***, TSLP-R**, TLR2*** and TLR4** were also expressed at a relatively higher level on hepatic cDCs than in splenic cDCs (**P < 0·01, ***P < 0·001). While the pDCs identified from the liver and spleen had no significant differences in the expression of co-stimulatory molecules, the hepatic pDCs expressed lower levels of CD4, CD11b, CD95,Sca-1 and TSLP-R than spleen pDCs (P < 0·05) (Fig. 2 and Table 1).
Fig. 2.
Surface phenotypes of the two dendritic cell (DC) subsets from liver and spleen. Hepatic mononuclear cells and splenocytes were isolated and stained with phycoerythrin (PE)-T cell receptor (TcR)β, PE/Cy5-CD11c, allophycocyanin (APC)-natural killer (NK)1·1 and APC/Cy7-B220, followed by staining with biotin-labelled antibodies for major histocompatibility complex (MHC) class II, CD80, CD86, CD40, or CD16/32. Others were stained with one of the following fluorescein isothiocyanate (FITC)-conjugated antibodies: isotype, CD4, CD11b, CD11c, CD95, Sca-1 and Toll-like receptor (TLR)2. The histograms depict the specific staining by mean fluorescence intensity (MFI) of the DC subsets in liver or spleen. This experiment has been repeated twice with similar results.
Table 1.
Comparison of the phenotypes of plasmacytoid dendritic cells (pDC) and conventional (cDC) in liver and spleen.
| pDC | cDC | |||
|---|---|---|---|---|
| Liver | Spleen | Liver | Spleen | |
| ISO | 2·42 ± 0·22 | 2·33 ± 0·09 | 3·01 ± 0·13 | 2·29 ± 0·06 |
| CD4 | 34·24 ± 5·58* | 45·39 ± 1·00 | 6·36 ± 0·23*** | 15·91 ± 0·76 |
| CD11b | 4·09 ± 0·04* | 8·05 ± 1·08 | 73·79 ± 3·35*** | 32·55 ± 2·52 |
| CD11c | 27·38 ± 0·38 | 25·83 ± 0·96 | 149·50 ± 35·29** | 298·70 ± 12·42 |
| CD95 | 3·65 ± 0·51* | 6·84 ± 0·98 | 12·38 ± 2·22* | 5·73 ± 0·25 |
| Sca-1 | 71·51 ± 18·29* | 122·6 ± 11·22 | 21·03 ± 2·16** | 7·30 ± 0·62 |
| TLR2 | 7·41 ± 0·06** | 5·72 ± 0·29 | 10·84 ± 0·12*** | 5·39 ± 0·15 |
| TLR4 | 3·14 ± 0·38 | 3·76 ± 0·16 | 4·46 ± 0·55** | 3·03 ± 0·13 |
| Class II | 40·94 ± 8·41 | 38·84 ± 2·03 | 127·9 ± 4·89*** | 177·6 ± 8·25 |
| CD80 | 3·74 ± 0·95 | 3·62 ± 0·28 | 9·59 ± 0·21* | 6·67 ± 0·72 |
| CD86 | 3·03 ± 0·18 | 3·31 ± 0·06 | 5·14 ± 0·18** | 4·12 ± 0·12 |
| B7-DC | 2·24 ± 0·06 | 2·30 ± 0·06 | 4·11 ± 0·11*** | 2·24 ± 0·09 |
| CD16/32 | 7·46 ± 0·18 | 6·73 ± 0·47 | 8·52 ± 0·11*** | 4·43 ± 0·37 |
| TSLP-R | 3·73 ± 0·32* | 4·91 ± 0·35 | 6·81 ± 0·37** | 5·02 ± 0·17 |
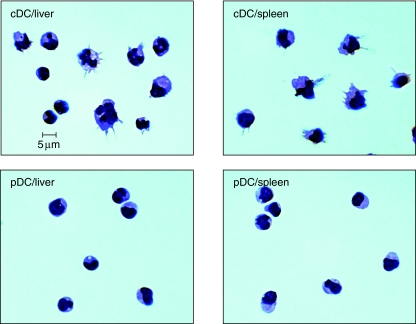
Both liver and spleen pDCs demonstrated a homogeneous morphology with a relatively smooth surface, round shape and a prominent large nucleus. In contrast to pDCs, hepatic cDCs have both a mature population with many pronounced dendrite processes and irregular or hyper-lobulated nuclei and an immature population that appear similar to pDCs (Fig. 3). Splenic cDCs in general appeared more homogeneous with mature features.
Fig. 3.
Morphology of dendritic cell (DC) subsets from liver and spleen. Cytospin preparations of fluorescence activated cell sorter (FACS)-sorted liver and spleen plasmacytoid dendritic cells (pDCs) and conventional (cDCs) were stained with May–Giemsa and photographed at 100×. Heterogeneous populations including immature cDCs with pronounced dendritic processes, compared to pDCs, were found in both liver and spleen.
Cell cycle analysis of DC subsets under normal steady state
A relatively higher frequency of hepatic cDCs (5·60 ± 0·82%) are in G2/M phase than spleen cDCs (2·57 ± 0·54%, P < 0·001). On the other hand, the percentage of hepatic pDCs in G2/M and S phase are lower (4·57 ± 1·18% and 0·84 ± 0·33%, P < 0·001) than spleen pDCs (8·65 ± 1·51% and 2·19 ± 0·88%, P < 0·05). A representative profile of hepatic and splenic cDCs and pDCs is shown in Fig. 4a and the summary in Fig. 4b.
Fig. 4.
Comparison of the cell cycle progression in dendritic cell (DC) subsets from liver and spleen. T cell receptor (TcR)β–NK1·1– were gated to determine bromodeoxybromidine (BrdU) and 7-amino-actinomycin D (7-AAD) expression on plasmacytoid dendritic cell (pDC) and conventional (cDC) subsets in liver and spleen. To simplify analysis, BrdU and 7-AAD-labelled cells were divided into three groups: G1/G0 phase, S phase and G2/M phase. (a) Each of the three phases in liver and spleen DC subsets from one representative experiment, of the BrdU positive cells are indicated by flow cytometry. (b) The percentage of cells in each phase is depicted in bar graph. This experiment has been repeated twice with similar results. Data were expressed as the mean ± standard deviation. *P ≤ 0·05 by t-test; **P ≤ 0·01; ***P ≤ 0·001 (Student's t-test).
Hepatic DCs engulf more antigen than splenic DCs in vivo
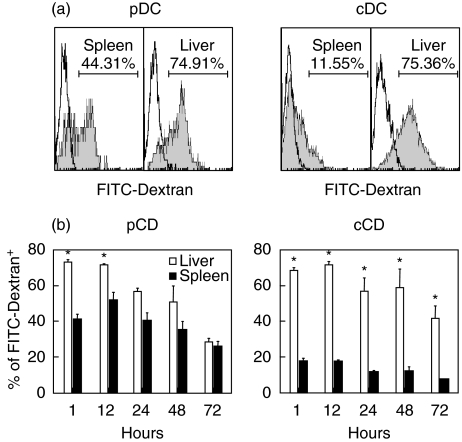
We reasoned that differences noted in the maturation status within different subsets of hepatic and liver DCs would correlate with the relative capability of each subset to engulf antigens. To test the endocytotic ability of cDCs and pDCs isolated from the liver and spleen, FITC-labelled dextran was injected into normal C57BL/6 mice in vivo. The relative MFI of FITC–dextran expression in hepatic DCs was clearly higher than splenic DCs by flow cytometry (Fig. 5a). We found that hepatic pDCs (74·91%) captured more dextran than splenic pDCs (44·31%) from 1 to 12 h post-injection. Similarly, cDCs from the liver (75·36%) had better capture ability than splenic cDCs (11·55%) up to 72 h after injection (Fig. 5b, P < 0·05).
Fig. 5.
Analysis of the function of endocytosis in plasmacytoid dendritic cells (pDCs) and conventional (cDCs) from liver and spleen. 2·5 mg/100 µl of fluorescein isothiocyanate (FITC)–dextran were injected per mouse. Dendritic cells (DCs) from mice injected with FITC–dextran were analysed various time-points for FITC-positive expression by flow cytometry. (a) The grey-filled histograms depict the positive FITC–dextran from the splenic and hepatic DCs compared with the unfilled histogram for isotype from one representative experiment. Mean fluorescence intensity (MFI) of dendritic cells (DCs) labelled with positive FITC–dextran is shown. (b) The percentage of pDCs or cDCs from liver (open bar) and spleen (solid bar) positive for FITC–dextran were measured and compared at various time-points (h). This experiment has been repeated twice with similar results. Data were expressed as the mean ± standard deviation and were consistent in two repetitions. *P ≤ 0·05 by Student's t-test.
More inflammatory cytokine production from hepatic DCs after TLR ligand stimulation
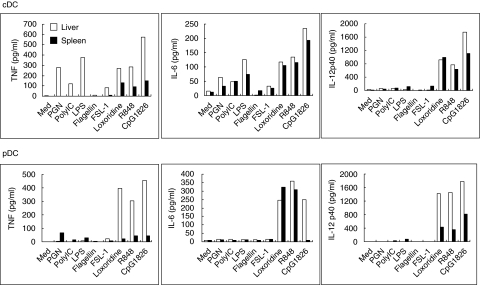
Freshly isolated cDCs and pDCs from the liver and spleen were cultured in vitro with TLR ligands and the levels of IFN-γ, IL-6, TNF-α and IL-12 p40 in the supernatant measured after 48 h of co-culture. TNF-α and IL-6 production by hepatic cDCs was up-regulated by all the TLR ligands tested except flagellin (TLR5). In contrast, only Loxorobrine (TLR7), R848 (TLR7, 8) and CpG1826 (TLR9) could stimulate TNF-α production from splenic cDCs (Fig. 6a). However, this production was less than that produced by hepatic cDCs. Similarly, hepatic pDCs produced more TNF-α (> 100-fold) and IL-12 p40 (two- to fourfold) after stimulation with Loxorobrine, R848 or CpG1826 than splenic pDCs (Fig. 6b). IFN-γ was undetectable in the supernatant fluid from either hepatic or splenic DCs (data not shown).
Fig. 6.
Liver (open bar) and spleen (solid bar) dendritic cells (DCs) in vitro cytokine secretion profiles. Liver and spleen DCs were isolated and cultured at a concentration of 1 × 105 cells/ml in complete RPMI-1640 with or without various T cell receptor ligands at 37°C. After 48 h, the supernatants were collected and tested for the presence of tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, interferon (IFN)-γ, monocyte chemoattractant protein (MCP)-1 and IL-12p40. IL-10, IFN-γ, and MCP-1 levels were undetectable. Bar graphs from one representative experiment are shown here. Data were consistent in three repetitions.
Less T cell allo-activation capacity by CpG-activated DC subsets from the liver than spleen
The levels of MHC class II expression and differences in the maturation state between hepatic and splenic DCs prompted us to examine the potential of these subsets to induce allogeneic T cell activation. Therefore, we compared the capacity to stimulate allogeneic T cells by both hepatic and splenic DC subsets in vitro. Highly enriched populations of hepatic pDCs and cDCs induced approximately half as much allogeneic T cell proliferation as did splenic pDCs and cDCs after CpG 1826 stimulation for 5 days (Fig. 7, P < 0·05).
Fig. 7.
Allogenic stimulation capacity of dendritic cell (DC) subsets from liver (open bar) and spleen (solid bar). DCs (2 × 104) were cultured with 105 allogenic CD4+ T cells for 4 days in the presence of 2 µM CpG 1826, with [3H]-thymidine added for the last 18 h. Cells were harvested and incorporation of [3H]-thymidine was measured. Data were consistent in three repetitions. *P ≤ 0·05 by Student's t-test.
Discussion
Hepatic DCs play a critical role in maintaining tolerance [11–13] and hence their role in autoimmune diseases has been a subject of intense study [14,15]. In the present study, we extend our earlier work by comparing the function of these hepatic cells to that of splenic DCs. Previously, subsets of DCs in mice have been identified phenotypically with the use of CD11b, CD11c, B220, CD8 and CD4 [16]. However, the use of CD11c as a DC marker is problematic because it is not expressed exclusively on DCs as it is also found on NK cells, but by a number of other cells types as well [17]. In the liver, NK cells make up about 7–8% of non-parenchymal cells from normal healthy mice [1] and both DCs and NK cells are central players in the early phase of an immune response. During an infection, DC-derived cytokines, such as IFN-γ, IL-2 and IL-12, can induce production of IFN-γ by NK cells and enhance NK cell cytotoxic function [18]. Contamination by CD11c+ NK cells accounts for IFN-γ production in CD11c+ DC cultures [19]. Thus, CD11c+ NK cell contamination can easily affect the interpretation of results related to the role of DCs in the liver. In order to avoid NK cell contamination, our freshly isolated CD11c+ DC were fluorescently labelled with NK1·1 antibody, allowing us to gate out NK cells (Fig. 1). This is consistent with the undetectable level of IFN-γ from either hepatic or splenic DCs in our study (data not shown).
An innate immune response is generated by DCs when pathogen-associated molecular patterns (PAMP) or pathogen-derived products are recognized via TLRs [20,21]. Upon TLR ligation, DCs up-regulate surface co-stimulatory molecules, secrete cytokines and chemokines, enhance antigen presentation and migrate to secondary lymphoid tissues. Like DCs in other organs, specific receptors are also required by hepatic DCs to recognize PAMP [22,23]. While these receptors, TLR2, 3, 4, 7 and 9, are found on both cDCs and pDCs in the liver, TLR3 is not expressed on hepatic pDCs [24,25]. In contrast to previous TLR4 reverse transcription–polymerase chain reaction (RT–PCR) data, we note herein a higher expression of TLR2 and TLR4 on hepatic cDCs than splenic cDCs (Fig. 2) [25]. In addition, our data show that hepatic cDCs produce more TNF-α and IL-6 after PGN and LPS stimulation, which correlates with their higher TLR2 and TLR4 expression, than splenic cDCs. In our system, hepatic DCs produced substantial amounts of inflammatory cytokines, such as TNF-α, IL-6 and IL-12, upon TLR ligand stimulation with Loxorobrine, R848 and CpG (Fig. 6a,b). This is consistent with previously published data where strong innate immune responses by hepatic DCs were observed [26].
Based on the higher levels of cytokine produced by hepatic compared with splenic DC in response to TLR stimulation, we expected hepatic DCs to be more effective stimulators of naive allogeneic CD4+ T cells than splenic DCs. However, we found that TLR ligand-stimulated, murine hepatic DCs are relatively weaker stimulators of naive allogeneic CD4+ T cells compared to splenic DCs (Fig. 7). It is of interest to note the heterogeneity of MHC class II expression by the cDCs but not pDCs from the liver and pDCs but not cDCs from the spleen. Thus, while the hepatic cDCs had at least two populations, one with a medium and the other with relatively lower levels of MHC class II-expressing cells, the splenic pDCs had a population of medium but with the second population showing a higher MFI of MHC class II expression. On the other hand, the hepatic DCs appeared to express higher relative densities of co-stimulatory molecules CD80/86 (Fig. 2). It is possible that such differences in the levels of MHC class II expression could contribute to the differences noted in the levels of allo-activation. In addition, B7-DC, one of the recently described B7 family members that has the capacity to inhibit T cell responses, also appeared to express higher MFI on hepatic cDCs than splenic cDCs [27]. The data are also consistent with the observation that hepatic CD4+ T cells do not respond to hepatic DCs as efficiently as they do to splenic DCs [28]. In addition, hepatic CD4+ T cells have been shown to proliferate to a similar extent upon antigen stimulation as do splenic CD4+ T cells [29]. Based on our results, hepatic CD4+ T cells are not activated directly by hepatic DCs because our hepatic DCs were poor T cell activators under culture conditions. However, hepatic CD4+ T cell activation may be assisted or enhanced by hepatic DC cytokine production. This supports the thesis that, within the liver, it is important to have DCs which can bridge innate and adaptive immune responses to the same antigen.
It is generally believed that only a mature DC can prime and drive T cell proliferation [30]. Therefore, weaker allo-stimulation may be due to the maturation state of the DCs involved. Indeed, upon examining the morphology of our DC subset, cDCs from the liver are more heterogeneous with both immature and mature features than splenic cDCs, which are homogeneous mature DCs (Fig. 3). Immature DCs are considered to possess the endocytosis capacity of soluble antigens and present them to T cells. This is consistent with the endocytosis data where hepatic cDCs had better capture ability than splenic cDCs up to 72 h after i.v. injection with FITC–dextran (Fig. 5). One explanation for the increased endocytosis by hepatic DCs compared with splenic DCs is that the injection of FITC–dextran may traffic differently to liver. Previous work has shown that immature DCs may play an important role in maintaining tolerance by preventing T cell auto-activation by mature DCs [31,32]. Because the maturation process of immature DCs occurs in response to antigen stimulation, the maturation and activation status of the DCs is critical at the time they encounter antigens [16]. We demonstrate that the subset compositions in liver and spleen are quite different. In terms of the percentage of total hepatic DCs (CD11c+, NK1·1–, TcRβ–), the heterogeneous populations with relatively high immature pDCs (44·2%) in liver may explain the low T cell responsiveness observed in our study.
Our present results vary from previously published results not only because we excluded NK1·1+ cells in the present study, but also because we did not treat our freshly isolated DCs with granulocyte–macrophage colony-stimulating factor (GM-CSF). GM-CSF is an important growth factor for DCs in vitro. Over-expression of GM-CSF leads to DC expansion in normal mice, with skewed increasing percentage of the myeloid DC (CD8α–CD11b+) subset, which is believed to be another subset of the more mature cDCs. This disparity may be the reason why, although we demonstrate that hepatic DCs are more immunogenic than splenic DCs in the early phase of an immune response, we show that hepatic DCs are less capable of inducing allogeneic CD4+ T cell immune response than splenic DCs. Finally, our conclusions support the thesis that hepatic DCs are important stimulators of innate immune responses to antigens within the portal circulation.
Acknowledgments
We thank Carol Oxford from the UC Davis Medical Center Flow Cytometry Core Facility for assistance with cell sorting. This study was supported by National Institutes of Health grant DK39588.
References
- 1.Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7:99–104. doi: 10.1097/00075198-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–90. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
- 3.Wiegard C, Frenzel C, Herkel J, Kallen KJ, Schmitt E, Lohse AW. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology. 2005;42:193–9. doi: 10.1002/hep.20756. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Hawiger D, Liu K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann NY Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 5.Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2006;56:243–52. doi: 10.1136/gut.2006.093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 7.Kamath AT, Pooley J, O'Keeffe MA, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–70. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 8.Lian ZX, Okada T, He XS, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–30. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 9.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 10.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–6. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 12.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 13.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and toll-like receptors. Crit Rev Immunol. 2006;26:291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 14.Lian ZX, Kikuchi K, Yang GX, Ansari AA, Ikehara S, Gershwin ME. Expansion of bone marrow IFN-alpha-producing dendritic cells in New Zealand Black (NZB) mice: high level expression of TLR9 and secretion of IFN-alpha in NZB bone marrow. J Immunol. 2004;173:5283–9. doi: 10.4049/jimmunol.173.8.5283. [DOI] [PubMed] [Google Scholar]
- 15.Okada T, Inaba M, Naiki M, Lian ZX, Gershwin ME, Ikehara S. Comparative immunobiology of thymic DC mRNA in autoimmune-prone mice. J Autoimmun. 2007;28:41–5. doi: 10.1016/j.jaut.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 17.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl. 1):S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 18.Granucci F, Zanoni I, Pavelka N, et al. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–95. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–7. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 21.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–7. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 24.Malmgaard L. Dendritic cells, toll-like receptors, and T-cell responses: lessons from viral infections in vivo. Viral Immunol. 2005;18:584–94. doi: 10.1089/vim.2005.18.584. [DOI] [PubMed] [Google Scholar]
- 25.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–45. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 26.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 27.Shin T, Yoshimura K, Crafton EB, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–41. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe M, Akbar SM, Horiike N, Onji M. Induction of cytokine production and proliferation of memory lymphocytes by murine liver dendritic cell progenitors: role of these progenitors as immunogenic resident antigen-presenting cells in the liver. J Hepatol. 2001;34:61–7. doi: 10.1016/s0168-8278(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 29.Katz SC, Pillarisetty VG, Bleier JI, et al. Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology. 2005;42:293–300. doi: 10.1002/hep.20795. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]