Abstract
Periodontitis is an inflammatory bone disease caused by Gram-negative anaerobic bacteria. Osteoclast differentiation is regulated by the balance between receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG). The purpose of this study was to examine the mechanism of OPG production in human gingival fibroblasts (HGF) stimulated by lipopolysaccharide (LPS) from periodontopathic bacteria. The expressions of Toll-like receptor 2 (TLR-2) and TLR-4 in HGF were examined using flow-cytometry. HGF were stimulated with whole cell extracts or LPS from Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis with or without polymyxin B, a LPS inhibitor. In addition, HGF were stimulated with LPS, prostaglandin E2 (PGE2), various agonists of PGE receptors (EP1, EP2, EP3 and EP4 agonists) with or without indomethacin (IND), a prostaglandin synthesis inhibitor. OPG and PGE2 production was measured using an enzyme-linked immunosorbent assay (ELISA). HGF expressed both TLR-2 and TLR-4. Both A. actinomycetemcomitans and P. gingivalis LPS augmented OPG expression in HGF. Whole cell extracts from A. actinomycetemcomitans and P. gingivalis augmented OPG production by HGF; the augmentation was suppressed by polymyxin B. IND suppressed OPG production in LPS-stimulated HGF. PGE2 stimulated HGF to produce OPG. EP1 and EP2 agonists, but not EP3 and EP4 agonists, increased OPG production by HGF. These results suggest that LPS-induced OPG production by HGF is regulated via EP1 and/or EP2 receptors by endogenously generated PGE2.
Keywords: HGF, LPS, OPG, periodontitis, PGE2
Introduction
Periodontitis is an inflammatory bone disease caused by Gram-negative anaerobic bacteria including Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans) and Porphyromonas gingivalis (P. gingivalis). Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, exhibits powerful immunostimulatory and inflammatory activities [1]. LPS is considered to be a major factor in the pathogenesis of periodontal disease; it is absorbed into the root surfaces and gingival tissues of patients with periodontal disease [2]. Although it was assumed initially that the LPS molecules obtained from different bacteria are similar, recent evidence suggests that there are structural and functional differences [3]. For example, the structure of A. actinomycetemcomitans LPS is known to resemble that of Escherichia coli (E. coli) LPS, whereas the structure of P. gingivalis LPS is different from that of E. coli LPS [2,4]. While the LPS of E. coli and A. actinomycetemcomitans are known to be toll-like receptor (TLR) 4 agonists [5], P. gingivalis LPS has been found to have an agonistic effect to TLR2 [6].
Under normal physiological conditions, bone is periodically resorbed by osteoclasts while new bone is formed by osteoblasts [7]. Osteoblasts regulate osteoclastic bone resorption, which involves the recruitment of new osteoclasts and the activation of mature osteoclasts. The recruitment of new osteoclasts is dependent upon the balance in osteoblasts between the activity of the receptor activator of nuclear factor kappa B ligand (RANKL) and its decoy receptor, osteoprotegerin (OPG) [7,8].
RANKL and OPG mRNA expressions have been detected in inflamed gingival tissue [9]. Human gingival fibroblasts (HGF) are a major constituent of gingival connective tissue. OPG, but not RANKL, mRNA was found to be expressed in HGF; E. coli LPS augmented OPG mRNA expression and OPG production in HGF [9]. The culture supernatant of E. coli LPS-stimulated HGF reduced the number of osteoclasts that were generated by culturing human peripheral blood monocytes with recombinant human RANKL and macrophage–colony stimulating factor (M-CSF); the suppression was inhibited by anti-OPG neutralizing antibody [9]. In periodontitis lesions, HGF may directly interact with bacteria and bacterial products; LPS and OPG production in HGF may be involved in the recruitment of new osteoclasts [10].
Prostaglandins (PGs), including PGE2, play a variety of actions under the physiological and pathological conditions [11,12]. In response to various stimuli, arachidonic acid released from membrane phospholipids is metabolized to PGs by cyclooxygenase (COX). PGE2 exerts its biological actions via specific PGE receptors on target cells and four distinct subtypes of PGE receptors, designated EP1, EP2, EP3 and EP4 [13,14]. EP1 receptor activation induces elevation of intracellular calcium levels via a poorly characterized mechanism involving G proteins. EP2 and EP4 receptors activate adenylate cyclase via stimulatory G protein, and result in the elevation of intracellular cAMP levels. Multiple isoforms of EP3 receptors with different C-terminal tails mediate several signalling pathways including inhibition and stimulation of adenylate cyclase, activation of phospholipase C and mobilization of intracellular calcium. Prostaglandins (PGs), including PGE2, are thought to be involved in the pathogenesis of periodontal disease [15,16].
LPS stimulates PGE2 production in target cells through the induction of mitogen-inducible COX 2 expression [17]. Recently, PGE2 synthesis was reported to suppress OPG production in both osteoblasts and periodontal ligament cells [18,19]; however, the effect of PGE2 synthesis on OPG production by HGF stimulated with LPS remains unknown.
The purpose of this study was to examine the OPG production in HGF stimulated with LPS obtained from periodontopathic bacteria and to clarify the involvement of PGE2 in the production of OPG by HGF.
Materials and methods
Reagents
E. coli LPS (serotype O55:B5), polymyxin B and 3,3′,5′,5-tetramethylbenzidine liquid substrate system were purchased from Sigma Chemicals (St Louis, MO, USA). Ultrapure LPS from P. gingivalis was purchased InvivoGen (San Diego, CA, USA). Fluorescein-conjugated anti-TLR-2, phycoerythrin-conjugated anti-TLR-4, fluorescein-conjugated mouse IgG2a isotype control antibody, and phycoerythrin-conjugated mouse IgG2a isotype control antibody were purchased from eBioscience (San Diego, CA, USA). Monoclonal anti-human OPG/TNFRSF11B antibody was purchased from R&D Systems (Minneapolis, MN, USA). Recombinant human OPG-Fc chimera and biotinylated anti-human OPG antibody were purchased from Genzyme (Cambridge, MA, USA). Streptavidin alkaline phosphatase was purchased from Techne Corporation (Minneapolis, MN, USA). Prostaglandin E2 enzyme immunoassay system was purchased from Amersham Pharmacia Biotech (Amersham, Bucks, UK). Indomethacin (IND) was purchased from Wako (Tokyo, Japan). Prostaglandin E2 was purchased from Cascade Biochem Ltd (Corston, UK); 17 phenyl-ω-trinor PGE2 (EP1 agonist) was purchased from Cayman Chemical (Ann Arbor, MI, USA). Butaprost (EP2 agonist), ONO-AE-248 (EP3 agonist) and ONO-AE1-329 (EP4 agonist) were provided by ONO Pharmaceutical Co. (Osaka, Japan).
Preparation of sonicated whole cell extracts and LPS
The study protocol was approved by the Ethic Committee of the Tokyo Medical and Dental University.
Sonicated whole cell extracts of A. actinomycetemcomitans ATCC43718 and P. gingivalis FDC381 were prepared as described previously [20]. The cells were suspended in saline (200 mg/ml, wet weight), placed on ice and disrupted by sonication at maximum output at 30-s intervals, for a total sonication time of 3 min. The sonicated cell suspensions were centrifuged, and the supernatants were lyophilized.
A. actinomycetemcomitans and P. gingivalis LPS were prepared as described previously [21]. A. actinomycetemcomitans ATCC43718 and P. gingivalis FDC381 were grown anaerobically (80% N2, 10% H2 and 10% CO2) using general anaerobic medium broth (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) supplemented with vitamin K1 and haemin for P. gingivalis and with yeast extract for A. actinomycetemcomitans. The bacteria were harvested by centrifugation, washed three times with distilled water, and lyophilized. LPS was isolated from the bacteria using the hot phenol–water method [22]. The purity of our LPS preparations has been studied; we have confirmed that little contamination is present in our preparations [23]. In addition, we have purchased ultra-pure P. gingivalis LPS, which is known to be a selective TLR-2 ligand, and confirmed that the results were the same as our P. gingivalis LPS. Thus, our LPS preparations appear to be comparable to the highly purified LPS.
Cell isolation and culture
All the patients had been diagnosed as having chronic periodontitis and had received initial periodontal therapy. After obtaining their written informed consent, samples of gingival tissue were obtained from chronic periodontitis patients during surgical therapy. Each sample of gingival tissue was cut into small pieces and cultured in α-minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS) (Bioserum, Victoria, Australia) in the presence of 100 U/ml of penicillin and 100 μg/ml of streptomycin (Sigma).
The fibroblast cells that grew from the explanted tissue were subcultured. HGF from passage levels 4–10 were used in this study.
The HGF were seeded into 96-well flat-bottomed culture plates at 1 × 104 cells per well and were grown to confluence. Once confluent, the HGF were cultured for 24 h with various additives, and the supernatants were harvested.
The HGF were cultured with or without polymyxin B or IND for 20 min. The HGF were stimulated with sonicated whole cell extracts (A. actinomycetemcomitans, P. gingivalis), LPS (A. actinomycetemcomitans, E. coli, P. gingivalis), PGE2 or agonists of PGE receptors (EP1, EP2, EP3 and EP4).
In the preliminary study, We titrated the sonicated extracts (0·01–10 μg/ml) from A. actinomycetemcomitans and P. gingivalis, and determined that 0·1 μg/ml of A. actinomycetemcomitans sonicated extracts and 0·1 μg/ml of P. gingivalis sonicated extracts were optimal for maximum level of OPG production in HGF (data not shown). Accordingly, we used 0·1 μg/ml of A. actinomycetemcomitans sonicated extracts and 0·1 μg/ml of P. gingivalis sonicated extracts in the experiments.
We have studied previously the effects of several doses of polymyxin B on the inhibition of LPS in sonicated extracts from A. actinomycetemcomitans and P. gingivalis[24]; and determined that 106 U/ml of polymyxin B was sufficient to inhibit LPS in 10 μg/ml of sonicated extracts of A. actinomycetemcomitans. We also confirmed that 106 U/ml of polymyxin B did not affect the viability of HGF in this study.
We have reported previously that A. actinomycetemcomitans LPS and P. gingivalis LPS induced PGE2 production in HGF and that the PGE2 production was inihibited completely by 1 μM of indomethacin [25]. Thus, we examined the effect of 1 μM indomethacin in this experiments.
After 24 h of stimulation, the OPG and PGE2 levels in the culture supernatants were measured using an enzyme-linked immunosorbent assay (ELISA).
Expression of TLR-2 and TLR-4 on HGF
HGF were treated with trypsin and suspended. The cells suspension was reacted with fluorescein-conjugated anti-TLR-2, phycoerythrin-conjugated anti-TLR-4 or the respective isotype control antibodies. Flow cytometric analysis was performed using a fluorescence activated cell sorter (FACScan) flow cytometer and CellQuest Software (Becton Dickinson, Mountain View, CA, USA).
Measurement of OPG and PGE2 production by HGF
The OPG level was measured as described previously [9]. In brief, anti-human osteoprotegerin antibody was coated onto a 96-well flat-bottomed plate. After blocking with 1% bovine serum albumin (BSA), samples were added to the well in triplicate. Recombinant human OPG-Fc chimera was used as the standard. Following washing, biotinylated anti-human OPG antibody was added. After washing, streptavidin alkaline phosphatase was added and the plate was incubated. Following washing, substrate solution was added, and the absorbance was recorded using an ELISA reader. PGE2 ELISA was performed according to the manufacturer's protocol (PGE2, Amersham Pharmacia Biotech).
Statistical analysis
Data are expressed as the mean ± standard deviation (s.d.). The Mann–Whitney U-test was used for statistical analysis. The level of significance was set at 0·01 and 0·05.
Results
Effect of LPS on OPG production
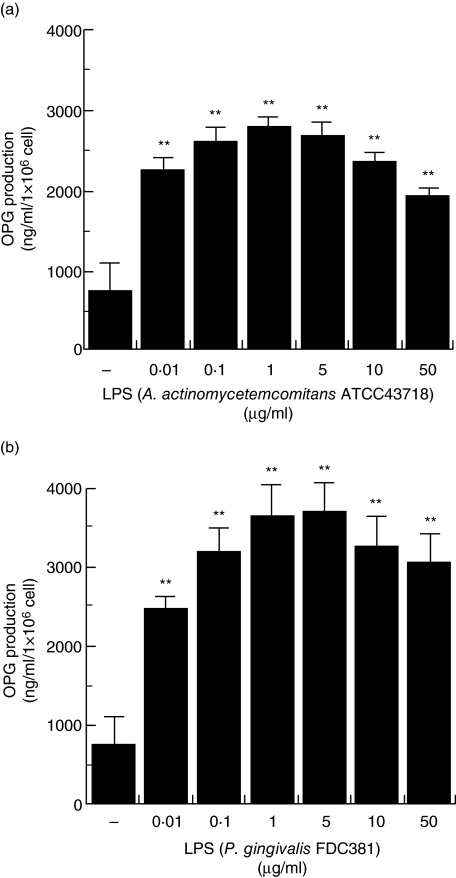
To examine the effect of LPS on OPG production in HGF, HGF were stimulated with various concentrations of A. actinomycetemcomitans and P. gingivalis LPS. A. actinomycetemcomitans ATCC43718 LPS augmented OPG production by HGF. P. gingivalis FDC381 LPS also augmented OPG production by HGF. The maximum production of OPG occurred with 1 μg/ml of A. actinomycetemcomitans LPS and 1–5 μg/ml of P. gingivalis LPS (Fig. 1). OPG production was higher in HGF stimulated with P. gingivalis LPS than in HGF stimulated with A. actinomycetemcomitans LPS.
Fig. 1.
Osteoprotegerin (OPG) production in human gingival fibroblasts (HGF) stimulated with lipopolysaccharide (LPS). (a) HGF stimulated with 0·01–50 μg/ml of Actinobacillus actinomycetemcomitans ATCC43718 LPS for 24 h. (b) HGF stimulated with 0·01–50 μg/ml of Porphyromonas gingivalis FDC381 LPS for 24 h. OPG in the culture supernatants was measured using enzyme-linked immunosorbent assay. The results are representative of three experiments. **P < 0·01.
Effect of sonicated whole cell extracts on OPG production in HGF
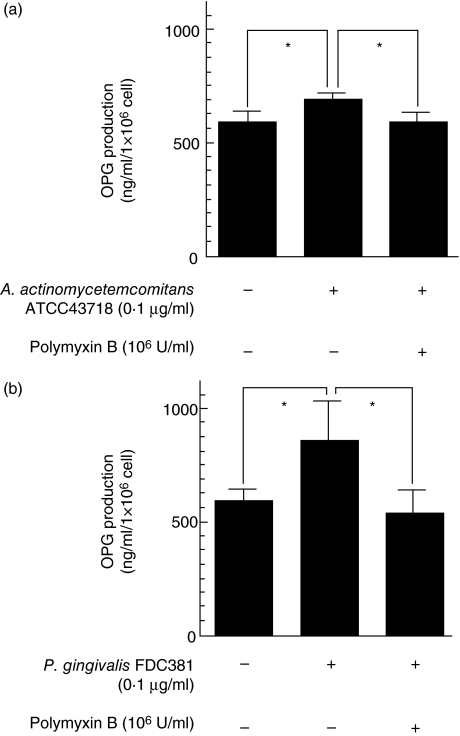
To examine the effect of other bacterial components on OPG production, HGF were stimulated with sonicated extracts of A. actinomycetemcomitans and P. gingivalis; 0·1 μg/ml of A. actinomycetemcomitans ATCC43718 sonicated whole cell extracts and 0·1 μg/ml of P. gingivalis FDC381 sonicated whole cell extracts augmented OPG production by HGF; 106 U/ml polymyxin B, a LPS inhibitor, almost completely abrogated the augmentation of OPG stimulated with A. actinomycetemcomitans and P. gingivalis sonicated whole cell extracts (Fig. 2). OPG production was higher in HGF stimulated with P. gingivalis sonicated whole cell extracts than in HGF stimulated with A. actinomycetemcomitans sonicated whole cell extracts.
Fig. 2.
Osteoprotegerin (OPG) production in human gingival fibroblasts (HGF) stimulated with 0·1 μg/ml of sonicated whole cell extracts. (a) HGF were stimulated with 0·1 μg/ml of Actinobacillus actinomycetemcomitans ATCC43718 sonicated whole cell extracts in the presence or absence of 106 U/ml polymyxin B for 24 h. (b) HGF were stimulated with 0·1 μg/ml of Porphyromonas gingivalis FDC381 sonicated whole cell extracts in the presence or absence of 106 U/ml polymyxin B for 24 h. OPG levels in the culture supernatants were measured using enzyme-linked immunosorbent assay. The results are representative of three experiments. *P < 0·05.
Expression of TLR-2 and TLR-4 on HGF
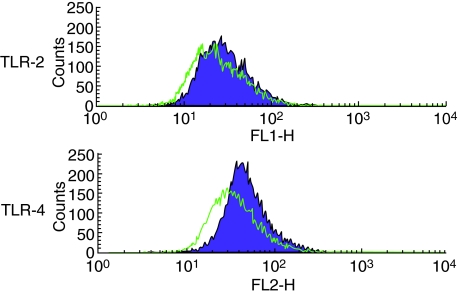
As LPS augmented OPG production in HGF, expression of TLR-2 and TLR-4 were examined. On HGF, both TLR-2 and TLR-4 were expressed (Fig. 3).
Fig. 3.
Expression of Toll-like receptor (TLR)-2 and TLR-4 in human gingival fibroblasts (HGF). HGF stained with anti-TLR-2, anti-TLR-4, and the respective isotype control antibodies. For flow cytometry, 10 000 cells were analysed in each case. Green line is stained with the respective isotype control antibodies. Purple line is stained with fluorescein-conjugated anti-TLR-2 and phycoerythrin-conjugated anti-TLR-4.
Effect of LPS on PGE2 production in HGF
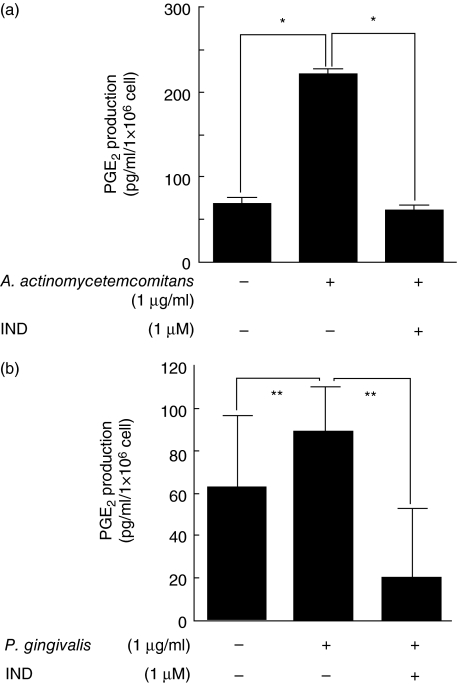
As PGE2 affects OPG production in osteoblasts, the effect of LPS on PGE2 production in HGF was examined.A. actinomycetemcomitans ATCC43718 LPS and P. gingivalis FDC381 LPS augmented PGE2 production by HGF; the amount of PGE2 was significantly higher with A. actinomycetemcomitans LPS stimulation than with P. gingivalis LPS stimulation. IND, a prostaglandin synthesis inhibitor, abrogated PGE2 production by HGF stimulated with A. actinomycetemcomitans LPS and P. gingivalis LPS (Fig. 4).
Fig. 4.
Prostaglandin E2 (PGE2) production in human gingival fibroblasts (HGF) stimulated with lipopolysaccharide (LPS). (a) HGF stimulated with 1 μg/ml of Actinobacillus actinomycetemcomitans LPS in the presence or absence of 1 μM indomethacin (IND) for 24 h. (b) HGF stimulated with 1 μg/ml of Porphyromonas gingivalis LPS in the presence or absence of 1 μM IND for 24 h. PGE2 levels in the culture supernatants were measured using enzyme-linked immunosorbent assay. The results are representative of three experiments. (a) *P < 0·05; (b) **P < 0·01.
Effect of PGE2 on OPG production in HGF
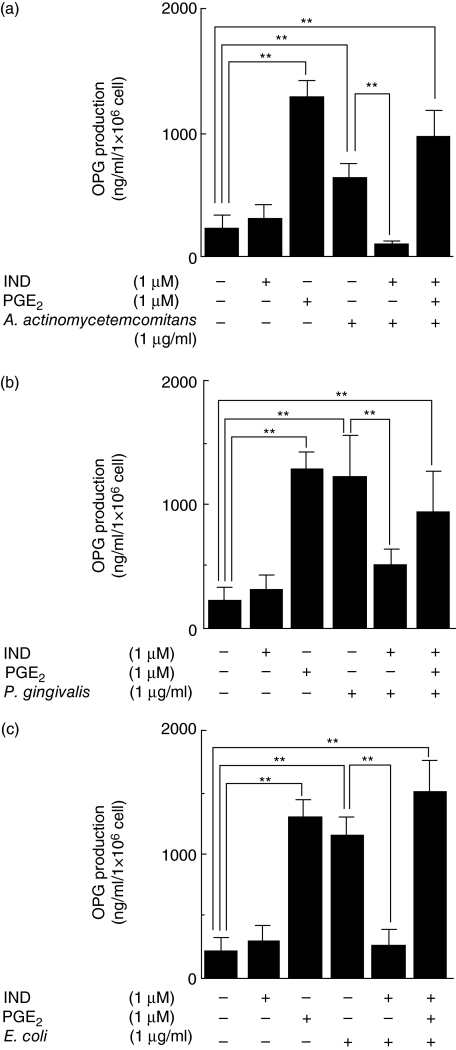
To examine the effect of PGE2 on OPG production in HGF, HGF were cultured with LPS and a prostaglandin synthesis inhibitor, IND. Without IND, OPG production was higher in HGF stimulated with P. gingivalis LPS than in those stimulated with A. actinomycetemcomitans LPS. IND significantly reduced the production of OPG by HGF stimulated with LPS (A. actinomycetemcomitans, E. coli, P. gingivalis). However, in the presence of IND and LPS (A. actinomycetemcomitans, E. coli, P. gingivalis), exogenous PGE2 augmented the production of OPG by HGF (Fig. 5).
Fig. 5.
Osteoprotegerin (OPG) production in human gingival fibroblasts (HGF) stimulated with lipopolysaccharide (LPS), indomethacin (IND) and prostaglandin E2 (PGE2). (a) HGF stimulated with 1 μg/ml of Actinobacillus actinomycetemcomitans LPS in the presence or absence of 1 μM IND and 1 μM PGE2 for 24 h. (b) HGF stimulated with 1 μg/ml of Porphyromonas gingivalis LPS in the presence or absence of 1 μM IND and 1 μM PGE2 for 24 h. (c) HGF stimulated with 1 μg/ml of Escherichia coli LPS in the presence or absence of 1 μM IND and 1 μM PGE2 for 24 h. The OPG levels in the culture supernatants were measured using enzyme-linked immunosorbent assay. The results are representative of three experiments. **P < 0·01.
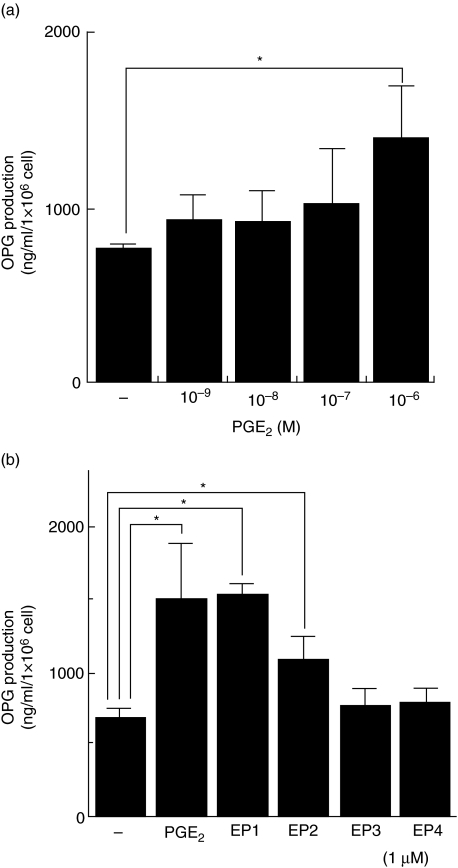
Effect of PGE2 and agonists of PGE receptors (EP1, EP2, EP3 and EP4 agonists) on OPG production in HGF
Exogenous PGE2 augmented OPG production in HGF. Therefore, the effect of various EP agonists on OPG production in HGF was examined. PGE2 augmented OPG production by HGF in a dose-dependent manner (Fig. 6a). EP1 agonist and EP2 agonist augmented OPG production by HGF, but EP3 and EP4 did not (Fig. 6b).
Fig. 6.
Osteoprotegerin (OPG) production in human gingival fibroblasts (HGF) stimulated with PGE2 and agonists of prostaglandin (PGE) receptors (EP1, EP2, EP3 and EP4 agonist). (a) HGF stimulated with 10−9−10−6 M PGE2 for 24 h. (b) HGF stimulated with 1 μΜ PGE2 and 1 μM EP agonists (EP1, EP2, EP3 and EP4 agonist) for 24 h. The OPG levels in the culture supernatants were measured using enzyme-linked immunosorbent assay. The results are representative of three experiments. *P < 0·05.
Discussion
In the present study, OPG production in HGF was augmented by stimulation with sonicated whole cell extracts derived from periodontopathic bacteria (A. actinomycetemcomitans, P. gingivalis). Polymyxin B inhibited the augmentation of OPG production caused by stimulation with A. actinomycetemcomitans and P. gingivalis sonicated whole cell extracts. These results suggest that LPS might be a potent agent to stimulate HGF to produce OPG.
In all Gram-negative bacteria, the structure of LPS includes a polysaccharide attached to a lipid component, called lipid A. Netea et al. [3] proposed that the shape of the lipid A component determines the bioactivity of LPS. Lipid A with a conical shape (e.g. from E. coli) induces cytokine production through TLR-4, whereas more cylindrical lipid A (e.g. from P. gingivalis) induces the expression of a different set of cytokines through TLR-2 [3]. Hirschfeld et al. [6] reported that signalling by TLR-2 and TLR-4 agonists results in differential gene expression in murine macrophages. In this study, it was found that HGF expressed TLR-2 and TLR-4. Both P. gingivalis LPS and A. actinomycetemcomitans LPS stimulated HGF to produce OPG (Fig. 2), suggesting that both TLR-2 and TLR-4 pathways are involved in LPS recognition in HGF.
It has been reported that PGE2 is involved in both cytokine-mediated osteoclast activation and osteoblastic bone formation [26,27]. Liu et al. [28] reported that PGE2 stimulated osteoclast formation through the inhibition of osteoblast OPG secretion. Suda et al. [19] reported that LPS inhibited osteoblast OPG expression, and that inhibition was mediated by PGE2 production. Sakata et al. [18] reported that interleukin (IL)-1β increased OPG and PGE2 expression in human periodontal ligament cells, and that the increase in the OPG level caused by stimulation with IL-1β was suppressed through PGE2 synthesis. These data suggest that PGE2 inhibits OPG production both in osteoblasts and in periodontal ligament cells. In the present study, HGF stimulated with LPS produced PGE2, and OPG production was inhibited partially by IND, a cyclooxygenase inhibitor (Fig. 4). This suggests that the increase in HGF OPG levels was mediated partially by PGE2 production. OPG production in HGF stimulated by P. gingivalis LPS was significantly higher than that in HGF stimulated by A. actinomycetemcomitans LPS (Fig. 1). PGE2 production in HGF stimulated with P. gingivalis LPS was lower than that in HGF stimulated with A. actinomycetemcomitans LPS (Fig. 4a), suggesting that the amount of PGE2 production did not account for the amount of OPG production in HGF stimulated with LPS. IND did not completely abrogate OPG production in HGF stimulated with P. gingivalis LPS. These results suggest that there might be PGE2-dependent and PGE2-independent pathways for OPG production in HGF stimulated with LPS.
EP receptors and PGE receptors are rhodopsin-type receptors that have seven transmembrane-spanning domains [29]. It has been reported that EP1, EP2 and EP4 mRNA were detected in HGF, but that EP3 mRNA was not detected [30]. EP1 is coupled to Gq/p, and ligand binding results in an increase in intracellular calcium levels and EP1-enhanced prorein kinsae C (PKC) signalling [29,31]. An intracellular calcium chelator suppressed OPG mRNA expression in osteoblasts, and the activator of PKC stimulated OPG mRNA in osteoblasts and bone marrow stromal cells [32,33]. OPG production via EP1 may be mediated by increased intracellular calcium and PKC activation. Both EP2 and EP4 were found to be coupled to Gs protein and to stimulate adenylate cyclase to trigger the cAMP–protein kinase A (PKA) signalling pathway [29,34]. The total formation of intracellular cAMP was greater in cells expressing the EP2 receptor than in those expressing the EP4 receptor [34]. We have reported previously that IL-1β-induced OPG production in HGF was different from that in PDL and osteoblasts, and that the activation of the PKA pathway is crucial for OPG production in HGF [35]. In this study, EP1 and EP2 agonists stimulated OPG production in HGF, but the EP4 agonist did not. EP1 and EP2 agonists might activate PKC and PKA in HGF, and the activation of PKC and PKA might result in OPG production in HGF.
Production of OPG in HGF plays a protective role in bone resorption of periodontitis lesions. Numerous reports have focused on the pathological role that HGF have in periodontitis, as HGF have been observed to produce inflammatory mediators, such as IL-1, IL-6 and PGE2, in response to bacterial products [36,37,38]. LPS and inflammatory mediators, including IL-1, IL-6 and PGE2, augment RANKL expression on the surface of osteoblasts [8,19]. As well, CD4+ T cells express RANKL in response to bacterial stimulation [39,40]. In periodontitis tissues, many B cells and the T cells were found to express RANKL [41]. Stimulation of PDL by IL-1α, IL-1β and PGE2 has been shown to induce RANKL mRNA [35,42]. It remains to be determined whether OPG produced by HGF is sufficient to suppress RANKL expression in osteoblasts, T lymphocytes and PDL that are activated by IL-1, IL-6, PGE2 and LPS in periodontitis tissue. The amount of OPG produced by HGF was higher than that produced by periodontal ligament fibroblasts [35]; HGF are better at inhibiting osteoclast differentiation than periodontal ligament fibroblasts [43]. HGF constitute 65% of the cellular population of gingival tissue [44]; this suggests that the total amount of OPG produced by HGF might be effective to suppress RANKL in gingival tissue.
The present study demonstrated that HGF produce OPG in response to LPS from A. actinomycetemcomitans and P. gingivalis. HGF stimulated with LPS produced PGE2, and PGE2 further augmented OPG production in HGF via EP1 and EP2 receptors. Induction of OPG in gingival fibroblasts might be a self-defence mechanism that inhibits alveolar bone destruction during periodontal inflammation. Further study is necessary to evaluate the in vivo relevance of OPG production from HGF in periodontitis tissue.
Acknowledgments
This work was supported by the Japan Society for Promotion of Science nos. 18592258, and Center of Excellence (COE) Program for Frontier Research on Molecular Destruction and Reconstruction of Tooth and Bone, Graduate School, Tokyo Medical and Dental University.
References
- 1.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 2.Mayrand D, Holt SC. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988;52:134–52. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, Van der Meer JW. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23:135–9. doi: 10.1016/s1471-4906(01)02169-x. [DOI] [PubMed] [Google Scholar]
- 4.Masoud H, Weintraub ST, Wang R, Cotter R, Holt SC. Investigation of the structure of lipid A from Actinobacillus actinomycetemcomitans strain Y4 and human clinical isolate PO 1021–7. Eur J Biochem. 1991;200:775–81. doi: 10.1111/j.1432-1033.1991.tb16244.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Kim KK, Rhyu IC, Koh S, Lee DS, Choi BK. Phenol/water extract of Treponema socranskii subsp. socranskii as an antagonist of Toll-like receptor 4 signalling. Microbiology. 2006;152:535–46. doi: 10.1099/mic.0.28470-0. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol Today. 2000;21:495–502. doi: 10.1016/s0167-5699(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 8.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa T, Kobayashi H, Kiji M, et al. LPS-stimulated human gingival fibroblasts inhibit the differentiation of monocytes into osteoclasts through the production of osteoprotegerin. Clin Exp Immunol. 2002;130:338–44. doi: 10.1046/j.1365-2249.2002.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa T, Kiji M, Yashiro R, et al. Roles of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontal. 2007;43:65–84. doi: 10.1111/j.1600-0757.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 11.DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991;1083:121–34. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- 12.Tilley SL, Coffman TM, Koller BH. Mixed messages. modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 14.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259:109–19. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 15.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64(5 Suppl.):432–44. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E in periodontal disease. Periodontology 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB J. 2001;15:2556–64. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 18.Sakata M, Shiba H, Komatsuzawa H, et al. Osteoprotegerin levels increased by interleukin-1beta in human periodontal ligament cells are suppressed through prostaglandin E(2) synthesized de novo. Cytokine. 2002;18:133–9. doi: 10.1006/cyto.2002.1026. [DOI] [PubMed] [Google Scholar]
- 19.Suda K, Udagawa N, Sato N, et al. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol. 2004;172:2504–10. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Kawashima Y, Nagasawa T, et al. Elevated serum IgG titer and avidity to Actinobacillus actinomycetemcomitans serotype c in Japanese periodontitis patients. Oral Microbiol Immunol. 2005;20:172–9. doi: 10.1111/j.1399-302X.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 21.Kubo K, Kamada T, Okamoto H, Izumi Y, Otsuji S, Sueda T. Lipopolysaccharide increases cell surface-associated fibronectin in fibroblasts in vitro. Oral Microbiol Immunol. 1996;11:29–34. doi: 10.1111/j.1399-302x.1996.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 22.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol water and further application of the procedure. Meth Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 23.Hiraoka T, Izumi Y, Sueda T. Immunochemical detection of CD14 on human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1998;13:246–52. doi: 10.1111/j.1399-302x.1998.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Nagasawa T, Aramaki M, Mahanonda R, Ishikawa I. Individual diversities in interferon gamma production by human peripheral blood mononuclear cells stimulated with periodontopathic bacteria. J Periodont Res. 2000;35:319–28. doi: 10.1034/j.1600-0765.2000.035006319.x. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi K, Shitashige M, Yanai M, et al. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–68. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone. 1997;20:521–6. doi: 10.1016/s8756-3282(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, Akatsu T, Murakami T, et al. Involvement of cyclo-oxygenase-2 in osteoclast formation and bone destruction in bone metastasis of mammary carcinoma cell lines. J Bone Miner Res. 2002;17:774–81. doi: 10.1359/jbmr.2002.17.5.774. [DOI] [PubMed] [Google Scholar]
- 28.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–8. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 29.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi K, Shitashige M, Endo H, Kondo H, Ishikawa I. Binary regulation of interleukin (IL)-6 production by EP1 and EP2/EP4 subtypes of PGE2 receptors in IL-1beta-stimulated human gingival fibroblasts. J Periodont Res. 2002;37:29–36. doi: 10.1034/j.1600-0765.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi A, Shimamoto C, Katsu K, Ito S, Imai Y, Nakahari T. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E(2) in guinea-pig antral mucous cells. Exp Physiol. 2001;86:451–60. doi: 10.1113/eph8602160. [DOI] [PubMed] [Google Scholar]
- 32.Takami M, Takahashi N, Udagawa N, et al. Intracellular calcium and protein kinase C mediate expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in osteoblasts. Endocrinology. 2000;141:4711–9. doi: 10.1210/endo.141.12.7852. [DOI] [PubMed] [Google Scholar]
- 33.Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17:1667–79. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 34.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–53. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Hormdee D, Nagasawa T, Kiji M, et al. Protein kinase-A-dependent osteoprotegerin production on interleukin-1 stimulation in human gingival fibroblasts is distinct from periodontal ligament fibroblasts. Clin Exp Immunol. 2005;142:490–7. doi: 10.1111/j.1365-2249.2005.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heath JK, Atkinson SJ, Hembry RM, Reynolds JJ, Meikle MC. Bacterial antigens induce collagenase and prostaglandin E2 synthesis in human gingival fibroblasts through a primary effect on circulating mononuclear cells. Infect Immun. 1987;55:2148–54. doi: 10.1128/iai.55.9.2148-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sismey-Durrant HJ, Hopps RM. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-beta release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991;6:378–80. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem Biophys Res Commun. 2000;273:1161–7. doi: 10.1006/bbrc.2000.3060. [DOI] [PubMed] [Google Scholar]
- 39.Teng YT, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahamed DA, Marleau A, Alnaeeli M, et al. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005;54:1477–86. doi: 10.2337/diabetes.54.5.1477. [DOI] [PubMed] [Google Scholar]
- 41.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–98. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nukaga J, Kobayashi M, Shinki T, et al. Regulatory effects of interleukin-1beta and prostaglandin E2 on expression of receptor activator of nuclear factor-kappaB ligand in human periodontal ligament cells. J Periodontol. 2004;75:249–59. doi: 10.1902/jop.2004.75.2.249. [DOI] [PubMed] [Google Scholar]
- 43.de Vries TJ, Schoenmaker T, Wattanaroonwong N, et al. Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts. J Cell Biochem. 2006;98:370–82. doi: 10.1002/jcb.20795. [DOI] [PubMed] [Google Scholar]
- 44.Lindhe J, Karring T, Lang PN. Clinical periodontology and implant dentistry. 4. Copenhagen: Blackwell Munksgaard; 2003. [Google Scholar]