Abstract
There has been no systematic study of the immune response of individuals aged over 60 years living in Schistosomiasis mansoni-endemic areas, although senescence is reportedly associated with susceptibility to infection and progressive decline in immune function. We have shown previously, in two endemic areas in Minas Gerais, Brazil, that the frequency of individuals aged over 60 years with chronic schistosomiasis is no longer negligible. Moreover, several elderly individuals who have always lived in these endemic areas stay protected from infection. An important question for studies of ageing and disease control in developing countries is which differences in the immunological profile of these negatively tested (non-infected) individuals can account for their resistance to either infection or reinfection. We show, in the present study, that non-infected (negative) elderly individuals develop innate immune mechanisms of protection that replace the age-associated decline in T cell function. Non-infected elderly individuals from endemic areas of schistosome infection present an increase in the frequency of the natural killer (NK) CD56low subset of NK cells expressing Toll-like receptors (TLR)-1, -2, -3 and -4 as determined by flow cytometry analysis. In addition, the proportion of dendritic cells expressing TLR-1 is elevated as well as the frequency of monocytes expressing TLR-1 and -4. These results suggest that TLR expression by cells of the innate immune system may be related to the negative status of infection in some elderly individuals who are constantly exposed to S. mansoni. Developing mechanisms of protection from infection may represent a biomarker for healthy ageing in this population.
Keywords: ageing, innate immunity, NK cells, schistosome infection, Toll-like receptors
Introduction
Studies on the relationship between age and schistosome infection in humans usually show that there is a typical rise in infection intensity during adolescence and a subsequent decline as the population in endemic areas ages. Usually, partial immunity to infection is acquired by adults between 25 and 49 years of age, with a further decline in infection seen in the following decades. Recently, both Webster and coworkers [1] and our group [2] have reported that another rise in infection intensity occurs among the elderly, suggesting a loss of this partially acquired immunity by elderly people. There has been no systematic study of the immune response of individuals aged over 60 years residing in Schistosomiasis mansoni-endemic areas, although senescence is reportedly associated with susceptibility to infection and progressive decline in immune function. Several age-related immunologically linked alterations have already been described in the medical literature, mainly regarding the T cell compartment [3]. They include involution of the thymus, reduction in the number of CD3+ cells with a parallel increase of oligoclonally expanded CD4+ cells with a memory phenotype, reduced potential to produce interleukin (IL)-2 and loss of CD28 expression [4,5]. Changes in B cells include a decline in production of naive B lymphocytes [6]. Therefore, ageing is associated with an accumulation of activated T cells and a decrease in the ability to mount immune responses to novel antigens.
The development of effector CD4+ T helper cells is important for the outcome of helminth infection and most chronically infected individuals in endemic areas of S. mansoni infection present a poor proliferative response to parasite antigens [7]. Reports on young and middle-aged individuals show that interferon (IFN)-γ production by T cells is important in the protective immune response to reinfection [8]. The role of B cells and specific IgE in resistance to infection is less established [9]. Recently, an increase in worm-IgE was seen mainly in > 15-year-olds and, unlike in children, was correlated inversely with infection intensity, suggesting that this response was associated with immune protection [10].
Because ageing is associated with a decline in T cell function, it is not surprising that individuals aged over 60 years would be more susceptible to infection. However, not all elderly individuals in endemic areas have a high intensity of infection; some of them display a negative stool-screening test for the presence of schistosome eggs, suggesting that they are not infected. An important question for studies of ageing and disease control in developing countries concerns which differences in the immunological profile of these negatively tested individuals can account for their resistance to either infection or reinfection.
It has already been documented that innate immune responses, differently from T cell function, are more resistant to change with ageing [11]. Natural killer (NK) cells are especially well preserved in healthy elderly subjects and the age-related increase in CD16+ CD57– NK cells with high cytotoxiciy capacity has been correlated with successful ageing [12,13]. We have reported recently that the frequency of IFN-γ-producing CD16+ NK cells in non-infected individuals over the age of 70 years is significantly higher than in infected individuals from an endemic area for schistosome infection [14]. As IFN-γ has been related to resistance to infection, our data suggest that age-associated changes in NK cells may counterbalance the decline in T cell function in these individuals and play a role in protective immunity. The nature of the interaction between NK cells and S. mansoni antigens is not known, but it is likely that components of innate recognition play a role in the response. Monocytes and macrophages as well as NK cells express pattern recognition receptors (PPR), namely scavenger and Toll-like receptors (TLR) [15].
The Toll-like receptor family is the best-characterized class of pattern recognition receptors that signal the presence of infection to the host. Most mammalian species have 10–15 TLRs and they detect multiple pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS, detected by TLR-4), bacterial lipoproteins and lipteichoic acids (detected by TLR-2), double-stranded RNA (detected by TLR-3), flagellin (detected by TLR-5), the unmethylated CpG DNA of bacteria and viruses (detected by TLR-9) and single-stranded viral RNA (detected by TLR-7) [16,17]. Expression of several types of TLRs has already been described in mammalian cells that participate in innate immunity (such as NK cells and macrophages) as well as in lymphocytes and dendritic cells (DC) [18,19]. Although there are data on the age-associated alterations on TLR expression in several cell types in mice [20], the influence of ageing in human TLR function and expression has not yet been well explored. A defect in production of tumour necrosis factor (TNF)-α and IL-6 by monocytes of elderly people after TLR-1/2 ligation, when compared with production by cells of young controls, has been reported recently. This defect in TLR-1/2 signalling may result from alterations in baseline TLR-1 surface expression in monocytes, which is decreased in older adults, whereas TLR-2 expression is unaffected by ageing [21].
Recent reports show that interaction of antigens from S. mansoni with TLR-2, -3 and -4 in DC promotes their differentiation into mature cells with distinct functions. Double-stranded RNAs activate inflammatory cytokine expression in DC through TLR-3 [22]. On the other hand, lysophosphatidylserine from eggs as well as from adult worms binds to TLR-2 [23], whereas the lacto-N-fucopentose III group of carbohydrates in S. mansoni is a ligand for TLR-4. Both types of antigen promote DC expression of the T helper 2 (Th2) cytokines IL-10 and IL-4 [24].
To investigate the putative role of these receptors in the immune response of elderly to S. mansoni infection, using flow cytometry we analysed the expression of TLR-1, -2, -3 and -4 in monocytes, NK and DC of infected and non-infected individuals of different age groups.
Subjects and methods
Ethical considerations
Our study was submitted and approved for ethical clearance by the Internal Review Board (IRB) of the World Health Organization (WHO-2002) and by the ethical committee of FIOCRUZ (Belo Horizonte). All subjects signed the informed consent and infected individuals received treatment with praziquantel 20 mg/kg of weight.
Subjects
Areas endemic for S. mansoni were identified from routine prevalence surveys undertaken by the Foundation for National Health (FUNASA). Fifty-five subjects who live in two endemic areas (Travessão and Caju, Minas Gerais, Brazil) were analysed in a cross-sectional study. From this total number of subjects, 17 individuals (nine non-infected and eight infected) live in Travessão and were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Thirty-six individuals (22 non-infected and 14 infected) live in Caju, and were divided into two age groups: 19–59 (adults) and 68–94 years (elderly).
Extensive water contact studies performed in these endemic areas showed that exposure measurements in total body minutes are not statistically different among age groups, nor are they different among individuals with distinct levels of infection. Participants were matched by gender and infection status in different groups (two age groups and two groups for infection status). Detailed information on the studied population is presented in Table 1. Negative individuals (non-infected) were considered to be those with no egg counts and only individuals with > 100 eggs/g of faeces were included in the positive (infected) group. Categories of infection are in accordance with the WHO/CDS/SIP (World Health Organization/Communicable Diseases/Schistosomiasis and Intestinal Parasitoses). Of note, two female individuals (aged 29 and 41 years) presented 1684 and 1724 eggs/g of faeces, and this can account for the large standard deviation shown by the adult positive group. These two individuals were not excluded from the study, because they showed average variability in the parameters tested compared to the others in the same group. None of the participants was affected by neoplastic or autoimmune diseases or was receiving chemotherapy that would impair immune function. All the infected (positive) subjects had the intestinal form of schistosomiasis, as determined by the absence of hepatic alterations on ultrasonography. Subjects classified as ‘intestinal’ were also defined as having eggs in their faeces with no clinical symptoms other than occasional intestinal discomfort.
Table 1.
Groups, age and egg counts of individuals in the study sample from Travessão and Caju, two areas in Brazil endemic for schistosomiasis.
| No. of individuals | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| hr/ | |||||||||
| Neg | Pos (> 100 egg) | EPG (average ± s.d.) | |||||||
| hr/ | hr/ | hr/ | |||||||
| Groups | Age (mean ± s.d.) | Total | F | M | Total | F | M | Neg | Pos (> 100 egg) |
| Adult | 15–59 (33·9 ± 11·8) | 16 | 12 | 4 | 16 | 9 | 7 | 0 | 416 ± 531·1 |
| Elderly | 60–94 (74·9 ± 8·7) | 15 | 6 | 9 | 8 | 4 | 4 | 0 | 139·56 ± 82·69 |
Neg = negative; Pos = positive.
Although infected individuals (adults and especially the elderly) are still common in some areas of Brazil, most of them have been already treated for S. mansoni infection at some point in their lives as a result of the extensive scientific research and health programmes that have been undertaken in past years on S. mansoni infection in Brazil. Our study aimed to investigate the immunological status of individuals living in unexplored endemic areas and who had never been treated for S. mansoni infection. Nowadays, these areas are few and untreated individuals rare, which is the main reason why we had to work with a low number of subjects (n = 3–5) in most of the studies (Figs 1–5).
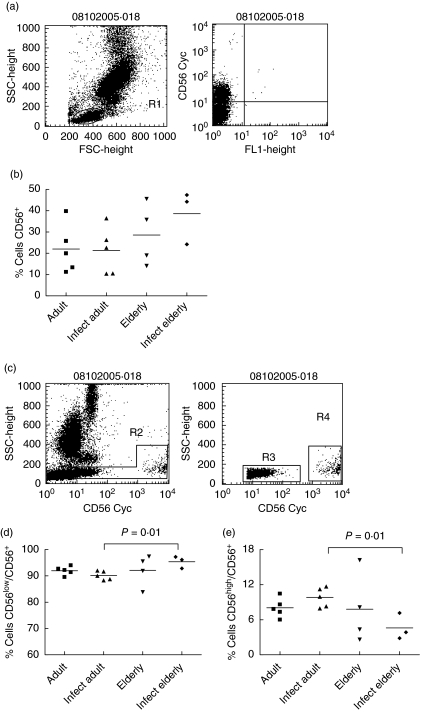
Fig. 1.
Frequency of CD56+, CD56low and CD56high natural killer (NK) cells in individuals from Travessão, an area in Brazil endemic for schistosomiasis. Nine non-infected and eight infected individuals were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. (a) NK cells were identified in flow cytometry studies by their light-scatter–side-scatter (SSC) and forward-scatter (FSC) characteristics and by the expression of CD56 (gated R1). (b) There is a trend to an increase in the frequency of CD56+ cells in non-infected and infected individuals aged over 60 years compared to adult individuals. (c) Two major NK subsets were also analysed: CD56low (gated R3) and CD56high (gated R4) cells. (d) The proportion of CD56low cells increased and (d) the proportion of CD56high declined in infected elderly individuals compared to individuals in the infected adult group. Infect adult = infected adult and Infect elderly = infected elderly.
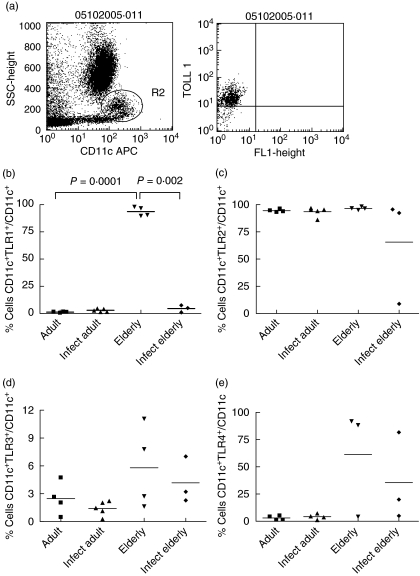
Fig. 5.
Frequency of Toll-like receptor (TLR)-1, TLR-2-, TLR-3- and TLR-4-expressing dendritic cells (DC) in individuals from Travessão, an area in Brazil endemic for schistosomiasis. Nine non-infected and eight infected individuals were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. (a) DC were detected in flow cytometry studies by their characteristic profile at the light scatter and by expression of CD11c. (b) Frequency of CD11c+ expressing TLR-1 was increased to almost 100% in elderly non-infected individuals compared to non-infected adult and infected elderly groups. Frequencies of TLR-2-, TLR-3- and TLR-4-expressing DC were not different among groups (c, d, e). Infect adult = infected adult and Infect elderly = infected elderly.
Test for infection status
Infection status was determined by the presence or absence of eggs on faecal samples. Individuals from the endemic area were asked for 3 consecutive days of faecal samples. Slide preparation of the stool samples occurred within 24 h of collection using the Kato–Katz thick smear technique, with two slides prepared from each day's stool sample. Faecal egg counts for S. mansoni were measured by counting the number of eggs per slide and then determining the arithmetic mean of the eggs found in six slides (i.e. two from each of 3-day samples) as described by Katz and coworkers [25].
Flow cytometry study
Fifty μL of the blood was incubated with cy-chrome-labeled anti-CD56, APC-labeled anti-CDIIC, fluorescein isothiocyanate (FITC)-labeled anti-CD14 (BD-pharmiycn, San Diego, CA, USA), phycoerythrinepe-labeled anti-TLR1, -TLR2, -TLR3, TLR4 (eBioscience, San Diego, CA, USA) monoclonal antibodies in 1 : 20 dilution. After incubation, the red cells were lysed with commercial solution [fluorescence activated cell sorter (FACS) lysing solution; Becton Dickinson, San Diego, CA, USA], washed and fixed using Macs Facs Fix solution. NK cells were detected by their characteristic profile in the light scatter and by the expression of CD56. DC and monocytes were identified by forward- and side-angle scatter on a FACScan flow cytometer (Becton Dickinson) and by the expression of CD11c and CD14, respectively. At least 20 000 events were analysed using cellquest software (Becton Dickinson).
Statistical analysis
Statistical analysis was performed using spss version 12·0 software (SPSS Inc., Chicago, IL, USA). Data were expressed in percentage. Each variable was converted to normal distribution by transformation into LN(X/100/(1-(X/100). Statistical differences were assessed using an unpaired t-test when comparing two groups. The analysis of variance was used with multiple measures. Significance was considered at the level of 5%.
Results
Frequency of CD56low NK cells was increased and CD56high NK cells was decreased in infected elderly individuals
To evaluate the impact of senescence and infection in innate immune components, we first analysed the frequency of NK cells of 17 individuals from Travessão, an area endemic for S. mansoni infection (details in Subjects section and Table 1).
NK cells were identified in flow cytometry studies by their light-scatter–side-scatter (SSC) and forward-scatter (FSC) characteristics and by the expression of CD56 and (Fig. 1a). Figure 1b shows that there was a significant increase in the frequency of CD56+ cells in non-infected (negative) individuals aged over 60 years compared to individuals of all other groups. Two major NK subsets differing in the expression of CD56 on the cell surface have been described so far: CD56low and CD56high (Fig. 1c). These two subsets represent functionally distinct populations of cells with different migration patterns [26]. To characterize further the changes in NK in these groups of individuals, frequencies of the two subsets were analysed separately. Our data show that the proportion of CD56low NK cells rose (Fig. 1d) and the frequency of CD56high NK cells declined in infected elderly individuals (Fig. 1e) compared to the infected adult group. Our results suggest that the frequency of NK cells and their subsets was not related to infection status in elderly individuals per se. On the other hand, there is a trend to an increase in the frequency of NK cells in the elderly group and the lack of significance may be due to the low number of individuals in the study sample.
Frequencies of TLR-1-, -2-, -3- and -4-expressing CD56+ NK, CD56low NK and CD56high NK cells varied with ageing and infection status
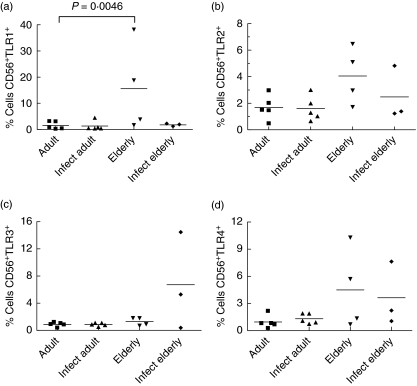
Although the number of individuals in the group was small, there was a significant increase in the frequency of TLR-1+ CD56+ cells in the non-infected elderly compared to the non-infected adult groups (Fig. 2a). No significant difference was observed in TLR-2-, -3- and -4-expressing CD56+ NK cells (Fig. 2b–d).
Fig. 2.
Frequencies of Toll-like receptor (TLR)-1, TLR-2, TLR-3 and TLR-4-expressing CD56+ natural killer (NK) cells in individuals from Travessão, an area in Brazil endemic for schistosomiasis. Nine non-infected and eight infected individuals were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. (a) There was a significant increase in the frequency of CD56+ TLR-1+ in non-infected elderly people compared to the non-infected adult groups. No difference was observed in the frequency of NK cells expressing either TLR-2, TLR-3 or TLR-4 among the groups (b, c, d). Infect adult = infected adult and Infect elderly = infected elderly.
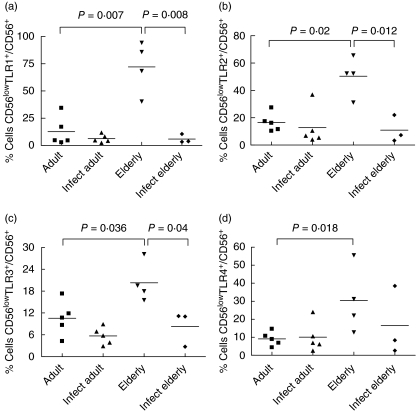
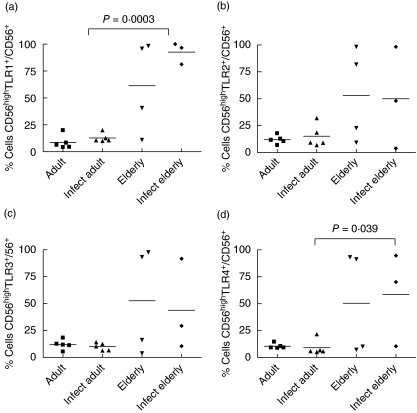
In Fig. 3, analysis of frequency of TLR-expressing cells within the subset of CD56low NK cells is shown. There was a significant increase in the non-infected elderly in the frequency of TLR-1+, TLR-2+, TLR-3+ and TLR-4+ cells of this subset compared to the adult non-infected group (Fig. 3a–d) and a rise in the frequency of TLR-1+, TLR-2+, TLR-3+ cells compared to infected elderly individuals (Fig. 3a–c).
Fig. 3.
Frequencies of Toll-like receptor (TLR)-, TLR2-, TLR3- and TLR4-expressing CD56low natural killer (NK) cells in individuals from Travessão, an area in Brazil endemic for schistosomiasis. Nine non-infected and eight infected individuals were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. There is an increase in the frequency of CD56low TLR-1+ (a), CD56low TLR-2+ (b) and CD56low TLR-3+ (c) cells in non-infected elderly individuals compared to non-infected adults and infected elderly subjects. Frequency of CD56low cells expressing TLR-4 was different only between healthy elderly and healthy adult individuals (d). Infect adult = infected adult and Infect elderly = infected elderly.
In the subset of CD56high NK cells, one major age-related alteration was observed: the increase in the frequency of TLR-1- and -4-expressing CD56high cells in infected elderly people when compared with infected adults (Fig. 4a,d). The differences in TLR-2- and -3-expressing cells among groups were not significant (Fig. 4b,c).
Fig. 4.
Frequencies of Toll-like receptor (TLR)-1, TLR-2-, TLR-3- and TLR-4-expressing CD56high natural killer (NK) cells in individuals from Travessão, an area in Brazil endemic for schistosomiasis. Nine non-infected and eight infected individuals were divided into two age groups: 15–59 (adults) and 60–77 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. There was an increase in the frequency of TLR-1- and TLR-4-expressing CD56high cells in infected elderly people compared to the infected adult group (a, d). Frequencies of CD56high cells expressing either TLR-2 and TLR-3 were not different among groups (b, c). Infect adult = infected adult and Infect elderly = infected elderly.
Frequency of TLR-1-expressing DC was up-regulated in the elderly
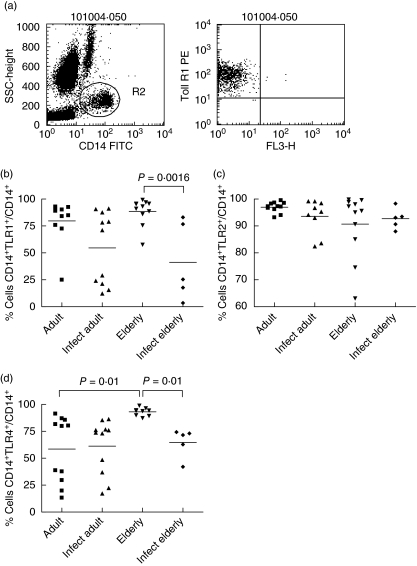
DC were detected by their characteristic profile at the light scatter and by the expression of CD11c (Fig. 5a). We observed a very low frequency of CD11c+ blood cells in adult individuals that express TLR-1, -3 or -4. At the same time, there was a high frequency of CD11c+ TLR-2+ cells in individuals from all groups. However, our data show that frequency of CD11c+ DCs expressing TLR-1 was increased to almost 100% in elderly non-infected individuals (Fig. 5b). Of note, the standard deviation was very low, indicating a consistent change in all individuals. A trend in the frequencies of TLR-3- and TLR-4-expressing DCs to rise was also observed.
Frequencies of TLR-1- and TLR-4-expressing monocytes were up-regulated in the elderly
We have analysed the frequency of CD14+ cells positive for TLR-1, -2 and -4 in peripheral blood cells of 36 individuals from Caju, another area endemic for S. mansoni infection in Brazil (details in the Subjects section and Table 1). Monocytes were detected by their typical profile at the light scatter and by the expression of CD14 (Fig. 6a). Contrary to what was observed in DC, there were high frequencies of CD14+ blood cells in adult individuals that express TLR-1, -2, -3 or -4. Frequencies of TLR-1- and TLR-4-expressing monocytes were both increased in non-infected elderly compared to infected elderly people (Fig. 6b,d) and there was also a significant difference between TLR-4-expressing monocytes in non-infected elderly compared to non-infected adults (Fig. 6d). Differences in TLR-2 expression among groups were not significant (Fig. 6c).
Fig. 6.
Frequencies of Toll-like receptor (TLR)-1, TLR-2 and TLR-4-expressing monocytes in individuals from Caju, an endemic area for schistosomiasis in Brazil. Twenty-two non-infected and 16 infected individuals were divided into two age groups: 19–59 (adults) and 68–94 years (elderly). Statistical analysis was performed as explained in the Subjects and methods section. Each point represents one individual. (a) Monocytes were detected in flow cytometry studies by their characteristic profile at the light scatter and by expression of CD14. (b) Frequency of CD14+ cells expressing TLR-1 was increased in elderly non-infected individuals compared to the infected elderly group. (c) The frequency of TLR-2-expressing monocytes was not different among groups. (d) The frequency of CD14+ cells expressing TLR-4+ was increased when compared to both the infected elderly and the non-infected groups. Infect adult = infected adult and Infect elderly = infected elderly.
Discussion
In times of demographic transition globally, studies on ageing and disease control in endemic areas have to address two major challenges. First, most endemic diseases, such as schistosomiasis, are lifelong conditions with a chronic phase that overlaps the process of senescence. Secondly, elderly individuals present several dysfunctions in their immune responses and vaccine strategies are usually designed for younger populations.
It is well documented that ageing is associated with a decline in immune function, mainly in the activities of T cells. Fewer early progenitor B cells are also correlated with ageing [6]. Dysfunction of APCs is observed and may represent a result of multiple failures of an ageing environment [27]. Recently, a new approach to the study of ageing emerged from the data collected from centenarians. These data show that senescence is not necessarily associated with deterioration of immune function, and that many immunological activities can be replaced by compensatory mechanisms [28,29]. The concept of healthy ageing comes from these studies and innate immune responses seem to be a key example of these mechanisms. Innate immunity is well preserved in senescence and this is reflected in the number and cytotoxicity activity of NK cells that seem to be increased in healthy elderly people and centenarians [12,30,31].
Developing countries, such as Brazil, are experiencing an unprecedented growth in the number of their elderly people; as such there is an increasing population of elderly people living in endemic areas for infectious diseases. Previous studies from our group have shown that there are individuals aged over 70 years in areas endemic for schistosome infection who are tested negative for the presence of S. mansoni eggs [14,32,33]. The concept of healthy ageing has an immediate consequence for studies on ageing and endemic diseases. Non-infected, negative individuals may be considered as an example of healthy ageing as they were able to develop compensatory mechanisms to cope with immune dysfunction and to generate protective responses against the constant threat of infection in these areas.
NK cells are defined commonly as CD3–/TCR– large granular lymphocytes that express CD56 and/or CD16 and mediate non-major histocompatibility complex (MHC)-restricted cytotoxic functions [34]. The two major NK cell subsets, CD56low and CD56high, have been described recently as separate populations of cells with distinct features [26]. We found no statistical significance in the frequency of CD56+ NK cells among the groups. However, a trend to elevated frequency of CD56+ NK cells was observed in the two groups of elderly people (infected and non-infected), confirming several previous studies that show a rise in the frequency of NK cells with ageing [11–13]. It is likely that the low number of individuals analysed in our study was a hindrance to reaching a statistically significant difference. The frequency of CD56low NK cells was augmented and CD56high diminished in infected individuals aged over 60 years. On one hand, our data suggest that a balance between the two populations may correlated with the infection status in the elderly. On the other hand, it has already been reported that the ageing-associated rise in the frequency of CD56+ NK cells is due mainly to an increase in the subset of CD56low cells [35]. Therefore, it is plausible that we found statistical significance only in infected subjects due to a lower dispersion of the individuals in this group compared to subjects in the non-infected group. Because in our previous study, as well in the present study, we found no association between negatively tested elderly individuals and increased numbers of NK cells, our main hypothesis was that the activity of NK cells may have a more profound impact in the protection against infection in elderly people than the number of cells.
Indeed, in a recent study on ageing and chronic schistosomiasis, we have shown that the frequency of IFN-γ-producing CD16+ NK cells in negative (non-infected) individuals over the age of 70 years is significantly higher than in positive (infected) individuals after in vitro stimulation with S. mansoni antigen extracts. Analysis of CD4+ and CD8+ T cells under the same culture conditions show no increase in IFN-γ+ cells in this age group, suggesting that the effect on CD16+ cells is not T cell-dependent [14]. Furthermore, cells were stimulated in vitro for only 5 h and this short period of time is not enough for antigen presentation and activation of T cells. Therefore, NK cells interacted directly with S. mansoni antigens to generate the IFN-γ response that was measured. These results indicate that IFN-γ-producing NK cells may be involved in the protective mechanisms that maintain the non-infected status in negative elderly individuals in spite of their constant exposure to infection.
Our previous data also opened the question of the nature of the interaction between NK cells and parasite antigens. One possibility is that NK cells bind IgG-bound antigens. However, the higher frequency of IFN-γ-producing NK cells in non-infected individuals [14] suggests that other molecules may mediate the direct interaction and activation of these cells. In the present study, we addressed this question by analysing the frequencies of subsets of NK cells that express different TLRs in elderly versus adult individuals. Our hypothesis was that these receptors participate in the interaction between NK cells and S. mansoni antigens as they are key elements of innate recognition of patterns in infectious agents. In addition, we examined the expression of TLRs in other cell types such as monocytes and DC. TLR-3 is usually expressed intracellularly. However, since extracellular dSRNA from S. Mansoni eggs can activate DC through TLR-3 ligation (22), we tested surface expression of TLR-3 in DC and NK cells.
Overall, our results show that the frequency of TLR-expressing NK cells vary mainly in the group of non-infected elderly subjects. There was a low frequency of CD56+ cells expressing TLR-1, -2, -3 or -4 in adult subjects (approximately 2%) and infection status did not change this situation. However, a 12-fold increase in the average frequency of CD56+ TLR-1+ NK cells was detected. A trend is also visible towards an increase in TLR-2-, TLR-3- and TLR-4-expressing CD56+ NK cells in elderly individuals of both groups, suggesting that TLR expression in NK cells correlates with ageing.
When CD56+ NK cells were analysed as separate subsets of CD56high and CD56low cells, we observed a rise in the frequency of CD56low cells expressing TLR-1, -2, -3 and -4. On the contrary, we have detected an increase in the frequency of NK CD56high cells expressing TLR-1 and TLR-4 in infected elderly individuals. As this latter population is present at a very low frequency (around 5%) in infected elderly individuals, the significance of this increase is limited and may not have a similar impact in immunity to the increase in the main CD56low NK cell population. There was also a general trend to increase in the frequencies of CD56high cells expressing all TLRs tested in elderly individuals (infected and non-infected), suggesting that this elevation may correlate better with ageing than with infection status.
Usually, CD56low cells predominate in blood whereas CD56high cells are more frequent in organs and tissues. CD56high cells express higher levels of molecules with co-stimulatory functions such as CD2, CD7, CD38 and CD44, and this may be related to a higher ability of these cells to respond to different stimuli. On the other hand, the absence or low intensity of expression of the Fc gamma receptor CD16 (a receptor for IgG-bound immune complexes) may explain why these cells are less efficient than CD56low cells in mediating antibody-dependent cytotoxicity (ADCC). Overall, NK56high cells exhibit a phenotype suggestive of cell activation that is IL-2-dependent as reflected by a higher fraction of cells expressing CD25 (IL-2Ra chain) and their constitutive expression of the IL-2Rb chain, CD122. They display increased cytotoxicity activity in the presence of low doses of IL-2 and higher proliferation capacity than CD56low cells. CD56low cells have a high ability for ADCC and high expression of CD57, an oligosaccharide that is expressed in the late stages of T cell activation [26]. Their phenotypic profile is compatible with a cell type involved mainly in functional activities that are T cell-independent. It is interesting that, in our study, non-infected elderly individuals presented an increase in the frequency of a subset of NK cells that act independently of IL-2, a T cell-related cytokine. As ageing is associated with a clear decline in T cell function and a reduced potential to produce IL-2 [36], CD56low cells may represent an alternative innate mechanism of cytotoxicity and protective immunity for elderly individuals living in endemic areas. In addition, the fact that we found previously increased numbers of IFN-γ+ NK cells [14] in this same group of individuals suggests that CD56low cells may act both via ADCC and IFN-γ production.
Similarly, we detected a significant increase in the frequency of CD11c+ DC expressing TLR-1 but not TLR-2+, TLR-3+ and TLR-4+ in non-infected elderly individuals. Of note, there is an increased trend in the frequency of TLR-3- and TLR-4-expressing DC and it is likely that the low number of individuals in our study groups hindered the statistically significance of these differences. Previous reports in murine [37] and human models [38,39] have demonstrated that DC enhance the cytolytic activity of resting NK cells. A recent study using human peripheral blood monocytes showed bidirectional cross-talk between NK cells and DCs, resulting in activation of both cell types [40]. It is plausible that, in healthy elderly individuals living in endemic areas, TLR-1 expression has a role in this type of interaction and mutual activation. As DC are in the interface between innate and adaptive immunity, a possibility exists that TLR-1-expressing DC may participate in age-related compensatory mechanisms not only by triggering innate immune responses, but also by optimizing antigen presentation that is known to be impaired in the elderly [41,42].
Higher frequencies of monocytes expressing TLR-1 and TLR-4 were also identified in non-infected elderly individuals within our study sample. In mice, ageing is associated with a profound decline in TLR expression by spleen and peritoneal macrophages. In addition, these cells secreted lower levels of IL-6 and TNF-α when stimulated with TLR ligands, supporting the hypothesis that these alterations may be related to the higher susceptibility to infection observed in elderly animals [20]. Although the secretion of proinflammatory cytokines such as IL-6, IL-1 and TNF-α by in vitro-stimulated peripheral mononuclear blood cells of elderly subjects is usually higher, their macrophages show impaired respiratory burst and reactive nitrogen intermediate production with a decreased ability to destroy pathogens [41,42]. In our study, increasing the frequencies of TLR-1- and TLR-4-expressing monocytes may represent a successful innate strategy to cope with pathogenic challenge by non-infected elderly individuals.
Although most of these TLRs are involved in the recognition of bacterial and viral products, their participation in the interaction with helminth antigens has been reported only recently. Using high-density oligonucleotide microarrays, Aksoy et al. demonstrated that two components of S. mansoni eggs, the glycolipid lysophosphatidylserine and the carbohydrate determinant lacto-N-fucopentaose III, activate TLR-2 and TLR-4, respectively, in myeloid DC [22]. In addition, they showed that egg-derived double-stranded RNAs activate inflammatory cytokine expression in DC through TLR-3. This signal triggers myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent pathways in DC.
In our study, we found consistently elevated frequencies of TLR-expressing cells in non-infected elderly individuals. According to the literature, TLR-3 is expressed predominantly by DC, and functions as a surface receptor for double-stranded RNA (dsRNA), a molecular pattern produced by most viruses. TLR-4 is expressed in a variety of human cell types but mainly in macrophages and DC. TLR-4 functions as the signal-transducing receptor for LPS and this binding requires interaction with other receptors such as CD14 and RP105 [43].
The most consistent increase in TLR-expressing cells observed in non-infected elderly individuals was in TLR-1+ cells that were elevated among CD56+ NK cells, CD56low NK cells, DC and monocytes. TLR-1 is expressed constitutively by monocytes and immature DC, binding to triacyl-lipopeptides of Gram-positive bacteria and other soluble bacterial products. TLR-2 is a member of the same subfamily of TLR-1 and TLR-6, and is expressed in monocytes and myeloid DC. Interestingly, TLR-1 and TLR-6 co-operate with TLR-2 to bind different bacterial products. The formation of heterodimers between TLR-2 and either TLR-1 or TLR-6 dictates the specificity of ligand recognition [44]. Therefore, the increase in the frequency of monocytes, NK and DC-expressing TLR-1 may act as a boost for innate immune responses mediated by other TLRs such as TLR-2. This would be a strategy for boosting the reactivity of these cells to a broad range of antigenic products.
In conclusion, our data show that non-infected elderly individuals from endemic areas of schistosome infection present an increased frequency of cells of innate immunity expressing TLRs. One major population up-regulated in these individuals is CD56low NK cells expressing TLR-1, -2, -3 and -4, and a consistent alteration observed was the elevated frequencies of TLR-1-expressing cells in the same group of subjects. These results suggest that elements of innate immunity may be related to the negative status of infection in some elderly individuals who are exposed constantly to S. mansoni, and they may represent biomarkers of healthy ageing in this population.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research Training in Tropical Diseases (TDR). Some of the authors are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq, Brazil (F. C., E. S., O. A. M. F., R. C. O and A. M. C. F).
References
- 1.Webster M, Roberts M, Fulford AJ, et al. Human IgE responses to rSm22·6 are associated with infection intensity rather than age per se, in a recently established focus of Schistosomiasis mansoni. Trop Med Int Health. 1998;3:318–26. doi: 10.1046/j.1365-3156.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- 2.Bethony J, Williams JT, Kloos H, et al. Exposure to Schistosoma mansoni infection in a rural area in Brazil. II. Household risk factors. Trop Med Int Health. 2001;6:136–45. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- 3.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–6. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaguarnera L, Ferlito L, Imbesi R, et al. Immunosenescence: a review. Arch Gerontol Geriatr. 2001;32:1–14. doi: 10.1016/s0167-4943(00)00086-8. [DOI] [PubMed] [Google Scholar]
- 5.Pawelec G, Solana R. Immunoageing − the cause or effect of morbidity. Trends Immunol. 2001;22:348–9. doi: 10.1016/s1471-4906(01)01956-1. [DOI] [PubMed] [Google Scholar]
- 6.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–9. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa-Oliveira R, Malaquias LC, Falcao PL, et al. Cytokines as determinants of resistance and pathology in human Schistosoma mansoni infection. Braz J Med Biol Res. 1998;31:171–7. doi: 10.1590/s0100-879x1998000100024. [DOI] [PubMed] [Google Scholar]
- 8.Henri S, Chevillard C, Mergani A, et al. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169:929–36. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 9.Webster M, Correa-Oliveira R, Gazzinelli G, et al. Factors affecting high and low human IgE responses to schistosome worm antigens in an area of Brazil endemic for Schistosoma mansoni and hookworm. Am J Trop Med Hyg. 1997;57:487–94. doi: 10.4269/ajtmh.1997.57.487. [DOI] [PubMed] [Google Scholar]
- 10.Walter K, Fulford AJ, McBeath R, et al. Increased human IgE induced by killing Schistosoma mansoni in vivo is associated with pretreatment Th2 cytokine responsiveness to worm antigens. J Immunol. 2006;177:5490–8. doi: 10.4049/jimmunol.177.8.5490. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–20. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 12.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–20. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 13.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–4. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Speziali E, Bethony J, Martins-Filho O, et al. Production of interferon-gamma by natural killer cells and aging in chronic human schistosomiasis. Med Inflamm. 2004;13:327–33. doi: 10.1155/S0962935104000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–80. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Jr, Medzhitov R. Lipoproteins take their toll on the host. Curr Biol. 1999;9:R879–82. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Reis e Sousa C. Toll-Iike receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006;84:333–41. doi: 10.1111/j.1440-1711.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 20.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 21.van Duin D, Mohanty S, Thomas V, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–5. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 22.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem. 2005;280:277–83. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 23.van der Kleij D, Latz E, Brouwers JF, et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 24.Thomas PG, Carter MR, Atochina O, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–41. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 25.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 26.Lima M, Teixeira MA, Queiros ML, et al. Immunophenotypic characterization of normal blood CD56+lo versus CD56+hi NK-cell subsets and its impact on the understanding of their tissue distribution and functional properties. Blood Cells Mol Dis. 2001;27:731–43. doi: 10.1006/bcmd.2001.0443. [DOI] [PubMed] [Google Scholar]
- 27.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 28.Cossarizza A, Ortolani C, Monti D, Franceschi C. Cytometric analysis of immunosenescence. Cytometry. 1997;27:297–313. doi: 10.1002/(sici)1097-0320(19970401)27:4<297::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi C, Valensin S, Bonafe M, et al. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–96. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 30.Sansoni P, Cossarizza A, Brianti V, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–73. [PubMed] [Google Scholar]
- 31.Solana R, Alonso MC, Pena J. Natural killer cells in healthy aging. Exp Gerontol. 1999;34:435–43. doi: 10.1016/s0531-5565(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 32.Gazzinelli A, Bethony J, Fraga LA, LoVerde PT, Correa-Oliveira R, Kloos H. Exposure to Schistosoma mansoni infection in a rural area of Brazil. I. Water contact. Trop Med Int Health. 2001;6:126–35. doi: 10.1046/j.1365-3156.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 33.Bethony J, Williams JT, Blangero J, et al. Additive host genetic factors influence fecal egg excretion rates during Schistosoma mansoni infection in a rural area in Brazil. Am J Trop Med Hyg. 2002;67:336–43. doi: 10.4269/ajtmh.2002.67.336. [DOI] [PubMed] [Google Scholar]
- 34.Lanier LL. The origin and functions of natural killer cells. Clin Immunol. 2000;95:S14–18. doi: 10.1006/clim.1999.4816. [DOI] [PubMed] [Google Scholar]
- 35.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 36.Hodes RJ. Molecular alterations in the aging immune system. J Exp Med. 1995;182:1–3. doi: 10.1084/jem.182.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Hagihara M, Ando K, et al. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol. 2001;166:1590–600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 39.Nishioka Y, Nishimura N, Suzuki Y, Sone S. Human monocyte-derived and CD83(+) blood dendritic cells enhance NK cell-mediated cytotoxicity. Eur J Immunol. 2001;31:2633–41. doi: 10.1002/1521-4141(200109)31:9<2633::aid-immu2633>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–9. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 42.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1998;64:703–12. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5:143–53. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 44.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]