Abstract
The ability of mesenchymal stem cells (MSC) to suppress alloresponsiveness is poorly understood. Herein, an allogeneic mixed lymphocyte response was used as a model to investigate the mechanisms of MSC-mediated immunomodulation. Human MSC are demonstrated to express the immunosuppressive cytokines hepatocyte growth factor (HGF), interleukin (IL)-10 and transforming growth factor (TGF)-β1 at concentrations that suppress alloresponses in vitro. MSC also express cyclooxygenase 1 and 2 and produce prostaglandin E2 constitutively. Blocking studies with indomethacin confirmed that prostaglandins contribute to MSC-mediated allosuppression. The proinflammatory cytokine interferon (IFN)-γ did not ablate MSC inhibition of alloantigen-driven proliferation but up-regulated HGF and TGF-β1. IFN-γ also induced expression of indoleamine 2,3, dioxygenase (IDO), involved in tryptophan catabolism. Use of an antagonist, 1-methyl-L-tryptophan, restored alloresponsiveness and confirmed an IDO contribution to IFN-γ-induced immunomodulation by MSC. Addition of the tryptophan catabolite kynurenine to mixed lymphocyte reactions (MLR), blocked alloproliferation. These findings support a model where IDO exerts its effect through the local accumulation of tryptophan metabolites rather than through tryptophan depletion. Taken together, these data demonstrate that soluble factors, or products derived from MSC, modulate immune responses and suggest that MSC create an immunosuppressive microenvironment capable of modulating alloresponsiveness even in the presence of IFN-γ.
Keywords: IFN-γ, inflammation, stem cells
Introduction
Mesenchymal stem cells (MSC), first recognized by Friedenstein and colleagues, are present at low numbers in normal adult bone marrow [1]. They have the capacity for controlled self-renewal and retain the potential to differentiate into a variety of specialized cell types, including cartilage, bone, tendon, ligament, adipose tissue, neural precursor cells, muscle and cardiomyocytes [2–4]. This potential makes MSC attractive candidates for applications in regenerative medicine. However, the use of autologous MSC may be limited in a clinical setting, due to availability, patient status and the demands required to make a personalized therapeutic approach feasible. Therefore, the possibility of engrafting standardized, allogeneic MSC would be very attractive therapeutically. The value of this approach has been demonstrated by the use of allogeneic MSC to treat children with osteogenesis imperfecta. Horwitz et al. reported that allogeneic MSC delivery resulted in both osteoblast engraftment and an increase in new bone formation, in the absence of marrow ablative chemotherapy [5]. Similar results have been observed in animal models showing MSC to be beneficial for the repair of tissue damage [3,6–8]. However, it is evident that the use of allogeneic, as opposed to autologous, stem cells in regenerative medicine poses more profound problems of immune rejection.
The capacity for MSC to escape immune recognition and to suppress activation of T cells, both in vivo and in vitro, has posed fundamental problems for immunologists in that there is little apparent reason why evolution would retain this property, given that allotransplantation is a recent phenomenon. However, a number of recent studies have suggested that allotransplantation of MSC may indeed be feasible [5,9,10]. Early clinical trials have demonstrated the potential of MSC therapy and provide evidence supporting tolerance to allogeneic MSC by recipients. Koc et al. showed no evidence of alloreactive T cells, and no incidence of graft-versus-host disease, when allogeneic MSC were infused into patients with Hurler's syndrome or metachromatic leucodystrophy [9]. Similarly, human MSC failed to elicit allogeneic T cell responses in mixed lymphocyte reactions (MLR) even when major histocompatibility complex (MHC) class II was up-regulated [11,12]. This effect is supported by observations that haploidentical MSC had striking immunosuppressive effects when transplanted into a patient with severe graft-versus-host disease [10]. Recently, work by Aggarwal and Pittenger showed that MSC altered the phenotype of specific immune cells, providing evidence that transplantation of allogeneic MSC is feasible [13]. Taken together, these results suggest that MSC exert their effect through the creation of a tolerogenic niche within the recipient.
Although data showing the successful use of allogeneic MSC in regenerative therapy is available, such approaches are unlikely to be broadly acceptable until the exact molecular mechanisms underlying the suppressive ability of MSC are revealed in combination with clinical studies. At present these mechanisms remain unclear. It is therefore a critical necessity to establish the precise mechanisms by which MSC exert their suppressive capabilities. Evidence from allogeneic co-cultures or MLR has demonstrated that both cell–cell contact and soluble factors contribute to the immunomodulatory function of MSC [14–17]. However, there are confounding data regarding the mechanisms involved. Previously, we and others have hypothesized that MSC retain features of the fetal allograft and have proposed that soluble factors including hepatocyte growth factor (HGF), interleukin (IL)-10, and the expression of indoleamine 2,3-dioxygenase (IDO), are candidate factors utilized by MSC to elicit immunosuppressive effects [18,19].
In the present study, we set out to determine the mechanisms by which human MSC exert immunosuppressive activity using MLR involving allogeneic peripheral blood mononuclear cells. The mechanism(s) underlying MSC-mediated suppression of alloantigen-driven proliferation were evaluated by assessing the role of different MSC-derived soluble factors, examining their ability to suppress an allogeneic MLR and investigating the capacity to restore proliferation through neutralization or chemical antagonism. Furthermore, as MSC may be required to retain immunosuppression in an inflammatory environment, the effect of the proinflammatory cytokine interferon (IFN)-γ on soluble factor production by MSC was examined. Results indicate that MSC have a distinctive immunosuppressive profile that modulates immune cell proliferation and IFN-γ does not ablate but enhances that capacity. Taken together, these findings support the use of allogeneic MSC in regenerative medicine for inflamed tissue.
Materials and methods
Cell isolation, purification and culture
Human MSC were isolated and expanded from aspirates of bone marrow from three different donors by direct plating, as described previously [2,20]. Cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (Invitrogen-Gibco, Paisley, Scotland, UK). Contamination in MSC populations is a key confounding variable, therefore rigorous quality control was adopted to ensure that MSC were not contaminated with haematopoietic or other cell contaminants and that cells retained differentiation capacity, as described previously [2,20]. In MSC culture experiments, human epithelial (A549), which do not secrete IL-10, transforming growth factor (TGF)-β, HGF or fibroblast-like (HDF-α) cells that do not express COX isoforms, or culture supernatants as appropriate were typically included as negative controls. Peripheral blood mononuclear cells (PBMC) were isolated from MHC mismatched donors by density gradient centrifugation, as described previously [21], and isolated cells were cryopreserved for use in mixed lymphocyte reactions. Study design and use of human mesenchymal stem cells was approved by the Bioethics Committees of the National University of Ireland Maynooth and the National University of Ireland Galway.
MLR
Allogeneic mixed lymphocyte reactions were performed with PBMC from MHC mismatched donors in flat-bottomed 96-well plates (Nunc, Roskilde, Denmark) in a total volume of 200 µl RPMI-1640 supplemented with 10% heat-inactivated FCS and 1% penicillin/streptomycin (Invitrogen-Gibco). Cells were cultured as follows: 1·5 × 104 MSC were cultured for 24 h prior to PBMC addition. PBMC from each donor were added at a concentration of 2 × 105 per well. The ratio of MSC to responding lymphocytes was selected after in-house optimization and reflects previous studies in the field. In various MLR (with or without MSC) cytokines, neutralizing antibodies, biochemical antagonists or metabolites were added. These included: recombinant IFN-γ (R&D Systems, Abingdon, UK; 200 ng/ml); IL-10 (Immunotools, Friesoythe, Germany; 200 pg/ml), TGF-β1 (R&D Systems; 500 pg/ml), HGF (R&D Systems; 20 ng/ml); polyclonal anti-human IL-10 (Peproptech, London, UK; 1–6 µg/ml), monoclonal anti-human TGF-β1 (R&D Systems; 1–6 µg/ml), monoclonal anti-human HGF (R&D Systems; 0·1–0·3 µg/ml); indomethacin (Sigma Aldrich, Dublin, Ireland; 20 µM); acetyl sialic acid (Sigma Aldrich; 1 µM) or 1-methyl l-tryptophan (Sigma Aldrich; 10 µM); or Kynurenine (Sigma Aldrich; 100 µM). Concentrations chosen reflected those seen in MSC cultures or after in-house optimization. For MLR containing MSC conditioned media, the supernatant used was taken from MSC that were cultured alone for 24 h. After 5 days' culture, 1 µCi [3H]-thymidine was added to each well and incubated for a further 6 h. Cells were then harvested and [3H]-thymidine incorporation measured by liquid beta scintillation counting. Proliferation was represented as the incorporated radioactivity in counts per minute (cpm). Results are expressed as the mean of triplicate values ± standard error (s.e.).
Determination of kynurenine concentration in cell culture
IDO activity was quantified by measuring kynurenine in supernatant from MLR with or without MSC. The medium was precipitated with 30% trichloroacetic acid (20% final volume) and centrifuged at 10 000 g for 10 min. Kynurenine was measured using reversed-phase high performance liquid chromatography (HPLC) (SP8800, Spectra Physics, Fremont, CA, USA). An RP18 column (250 × 4 mm) with a 5-µM pore was used to separate samples and absorbance measured at 360 nm. The mobile phase was a mixture of 5 mM aqueous zinc acetate solution and acetronitrile (Sigma Aldrich) at a ratio of 92 : 8 (v/v) pH 4·9; a sample volume of 20 µl was used. The concentration of kynurenine was determined by comparison against standard kynurenine (Sigma-Aldrich) solutions (0–100 µM). Chromatographic data was processed using a SP4290 integrator (Spectra Physics).
Cytokine measurement
MSC were cultured in 24-well plates (Nunc) in a total of 0·5 ml DMEM culture media (2 × 105/ml). Cells were harvested after 24 h and supernatants collected and assayed by commercial enzyme immunoassay (EIA) for IL-10 (Immunotools), TGF-β1 (R&D Systems) or HGF (Immunotools; recombinant HGF, R&D Systems). Characterization of candidate cytokine mRNA expression by human MSC was performed using reverse transcription–polymerase chain reaction (RT–PCR). MSC were cultured as above for 6 h, or in some cases 24 h. Total RNA was extracted from cells using Tri-Reagent (Molecular Research Centre Inc, Cincinnati, OH, USA) according to the manufacturer's instructions. Briefly, RNA was reverse-transcribed using Superscript II (Invitrogen-gibco) to cDNA. cDNA was analysed for the expression of human IL-10, HGF, TGF-α1, cyclooxygenase (COX)-1, COX-2 and IDO mRNA by semiquantitative RT–PCR. Primer sequences and annealing temperatures are shown in Table 1.
Table 1.
Sequences of primers employed for reverse transcription–polymerase chain reaction (RT–PCR) studies.
| Target | Primer sequence1 | Product size (bp) | Anneal temp °C |
|---|---|---|---|
| TGF-β1 | F 5′-CAGATCCTGTCCAAGCTG-3′ | 270 | 54·8 |
| R 5′-TCGGAGCTCTGATGTGTT-3′ | |||
| HGF | F 5′-ATGCATCCAAGGTCAAGGAG-3′ | 349 | 56 |
| R 5′-TTCCATGTTCTTGTCCCACA-3′ | |||
| IL-10 | F 5′-ATCCAAGACAACACTACTAA-3′ | 588 | 55 |
| R 5′-TAAATATCCTCAAAGTTCC-3′ | |||
| IDO | F 5′-CGCTGTTGGAAATAGCTTC-3′ | 234 | 55 |
| R 5′-CAGGACGTCAAAGCACTGAA-3′ | |||
| COX 1 | F 5′-GAGTTTGTCAATGCCACCT-3′ | 215 | 58·5 |
| R 5′-CAACTGCTTCTTCCCTTTG-3′ | |||
| COX 2 | F 5′-TCCTTGCTGTTCCCACCCATG-3′ | 847 | 55·5 |
| R 5′-CATCATCAGACCAGGCACCAG-3′ |
Forward (F) and reverse (R) primers used to detect mRNA expression for the targets described. TGF: transforming growth factor; HGF: hepatocyte growth factor; IL: interleukin; IDO: indoleamine 2,3, dioxygenase; COX: cyclooxygenase.
Prostaglandin E2 (PGE2) determination
The PGE2 concentration from cultured MSC supernatant was measured by a competitive enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer's instructions. Briefly, this assay was accomplished by converting PGE2 metabolites into a single derivative measured by enzyme-linked immunosorbent assay (ELISA). Inhibition of PGE2 was achieved using indomethacin (Sigma Aldrich), an anti-inflammatory agent that blocks biosynthesis by inhibiting COX [22] at a previously optimized concentration (20 µM, data not shown). MLR were also performed with/without MSC and indomethacin (20 µM) or the alternative inhibitor acetyl sialic acid (Sigma Aldrich; 1 µM). Proliferation assays were performed as above.
Statistical methods
The statistical significance was assessed using Prism3 software (GraphPad Software, San Diego, CA, USA). Data were presented as the mean ± s.e. Statistical analysis was performed by paired t-test. A P-value of P < 0·05 (*), P < 0·01 (**) or P < 0·001 (***) were considered statistically significant.
Results
Human MSC constitutively express HGF, IL-10 and TGF-β1 at immunosuppressive concentrations
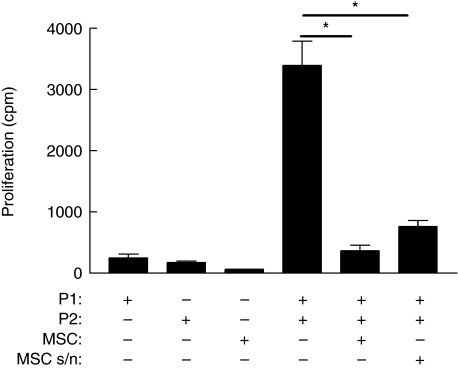
In order to investigate the contribution of soluble factors to immunosuppression, human MSC were examined for their ability to modulate a mixed lymphocyte reaction (MLR), a well-characterized model of allogeneic interaction. MSC were co-cultured with PBMC from two MHC mismatched donors. In line with previous observations, MSC significantly reduced proliferation of responder PBMC (Fig. 1). However, there is controversy as to whether the suppressive effects of MSC are contact-dependent or mediated through the release of soluble factors [14–17,23,24]. Therefore, two-way MLR were performed supplemented by conditioned media from 24-h MSC cultured alone. MSC-derived supernatant suppressed proliferation significantly (Fig. 1) (P < 0·05), suggesting that soluble factors were involved in mediating inhibition of T cell proliferation. However, suppression was less than when cell–cell contact between MSC and effector cells was permitted, suggesting that both contact-dependent and -independent mechanisms operate.
Fig. 1.
Mesenchymal stem cells (MSC) suppress alloantigen-driven proliferation. MSC were co-cultured with peripheral blood mononuclear cells (PBMC) from major histocompatibility complex (MHC) mismatched donors (P1 or P2) for 96 h and alloantigen-driven proliferation measured by [3H]-thymidine incorporation expressed as mean counts per minute (cpm) ± standard error. Significantly reduced proliferation by responder PBMC (*P = 0·012) was determined by paired t-test analysis. Conditioned media (MSC s/n) obtained from 24-h MSC cultures suppressed alloantigen-driven proliferation (*P = 0·027). MSC or PBMC cultured alone are shown for comparison. Results are representative of three independent experiments each performed in triplicate. No significant loss of viability or induction of apoptosis was detected in this or subsequent experiments.
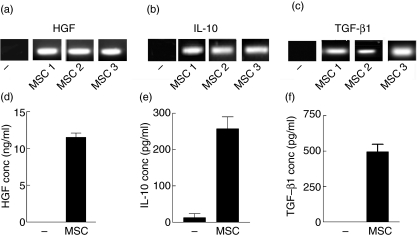
In order to dissect these effects, MSC cultures were examined for the production of three potent immunomodulatory mediators. IL-10 and TGF-β1 are known immunosuppressive cytokines that have the ability to inhibit proinflammatory cytokine secretion by antigen-presenting cells (APCs) and both are involved in the induction of regulatory T cells [25,26]. Similarly, HGF, a cytokine with anti-apoptotic and haematopoietic activity, is emerging as a potent immunomodulator [27]. Cultured human MSC constitutively expressed mRNA for HGF, IL-10 and TGF-β1 (Fig. 2a–c), and all three cytokines could be detected by EIA in supernatants from 24-h cultures by cytokine-specific EIA (Fig. 2d–f).
Fig. 2.
Mesenchymal stem cell (MSC) production of immunomodulatory mediators determined by reverse transcription–polymerase chain reaction (RT–PCR) (a–c) and enzyme immunoassay (EIA) (d–f). mRNA was isolated from 6-h cultures of unstimulated MSC and subjected to semiquantitive reverse transcription–polymerase chain reaction (RT–PCR) as detailed in Methods for hepatocyte growth factor (HGF) (a), interleukin (IL)-10 (b) and transforming growth factor (TGF)-β1 (c). As human results may show variability, MSC derived from three separate donors (a–c: MSC 1, 2, 3) are shown. Supernatants from parallel cultures were also examined at 24 h for HGF (d), IL-10 (e) and TGF-β1 (f) protein expression by EIA. – indicates mRNA (a–c) from 6-h epithelial (A549) non-cytokine-secreting cell cultures or 24-h culture supernatants (d–f) as negative controls. EIA results are representative of three independent experiments using MSC from donor 1. EIA determinations were each performed in triplicate and expressed as mean concentration ± standard error.
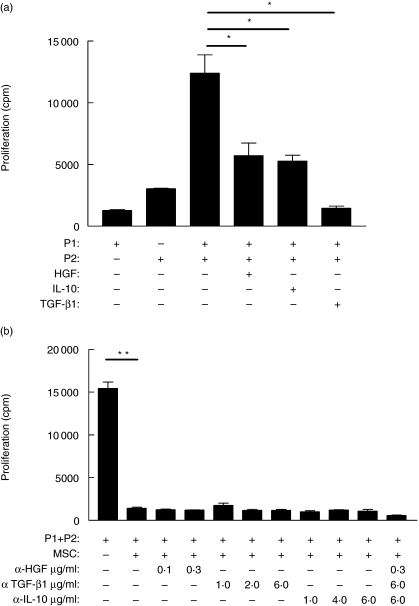
Human IL-10, TGF-β1 and HGF are known to have immunomodulatory properties; however, the activity of these cytokines is concentration-dependent and there is a danger of interpreting data based on observations relating to cytokine concentrations that are not physiologically relevant. Therefore, the effect of these cytokines on allogeneic MLR was examined using concentrations that reflected those seen in MSC culture and which were achievable in vivo (Fig. 2). All three cytokines reduced proliferation repeatedly (Fig. 3), suggesting that these cytokines contribute to MSC-mediated suppression through soluble factors.
Fig. 3.
Immunosuppressive cytokines reduced alloantigen-driven proliferation but could not be reversed by neutralizing these factors. (a) mixed lymphocyte reactions (MLR) were performed with major histocompatibility complex (MHC) mismatched peripheral blood mononuclear cells (PBMC) (P1 and P2) but not mesenchymal stem cells (MSC). Selected cultures also contained recombinant human hepatocyte growth factor (HGF) (20 ng/ml), interleukin (IL)-10 (200 pg/ml) or transforming growth factor (TGF)-β1 (500 pg/ml). Cytokine concentrations were selected as comparable to that observed in Fig. 2. HGF, IL-10 and TGF-β1 significantly decreased alloantigen-driven proliferation (*P = 0·04, P = 0·044 and P = 0·018, respectively). In blocking studies (b) neutralizing antibodies against these cytokines (anti-HGF, 0·1–0·3 µg/ml; anti-TGF-β1, 1–6 µg/ml; anti-IL-10, 1–6 µg/ml) alone or in combination were added to MLR from MHC mismatched donors as above. Blocking any one of these factors alone or in combination did not reverse the inhibitory effect of MSC. Proliferation by responder PBMC are expressed as mean counts per minute ± standard error. Results are representative of at least three independent experiments, each performed in triplicate.
To clarify whether these soluble factors were responsible for MSC-mediated suppression, neutralizing monoclonal antibodies directed against IL-10, TGF-β1 and HGF were added into an MLR containing MSC. In these experiments concentrations of antibody were chosen to reflect the optimium range that effectively neutralized concentrations greater than the amount of cytokine released by MSC as seen in Fig. 2. Consistent with other findings [12,24], neutralization of these soluble factors either alone or in combination failed to prevent MSC-mediated suppression, suggesting that there is either redundancy in the mechanisms of immunosuppression employed by MSC or that other mechanisms take precedence in this function.
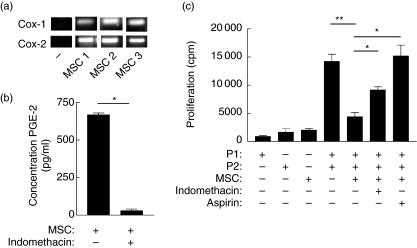
Human MSC constitutively express COX-1, COX-2 and PGE2
Prostaglandins are lipid mediators known to suppress T cells [28]. These mediators are derived from the conversion of arachidonic acid to prostaglandins by isoforms of COX [28]. Therefore, the expression of COX-1 and COX-2 by unstimulated MSC were examined. mRNA for both COX-1 and COX-2 were expressed constitutively by MSC detectable by RT–PCR (Fig. 4a), and significant levels of PGE2 were detected in supernatants from MSC cultures (Fig. 4b). To determine whether PGE2 produced by MSC could account for the inhibition of T cell proliferation, the effect of blocking COX activity was examined. Indomethacin is a non-steroidal anti-inflammatory drug that blocks prostaglandin biosynthesis by inhibiting cyclooxygenase [22]. Figure 4b shows that PGE2 production was decreased dramatically when MSC were treated with indomethacin. It was therefore necessary to determine whether blocking PGE2 synthesis interfered with MSC-mediated suppression. Figure 4c shows that allogeneic proliferation was significantly restored once PGE2 production was prevented in MSC co-culture. Similarly, addition of aspirin (acetyl sialic acid), an alternative COX inhibitor, showed a similar effect [29]. Taken together, these results demonstrate a functional role for PGE2 in suppression mediated by unstimulated MSC through soluble factors.
Fig. 4.
Human mesenchymal stem cells (MSC) modulate alloresponses through prostaglandin production. (a) Expression of cyclooxygenase (COX)-1 and COX-2 mRNA by MSC or control prostaglandin E (PGE) non-producing fibroblast (–) cells was determined by reverse transcription–polymerase chain reaction (RT–PCR). MSC derived from three separate donors (MSC 1, 2, 3) are shown. (b) Constitutive PGE2 was detected in supernatants sampled from 24-h cultures of MSC but antagonized by indomethacin, as determined by specific enzyme immunoassay (EIA). (c) MSC were co-cultured in mixed lymphocyte reactions (MLR) from major histocompatibility complex (MHC) mismatched donors (P1 or P2) as in Fig. 1, but additionally cultures were established containing either indomethacin (20 µM) or acetyl sialic acid (aspirin, 1 µM). PGE2 inhibition by either indomethacin or aspirin significantly restored alloantigen driven proliferation (*P = 0·015 and P = 0·0158, respectively). Proliferation by responder peripheral blood mononuclear cells (PBMC) are expressed as mean counts per minute ± standard error. Results are representative of at least three experiments, each performed in triplicate.
IFN-γ does not ablate MSC inhibition of T cell proliferation but induces high-levels of HGF and TGF-β1 expression
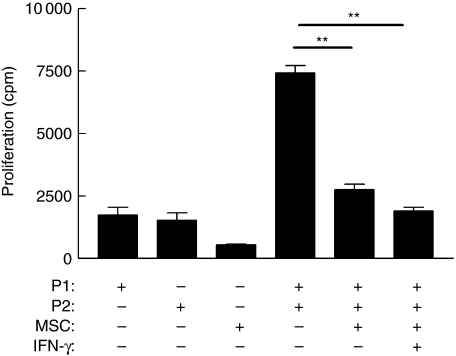
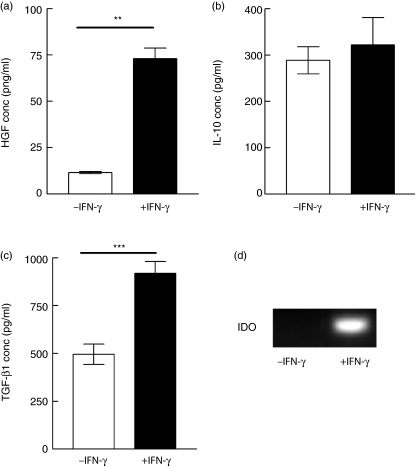
For clinical utility, allogeneic MSC must remain immunosuppressive in an inflamed environment. Therefore, the effect of IFN-γ stimulation on MSC-mediated inhibition of alloantigen-driven proliferation was examined. IFN-γ did not ablate the immunosuppressive effect of MSC in MLR; rather, it suppressed alloantigen-induced T cell proliferation. Figure 5 shows that the suppression of alloresponses, mediated by MSC, was not reversed by IFN-γ stimulation. As IFN-γ exerts potent immunomodulatory and anti-proliferative effects both in vitro and in vivo[30,31], the effects of IFN-γ on MSC cultures were examined. Surprisingly, IFN-γ stimulation resulted in a significant increase in MSC production of HGF and TGF-β1, and no significant reduction in the high levels of IL-10 (Fig. 6a–c). These findings are consistent with a model in which MSC can be activated by inflammatory signals and that such activation supports potent immunosuppressive activity.
Fig. 5.
Interferon (IFN)-γ does not prevent mesenchymal stem cells (MSC) suppression of alloresponsiveness. Mixed lymphocyte reactions (MLR) were performed in the presence or absence of MSC as described in the legend to Fig. 1. MSC significantly suppress alloantigen-driven proliferation (**P = 0·0031). Additionally selected wells contained interferon (IFN)-γ (200 ng/ml) for the duration of the cultures. A significant decrease in proliferation by responder peripheral blood mononuclear cells (PBMC) was observed even in the presence of IFN-γ (**P = 0·0051). Proliferation by responder PBMC are expressed as mean counts per minute ± standard error. Results are representative of three independent experiments, each performed in triplicate.
Fig. 6.
Interferon (IFN)-γ increases expression of hepatocyte growth factor (HGF) and transforming growth factor (TGF)-β1 and induces indoleamine 2,3, dioxygenase (IDO). Supernatants from 24-h cultures of mesenchymal stem cells (MSC) in the absence or presence of IFN-γ (200 ng/ml) were also examined for HGF (a), interleukin (IL)-10 (b) and transforming growth factor (TGF)-β1 (c); 24-h cultures of unstimulated or IFN-γ-stimulated MSC were lysed and assayed by semiquantitive transcription–polymerase chain reaction (RT–PCR) for IDO mRNA expression (d) as detailed in Methods. IFN-γ significantly increased HGF (**P = 0·0097) and TGF-β1 (***P = 0·0008) and did not reduce IL-10 expression following stimulation (P = 0·5). Enzyme immunoassay (EIA) results are expressed as mean counts per minute ± standard error and are representative of at least three independent experiments, each performed in triplicate.
IFN-γ-induced expression of IDO by MSC contributes to immunomodulation
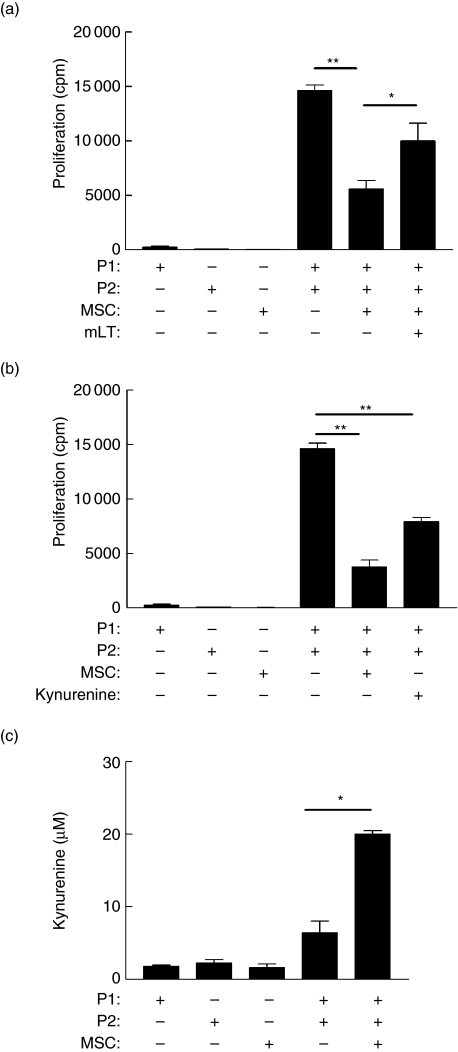
Expression of the tryptophan degrading enzyme IDO has been documented as playing a role in the suppression of T cell activity at the feto–maternal interface [32,33]. Recently, we and others proposed that MSC retain features of this frequent form of allograft-mediated immune modulation [19,34]. The expression of IDO by MSC was therefore investigated. RT–PCR demonstrated that human MSC do not constitutively express IDO (Fig. 6d). However IFN-γ stimulation of MSC induced enzyme expression detectable by RT–PCR (Fig. 6d). Again, the presence of mRNA need not correlate with protein or functional activity, therefore a chemical antagonist, 1 methyl l-tryptophan (mLT), was introduced to MLR containing MSC. Addition of mLT restored proliferation (Fig. 7a), suggesting that IDO activity contributes to IFN-γ-mediated MSC immunomodulation. Previously, we and others have proposed that local tryptophan depletion may lead to a ‘tryptophan desert’, a condition that is putatively suppressive for T cell priming [19,35]. However, an alternative possibility was that tryptophan depletion was a surrogate marker and that the local accumulation of tryptophan metabolites was acting as the direct immunomodulator. Consequently the tryptophan metabolite kynurenine was added to MLR in the absence of MSC. Cultures showed no apoptosis or decreased viability (data not shown); however, kynurenine blocked alloantigen-driven proliferation (Fig. 7b). Thus IFN-γ-mediated suppression of alloresponsiveness by MSC involves IDO-mediated mechanisms, but this may be driven by the accumulation of local kynurenine rather than tryptophan depletion. Ideally, antibody neutralization or siRNA studies would confirm these findings; however, the small size of kynurenine and its position as a metabolic degradation product preclude these approaches. However, in addition to the metabolic inhibitor study (Fig. 7a), a testable prediction from this hypothesis was that increased levels of kynurenine would be observed in MLR that contained MSC; to test this possibility, kynurenine concentration was measured by HPLC from different cultures with or without MSC. Consistent with the hypothesis above, Fig. 7c shows that kynurenine did accumulate when MLR were performed in the presence of MSC.
Fig. 7.
Indoleamine 2,3, dioxygenase (IDO) plays a role in mesenchymal stem cells (MSC)-mediated suppression of allo-responsiveness. Mixed lymphocyte reactions (MLR) were performed with mismatched donors (P1 and P2), as described in the legend to Fig. 1. Additionally, selected wells were treated with the IDO antagonist, 1-methyl l-tryptophan (mLT) (10 µM) (a) or with MSC replaced by the tryptophan breakdown product kynurenine (100 µM) (b). mLT significantly restored proliferation (a; *P = 0·047); conversely, addition of exogenous kynurenine significantly reduced allo-driven proliferation (b; **P = 0·0084). Kynurenine also accumulated in MLR containing MSC (c). Kynurenine concentration in supernatant from MLR with or without MSC was determined by high performance liquid chromatography (HPLC) as described in Methods. Kynurenine was significantly increased in MLR performed in the presence of MSC (*P = 0·022), peripheral blood mononuclear cells (PBMC) and MSC cultures are shown for comparison. Results are representative of three independent experiments, expressed as mean ± standard error, each performed in triplicate.
Discussion
The mechanisms through which human mesenchymal stem cells suppress alloresponses are poorly understood. This study, using an MLR model, does not exclude a role for cell-contact-dependent mechanisms, but clarifies how MSC contribute to conditioning an immunosuppressive milieu through cytokine secretion and expression of enzymatic pathways associated with immune modulation. Human MSC constitutively express levels of HGF, IL-10 and TGF-β1 that can modulate alloresponses. MSC are also shown to constitutively express COX-1 and -2. Antagonists of prostaglandin biosynthesis significantly reduced the suppressive activity of MSC, supporting a role for prostaglandins in this effect. Exposure to IFN-γ did not ablate MSC inhibition of T cell proliferation but induced expression of HGF and TGF-β1 at concentrations that suppressed alloresponsiveness. IDO was not expressed constitutively by MSC but could be induced by IFN-γ, and expression contributed to immunomodulation as demonstrated by use of an antagonist. However, addition of kynurenine to MSC cultures also blocked allodriven proliferation, suggesting that immunosuppression may be mediated through the local accumulation of tryptophan metabolites rather than tryptophan depletion. The observation of increased kynurenine concentration in MLR containing MSC supported this hypothesis. Taken together, these results demonstrate that MSC could be activated by inflammatory signals and that such activation supported up-regulation of potent immunosuppressive activity consistent with the creation of an immunosuppressive microenvironment through soluble factors.
The mechanisms by which MSC suppress alloresponsiveness has been the subject of intense recent research [18,19,34,36]. From an evolutionary standpoint, the capacity of MSC to perform such a function is counterintuitive. However, we and others have suggested recently that MSC retain the immunomodulatory properties of the fetal allograft [18,34]. This concept is intriguing, given that both entities have to retain the capacity for controlled cell division and multi-potent differentiation. Thus, the reason that MSC possess such immunomodulatory capacity may be that it is a retained attribute of mesenchymal ‘stemness’ and differentiation capacity. It is known that the study of MSC is confounded by species and methodological differences, and different groups have reported differences in immunomodulatory capacity [6,12–17,23]. Despite these differences, there is agreement that MSC use a surprising array of mechanisms to avoid deletion by the mismatched host [18,19].
There is further conflicting opinion regarding the contribution from cell-contact-dependent and -independent mechanisms to suppression [13–17]. While this study has focused on the soluble factors from MSC that could condition the cellular microenvironment, our antagonist studies suggest that contact-dependent mechanisms are also involved. A dual mechanism that involves both contributions is reminiscent of previous scientific debates regarding the mechanisms by which CD4+ T cells provide help for B cell production of antibody [37,38]. That process is now known to require contact-dependent and -independent mechanisms operating in concert [39]. The data provided herein are consistent with MSC mediating suppression via both mechanisms, albeit through different soluble mediators and ligand–receptor interactions.
This study demonstrates that a well-characterized human MSC population constitutively secretes the immunomodulatory cytokines HGF, IL-10, TGF-β1 at concentrations that can suppress alloresponsiveness (Figs 2 and 3). Interestingly, comparison of the mechanisms operating at the materno–fetal interface show striking similarities, where HGF, IL-10, TGF-β1 play an immunomodulatory role that serves to contribute to tolerance of the fetal allograft [18]. The observation that MSC supernatant suppressed MLR indicates that cell–cell contact is not an absolute requirement for suppression. In contrast, Maitra et al. observed significant inhibition of MLR only when conditioned medium from MSC and unrelated lymphocytes was used, suggesting that an MSC–lymphocyte interaction results in the release of inhibitory mediators either by MSC or lymphocytes [40]. Both data are consistent with soluble factor production requiring an as-yet unidentified activation event. However, as lymphocytes were not present in the system to develop conditioned medium in this work, the findings herein suggest that it is MSC-derived factors that are mediating suppression. Similarly, animal studies by Krampera et al. and Augello et al. stress the significance of cell–cell contact for specific inhibition of T cell activation [16,41]. However, Augello et al. showed that soluble factors are involved in suppression of B cells [41].
The present study found that the immunosuppressive effect mediated by MSC is not reversed by neutralizing IL-10, HGF and/or TGF-β1; however, a role for their involvement cannot be ruled out completely. There is a possibility that these factors play a supporting but redundant role as immunosuppressive mediators. Previous studies have suggested that HGF played a role in the acceptance of allogeneic grafts. Although Le Blanc et al. did not detect HGF in MSC co-cultures [24], Di Nicola et al. suggest that HGF works in synergy with TGF-β1 to resist T cell recognition [14]. Simultaneous neutralization of HGF and TGF-β1 in the latter study restored T cell proliferation; however, even using combinations of neutralizing antibodies, this effect was not observed herein. This discrepancy may be explained by differences in MSC and MLR populations. Di Nicola et al. used CD2+ separated allogeneic cells cultured at a ratio of 1 : 1 MSC : PBMC. Nevertheless, the possibility of the involvement of these soluble factors is not excluded by our findings; rather we propose that they have a subsidiary or redundant role.
This study clarifies the role of prostaglandins in MSC-mediated immunomodulation. In humans, expression of COX-1 is generally considered to be widespread, whereas COX-2 expression is more restricted [28]. Here, we confirm that human MSC express both COX-1 and -2. By blocking these isoforms, we have shown effectively that PGE2 has a role in mediating suppression (Fig. 4c). Although this conflicts with some studies suggesting that PGE2 is not a significant component of suppression [12,23], Aggarawal and Pittenger have shown that inhibition of PGE2 ablated MSC-mediated immunosuppressive effects on T cells [13]. This finding has two important implications. First, it demonstrates another parallel between the tolerance of the fetal allograft and MSC, as PGE2 performs both physiological and developmental functions during pregnancy with an immunomodulatory role that protects the fetal allograft [42,43]. The second implication is of potential clinical importance and has received little attention. MSC have been proposed as potential therapeutic agents in ischaemic heart disease; however, many of these patients will be on pre-existing medications which may influence PGE2 expression [44,45]. Caution will need to be taken in the use of allogeneic MSC in such situations to ensure that pre-existing therapies do not interfere with MSC capacity to suppress alloreactivity.
A key question that will decide the utility of clinical use of MSC for regenerative medicine centres on whether MSC retain their immunomodulatory capacity in an inflamed environment. Very recent work by Krampera et al. suggests that IFN-γ produced upon T cell activation contributes to MSC suppressive activity [46]. The present study shows that addition of the proinflammatory cytokine IFN-γ, even at a high concentration, does not ablate MSC immunosuppressive activity. More importantly, we demonstrate that IFN-γ stimulation of MSC enhances the secretion of the immunosuppressive mediators, HGF and TGF-β1, which can potentially inhibit T cell proliferation (Fig. 2), supporting a role for IFN-γ, and in line with studies showing that MSC exposed to IFN-γ fail to up-regulate MHC class II and do not elicit alloreactive lymphocyte responses [11].
Tissue damage (presumably requiring the recruitment of stem cells/induction of repair mechanisms) and inflammation are coincident and co-localized processes. It is therefore not surprising that MSC can be activated by inflammatory signals and that such activation should support up-regulation of potent immunosuppressive activity. The current study therefore reinforces the potential use of MSC in the clinical setting where inflammation is a feature, but where therapeutic prostaglandin inhibition is not an underlying requirement.
The immunological role of IDO, a tryptophan catabolizing enzyme [35], has also been the subject of recent debate. IDO expression in the syncytiotrophoblast contributes to the tolerance of the fetal allograft, where expression is thought to deplete the local environment of l-tryptophan, an essential amino acid for cell growth [47]. Previously, we and others have suggested that IDO expression leads to the formation of a ‘tryptophan desert’, a microenvironment in which tryptophan is depleted, that modulates T cell function, and during infection impairs microbial activity [19,48,49]. However, our results presented herein refine that model. We show that IFN-γ up-regulates IDO expression by MSC. Response elements for IFN-γ have been identified in the human IDO promoter and shown to be essential for induction of reporter gene expression in vitro[50]. Two IFN-stimulated response elements (ISREs) and an IFN-γ-activated site (GAS) element are found in the 5′-flanking region of the IDO gene, supporting a role for IFN-γ in regulating IDO [50]. IDO expression by activated MSC may play a role in allo-MSC acceptance and could represent a novel means of immunoregulation for both MSC and the fetal allograft. IDO involvement has been suggested by studies showing restoration of T cell proliferation by addition of tryptophan to an MLR [51]. In the current study, we extend this further to show that an IDO antagonist, 1-methyl l-tryptophan, ablates MSC immunomodulation, indicating the IDO functional activity is a mechanism contributing to suppression. In addition to this, we show that inhibition of T cell proliferation may be mediated through the accumulation of kynurenine, a tryptophan breakdown product [52]. Furthermore, we show that kynurenine concentration increases in MSC-suppressed MLR consistent with this hypothesis. This study may resolve confounding observations concerning the role of IDO in suppression using tryptophan supplementation [12]. IDO may exert its effect not through depletion (the creation of a ‘tryptophan desert’), but rather through local accumulation of kynurenine, a product of tryptophan catabolism.
In conclusion, we demonstrate that MSC secrete factors consistent with the creation of an immunosuppressive milieu or microenvironment. The similarity to the materno–fetal interaction is striking. The carriage of the fetal allograft requires a range of co-ordinated immunomodulatory activities involving cell–cell contact and soluble factors. The data herein are consistent with MSC retaining not just early life features of differentiation, multipotency and controlled renewal, but also mechanisms of immunomodulation associated normally with the survival of the fetal allograft.
Acknowledgments
This work was funded by a Science Foundation Ireland, Centre for Science Engineering and Technology award (CSET) in Regenerative Medicine.
References
- 1.Friedenstein AJP, Petrokova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morph. 1966;16:381–90. [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–92. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 6.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 7.Arinzeh TL, Peter SJ, Archambault MP, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–35. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 9.Koc ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 10.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 12.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 14.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 15.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 17.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–19. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 18.Barry FP, Murphy JM, English K, et al. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Devel. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 19.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran A, Mahon BP, McParland P, et al. Ex vivo cytokine responses against parvovirus B19 antigens in previously infected pregnant women. J Med Virol. 2003;70:475–80. doi: 10.1002/jmv.10420. [DOI] [PubMed] [Google Scholar]
- 22.Kulmacz RJ. Topography of prostaglandin H synthase. Antiinflammatory agents and the protease-sensitive arginine 253 region. J Biol Chem. 1989;264:14136–44. [PubMed] [Google Scholar]
- 23.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–15. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 25.Gollnick SO, Cheng HL, Grande CC, et al. Effects of transforming growth factor-beta on bone marrow macrophage Ia expression induced by cytokines. J Interferon Cytokine Res. 1995;15:485–91. doi: 10.1089/jir.1995.15.485. [DOI] [PubMed] [Google Scholar]
- 26.Asseman C, Powrie F. Interleukin 10 is a growth factor for a population of regulatory T cells. Gut. 1998;42:157–8. doi: 10.1136/gut.42.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroiwa T, Kakishita E, Hamano T, et al. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J Clin Invest. 2001;107:1365–73. doi: 10.1172/JCI11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris SG, Padilla J, Koumas L, et al. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 29.Wu KK. Aspirin and salicylate: an old remedy with a new twist. Circulation. 2000;102:2022–3. doi: 10.1161/01.cir.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 30.Dai W, Gupta SL. Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem Biophys Res Commun. 1990;168:1–8. doi: 10.1016/0006-291x(90)91666-g. [DOI] [PubMed] [Google Scholar]
- 31.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–6. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonney EA, Matzinger P. Much IDO about pregnancy. Nat Med. 1998;4:1128–9. doi: 10.1038/2624. [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 34.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–79. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–9. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 37.Croft M, Swain SL. B cell response to fresh and effector T helper cells. Role of cognate T–B interaction and the cytokines IL-2, IL-4, and IL-6. J Immunol. 1991;146:4055–64. [PubMed] [Google Scholar]
- 38.Noelle RJ, Snow EC. Cognate interactions between helper T cells and B cells. Immunol Today. 1990;11:361–8. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- 39.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–82. [PubMed] [Google Scholar]
- 40.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 41.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 42.Arosh JA, Banu SK, Chapdelaine P, et al. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology. 2004;145:2551–60. doi: 10.1210/en.2003-1607. [DOI] [PubMed] [Google Scholar]
- 43.Denison FC, Kelly RW, Calder AA, et al. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod. 1998;13:3560–5. doi: 10.1093/humrep/13.12.3560. [DOI] [PubMed] [Google Scholar]
- 44.Guazzi M, Brambilla R, Reina G, et al. Aspirin-angiotensin-converting enzyme inhibitor coadministration and mortality in patients with heart failure: a dose-related adverse effect of aspirin. Arch Intern Med. 2003;163:1574–9. doi: 10.1001/archinte.163.13.1574. [DOI] [PubMed] [Google Scholar]
- 45.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 46.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 47.Sedlmayr P, Blaschitz A, Wintersteiger R, et al. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385–91. doi: 10.1093/molehr/8.4.385. [DOI] [PubMed] [Google Scholar]
- 48.Frumento G, Rotondo R, Tonetti M, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahon BP, Mills KH. Interferon-gamma mediated immune effector mechanisms against Bordetella pertussis. Immunol Lett. 1999;68:213–17. doi: 10.1016/s0165-2478(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 50.Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon-gamma-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–52. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- 51.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 52.Heyes MP, Chen CY, Major EO, et al. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326:351–6. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]