Abstract
Modification of intestinal microbiota early in life by administration of probiotic bacteria may be a potential approach to prevent allergic disease. To select probiotic bacteria for in vivo purposes, we investigated the capacity of probiotic bacteria to interact with neonatal dendritic cells (DC) and studied the ensuing T cell polarizing effect. Immature DC were generated from cord blood-derived monocytes and maturation was induced by maturation factors (MF), lipopolysaccharide (LPS) plus MF and Bifidobacterium bifidum, B. infantis, Lactobacillus salivarius, Lactococcus lactis alone or combined with MF. After 12 days of co-culture with DC and Staphylococcus aureus enterotoxin B (SEB) as antigenic stimulus, cytokine production by autologous T cells was determined by intracellular cytokine staining. Additionally, cells were stimulated with CD3 and CD28 monoclonal antibodies and cytokines were measured in supernatants by multiplex assay. The probiotic strains induced partial maturation of DC. Full maturation of DC was induced for all strains tested when MF was added. The percentage of interleukin (IL)-4 producing T cells was lower in T cell cultures stimulated with B. bifidum matured DC compared to MF and LPS matured DC, which coincided with a higher percentage of interferon (IFN)-γ-producing T cells. Furthermore, T cells stimulated by B. bifidum matured DC produced significantly more IL-10 compared to MF matured DC. Selected species of the Bifidobacterium genus prime in vitro cultured neonatal DC to polarize T cell responses and may therefore be candidates to use in primary prevention of allergic diseases.
Keywords: cord blood, dendritic cells, immune modulation, probiotics, Th1 skewing
Introduction
The hygiene hypothesis states that decreased exposure to microbial stimuli early in life contributes to the increasing prevalence of atopic disease [1,2]. Much attention has focused upon the intestinal microbiota and its potential role in the development of atopic disease [3]. Enhanced presence of probiotic bacteria in the intestinal microbiota seems to correlate with protection against atopy, as bifidobacteria and lactobacilli are found more commonly in the composition of the intestinal flora of non-allergic children [4–6]. Intervention studies in which lactobacilli and bifidobacteria were studied for possible beneficial effects in prevention and treatment of atopic disease support the association between the intestinal microbiota and the development of atopic disease [7–10].
It is not clear how intestinal microbes, including probiotic bacteria, interact with the intestinal mucosal immune system and which bacteria or bacterial products are beneficial. Probiotic bacteria may be capable of modulating the immune system [3,11]. For example, reconstitution of the intestinal microbiota of germ-free rodents restored their development of oral tolerance [12]. In the gut, antigen-presenting cells (APC), in particular dendritic cells (DC), play a crucial role in innate as well as adaptive immune response against microbial antigens. DC are the main stimulators of naive T cells and the nature of the T cell polarizing signals is determined largely by the type of microbial products encountered in the peripheral tissues [13]. Previous studies have shown that viable and killed probiotic bacteria demonstrate strain-specific effects on the phenotype of human and murine DC [14–16], and on polarizing T helper cell responses via modulation of dendritic cell function [17–20]. Therefore, we speculate that specific strains may have higher potential to target specific diseases such as atopic diseases. To this end, we chose to select probiotic bacteria based on their capacity to modulate immune responses in vitro in preparation of our clinical trial on primary prevention of atopy and allergic disease (NCT00200954). Previously, we have examined the effects of 13 strains of probiotic bacteria on their capacity to modulate cytokine production by adult peripheral blood mononuclear cells (PBMC) [21]. We selected four strains to investigate further the effect of probiotic bacteria on neonatal immune cells. In this study, we investigated the effects of four selected probiotic strains on maturation of cord blood monocyte-derived DC. Furthermore, the effect of DC matured in the presence of probiotic bacteria on polarization of the neonatal T cell response was examined.
Methods
Bacterial strains and preparation of bacteria
Four strains were selected for the present study based on their capacity to modify cytokine production of PBMC [21]. These strains are: Bifidobacterium (B) bifidum W23; B. infantis W52; Lactobacillus (Lb.) salivarius W24; Lactococcus (Lc.) lactis W58. B.bifidum, B. infantis and Lc. lactis were selected based on their capacity to induce the production of interleukin (IL)-10 and reduction of IL-5 and IL-13 production. Lb. salivarius was included because of its contrasting effect, i.e. no induction of IL-10 production. All strains were supplied and prepared by Winclove Bio Industries®, Amsterdam, the Netherlands. Pure strains were cultured from frozen stocks as described previously [21]. One fresh aliquot was thawed for every new experiment to avoid variability in the cultures.
Cell preparation
Umbilical cord blood was obtained from deliveries of healthy children. The study was approved by the Medical Ethics Committee for Human Research of the University Medical Centre, Utrecht. Blood samples were collected in cord blood collection bags (MacoPharma, Utrecht, the Netherlands) and mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). The cells were washed and resuspended in RPMI-1640 containing l-glutamine (2 mM) and penicillin (100 U/ml)/streptomycin (100 µg/ml) (all obtained from Invitrogen Life Technologies, Breda, the Netherlands) and supplemented with 2% heat-inactivated fetal calf serum (FCS). CD14 monocytes were purified by positive selection using anti-CD14 conjugated magnetic microbeads according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladback, Germany). Flow cytometric analysis showed that CD14 positive monocytes were recovered with a purity of > 90%. Subsequently, the negatively selected cells were used to isolate naive T cells from cord blood mononuclear cells by positive selection with anti-CD4-conjugated magnetic microbeads (Miltenyi Biotec).
In vitro generation and maturation of DC
Immature DC (IDC) were generated by culturing cord blood CD14+ monocytes, as described previously [22]. At day 6, maturation was induced by culturing the cells for 2 days with 50 ng/ml IL-1β and 50 ng/ml tumour necrosis factor (TNF)-α (both Strathmann, Hamburg, Germany), referred to subsequently as maturation factors (MF), lipopolysaccharide (LPS) Escherichia coli (Sigma-Aldrich, St. Louis, MO, USA) plus MF (LPS-DC), and the different probiotic bacteria [20 × 106 colony-forming units (CFU)/ml; bacteria: cell ratio 10 : 1] in the presence or absence of MF.
Expression of cell surface molecules and cytokine production of dendritic cells
Expression of cell surface molecules and cytokine production was studied in in vitro generated DC as described above. The maturation status was determined by cell surface analysis. DC were washed in fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS) containing 0·02% azide, 2% fetal calf serum (FCS) and 2 mM ethylenediamine tetraacetic acid (EDTA)] and to block non-specific binding of antibody reagents incubated with heat-inactivated human serum (30 min at 4°C). Subsequently, cells were incubated in 50 µl of FACS buffer containing appropriately diluted fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)- or allophycocyanin (APC)-labelled monoclonal antibodies (mAbs) against human CD86, CD80, CD14, CD40 and HLA-DR (all from BD Biosciences, Mountain View, CA, USA). Cells were analysed using FACS-Calibur and CellQuest software (BD Biosciences). For cytokine production, at day 8 of the DC cultures, mature DC (2 × 104 cells) were stimulated with mouse CD40-ligand (CD40L)-expressing mouse plasmacytoma cells (J558, 2 × 104 cells; a gift from Dr P. Lane, University of Birmingham, UK) overnight. Supernatants were collected and stored until further use.
Stimulation and culture of CD4+ T cells by mature DC
Autologous CD4+ T cells (2 × 104 cells) were co-cultured with mature DC (5 × 103 cells) in the presence of the superantigen Staphylococcus aureus enterotoxin B (SEB) (100 pg/ml; Sigma-Aldrich) in 96-well flat-bottomed culture plates (Nunc, Roskilde, Denmark). At day 5, 10 U/ml recombinant (r)IL-2 (Roche, Basel, Switzerland) was added to the cultures and the cultures were expanded for the next 7 days. At day 5, [3H]-thymidine incorporation was measured to investigate the capacity of the differently matured DC to induce T cell proliferation.
Cytokine production by CD4+ T cells
On day 12, T cells were restimulated with 10 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) and 1 µg/ml ionomycin (Calbiochem, San Diego, CA, USA) for 6 h, the last 5 h in the presence of 10 µg/ml Brefeldin A (Sigma-Aldrich). Cells were fixed in 2% paraformaldehyde (PFA; Fluka, Deisenhofen, Germany), permeabilized with saponin buffer 0·5% [PBS containing 0·5% bovine serum albumin (BSA)], 0·05% azide and 0·5% saponin (Sigma-Aldrich), and stained with anti-human IFN-γ-FITC and anti-human IL-4-PE (both from BD Pharmingen) to measure intracellular IL-4 and IFN-γ production by flow cytometry. In parallel, T cells were restimulated overnight with 0·4 µg/ml anti-CD3 mAbs and 1 µg/ml anti-CD28 mAbs (both from BD Pharmingen). Supernatants were collected and cytokines were detected by multiplex immunoassay on a Luminex-100 system [23].
Incubation of bacteria with transfected Chinese hamster ovary (CHO)-cell lines
CHO cells transfected with human CD14 (CHO/CD14), human Toll-like receptor (TLR)-2 (CHO/CD14/TLR-2) and human TLR-4 (CHO/CD14/TLR-4) were kindly provided by Liana Steeghs (University Medical Centre Utrecht) and Douglas Golenbock (Division of Infectious Diseases and Immunology, University of Massachusetts Medical School). Upon activation via TLRs or CD14, expression of CD25 on the cell surface induced via a transfected nuclear factor kappa B (NF-kB) construct, was used as a read-out [24]. PE-labelled anti-CD25 mAb was purchased from Becton Dickinson. Cells were cultured and stimulated as described in detail elsewhere [25]. In pilot experiments, viable, heat-inactivated (incubated 30 min at 56°C) or PFA fixed bacteria were added in different bacteria : cell ratios, i.e. 50 : 1, 20 : 1 and 10 : 1. Viable and heat-inactivated probiotic bacteria in a bacteria : cell ratio of 50 : 1 were found to induce to the highest expression of CD25 and were used in further experiments. Heat-inactivated Neisseria meningitides was used as a positive control for all three cell lines. CD25 expression was determined by flow cytometry.
Statistical analysis
All cultures were carried out in duplicate or triplicate. Values and error bars in the figures represent the mean ± standard error of the mean (s.e.m.). Cytokine data were analysed as continuous data. Non-parametric statistical analyses were performed with the Mann–Whitney U-test to reveal significant differences in capacity to induce cytokine production. Differences were considered significant at P < 0·05. Statistical calculations were performed with spss 11·5 for Windows.
Results
Probiotic bacteria induce partial maturation of in vitro-cultured DC
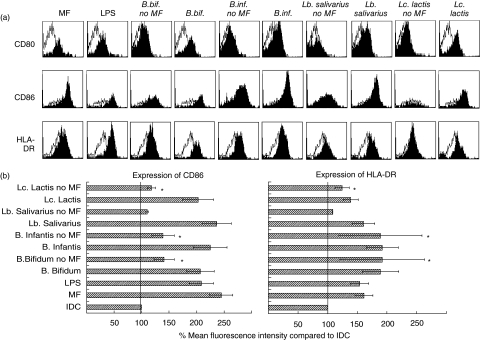
We investigated the effect of four probiotic bacterial strains on the expression of CD40, CD80, CD86 and human leucocyte antigen D-related (HLA-DR) as maturation markers of DC. First, we determined the optimal bacteria : DC ratio. A bacteria : DC ratio of 10 : 1 induced higher expression of co-stimulatory molecules compared to a ratio of 1 : 1. Higher bacterial doses had a cytotoxic effect on the DC (data not shown). Therefore, a 10 : 1 bacteria : DC ratio was selected for further experiments. Exposure of IDC to probiotic bacteria resulted in partial DC maturation (Fig. 1a) and expression of CD86 and HLA-DR was enhanced significantly compared to IDC with B. bifidum, B. infantis and Lc. lactis (Fig. 1b). No differences in expression of CD40 and CD80 were observed between IDC and DC cultured with the four tested strains. However, full maturation of DC comparable to MF-DC and LPS-DC was induced for all strains tested when MF were added (Fig. 1a,b). In the presence of MF no statistically significant differences were observed in the expression of CD86 and HLA-DR, whether or not bacteria were added. In subsequent experiments, IDC were cultured with probiotic strains in the presence of MF to induce equal maturation in all groups.
Fig. 1.
Phenotype and maturation status of cord blood monocyte-derived dendritic cells (DC) upon exposure to selected probiotic bacteria. Immature DC were matured with maturation factors (MF), lipopolysaccharide (LPS) and probiotic bacteria (bacteria : cell ratio 10 : 1) in the presence or absence of MF. After 48 h expression of CD14, CD40, CD80, CD86 and human leucocyte antigen D-related (HLA-DR) was analysed by flow cytometry. (a) Open histograms represent expression by immature DC, solid histograms show the level of expression as a result of treatment. One representative experiment of nine is shown. (b) Mean expression of CD86 and HLA-DR in differently matured DC. The expression of CD86 and HLA-DR of immature DC was set at 100% and the relative expression in other culture conditions was compared to this value. Data represent mean expression ± standard error of the mean (n = 9). Statistical analysis was performed by Mann–Whitney U-test. *P < 0·05 compared to immature DC. Expression of CD86 and HLA-DR of all fully matured DC (MF, LPS, Bifidobacterium bifidum, B. infantis, Lactobacillus salivarius and Lactococcus lactis) was significantly higher (P < 0·01) compared to immature DC.
Probiotic bacteria do not modify production of cytokines by in vitro-cultured DC
Next, we measured the capacity to produce cytokines by DC matured with different stimuli after ligation of CD40 by CD40L, mimicking the engagement by T cells. Compared to MF-DC and LPS-DC, production of IL-12 and TNF-α was not significantly (P > 0·05) affected by the presence of the four tested strains during maturation (Fig. 2a,b). Furthermore, B. bifidum stimulated the production of IL-6 fourfold compared to MF and LPS DC, but not statistically significantly (P= 0·909 and P= 0·277, respectively) (Fig. 2c). Other cytokines were also measured, but production of IL-10 was minimal. No production of IL-1α, IL-1β, IL-2, IL-4, IL-5 and IFN-γ was observed (data not shown).
Fig. 2.
Cytokine production by dendritic cells (DC) upon exposure to probiotic bacteria. Mature DC (day 8 of culture) were stimulated overnight with CD40-ligand transfected J558 cells to induce production of cytokines. Interleukin (IL)-12, tumour necrosis factor (TNF)-α and IL-6 were measured in supernatants by multiplex assay. Data represent mean cytokine production ± standard error of the mean (n = 7). Compared to maturation factors (MF) matured DC or lipopolysaccharide (LPS)-matured DC the presence of the four tested strains during maturation did not have any statistically significant effect (P > 0·05) on the production of IL-12, TNF-α and IL-6 by DC. Statistical analysis was performed by Mann–Whitney U-test.
B. bifidum polarizes in vitro-cultured DC to drive Th1 responses
To investigate the T cell polarizing capacity of DC matured with probiotic bacteria, we performed co-cultures with autologous CD4+ T cells. CD4+ T cells proliferated to a similar extent, independently of a prior incubation of the DC with probiotic bacteria. In parallel, the expression of CD25 as cell surface marker of activation of CD4+ T cells was similar in all co-cultures, indicating equally activated T cells (data not shown).
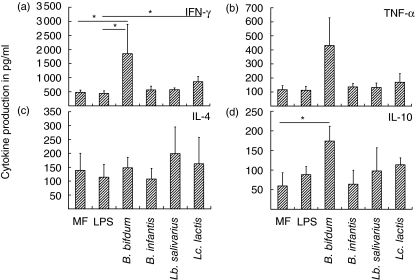
Next, the intracellular cytokine profile of the Th1 cytokine IFN-γ and the Th2 cytokine IL-4 was determined, B. bifidum-stimulated DC reduced the number of IL-4 producing Th cells and increased the number of IFN-γ-producing Th cells (Fig. 3a). The mean percentage of IL-4-producing T cells was decreased by up to 50% in T cell cultures stimulated with B. bifidum compared to T cells activated by MF-DC and LPS-DC. In parallel, the mean percentage of IFN-γ-producing T cells was increased (Fig. 3b). The differences observed in percentages of IL-4- and IFN-γ-positive T cells did not reach statistical significance (P > 0·05) due to large donor variability in the number of IL-4 positive and IFN-γ-positive T cells, but consistent effects were observed in individual experiments. Lc. lactis-stimulated DC seemed to have similar effects on CD4+ cells to B. bifidum but to a much lesser extent. The effect of B. infantis-DC on the number of IL-4-producing T cells was mainly donor-dependent, but B. infantis-DC consistently increased the percentage of IFN-γ producing T cells (data not shown). Lb. salivarius-DC did not affect IL-4 or IFN-γ production. Similar results were obtained when comparing the IFN-γ/IL-4 ratio. For MF and LPS matured DCs the IFN-γ/IL-4 ratio was 3·44 and 3·70, respectively. B. bifidum matured DC induced the highest IFN-γ/IL-4 ratio, 9·44. IFN-γ/IL-4 ratio for B. infantis was 4·18, for Lb. salivarius 4·34 and for Lc. lactis 5·25. Besides IFN-γ and IL-4 we also aimed to investigate the production of other cytokines by CD4+ T cells. To this end, cells were restimulated for 18 h with anti-CD3 mAb and anti-CD28 mAb and we measured levels of cytokines in the supernatants by multiplex assay. Indeed, in cultures of DCs co-cultured with B. bifidum, levels of IFN-γ were increased significantly (Fig. 4a). B. bifidum DC also stimulatedproduction of TNF-α by CD4+ T cells (Fig. 4b). A significant, although weaker, effect on IFN-γ production was also observed for Lc. lactis co-cultured DCs (Fig. 4a). Cytokine measurements in supernatants did not confirm reduced production of IL-4 for B. bifidum and Lc. lactis-DC, as demonstrated with intracellular cytokine analysis (Fig. 4c). Furthermore, production of IL-10 by T cells stimulated with B. bifidum-DC was significantly increased compared to MF-DC (Fig. 4d).
Fig. 3.
Intracellular production of interferon (IFN)-γ and interleukin (IL)-4 by CD4+ T cells. Dendritic cells (DC) matured by different stimuli (5 × 103 cells) were co-cultured with CD4+ T cells (2 × 104 cells) and Staphylococcus aureus enterotoxin B (SEB) (100 pg/ml). After 12 days, CD4+ T cells were restimulated with phorbol myristate acetate (PMA) and ionomycin (last 5 h in the presence of Brefeldin A) and IFN-γ and IL-4 production per cell was measured by intracellular staining and flow cytometry. (a) One representative experiment of nine is shown. The number in the dot plots are the percentages of cells in the corresponding quadrant of the representative experiment. (b) Data represent mean percentage ± standard error of the mean of IFN-γ and IL-4 producing T cells (n = 9) after co-culture with maturation factors (MF), lipopolysaccharide (LPS) and Bifidobacterium bifidum DC. The differences observed in percentages of IL-4 and IFN-γ-positive T cells did not reach statistical significance (P > 0·05) by Mann–Whitney U-test due to large variability in the number of IL-4 positive and IFN-γ-positive T cells, but consistent effects were observed in individual experiments.
Fig. 4.
Production of cytokines by CD4+ T cells. CD4+ T cells were stimulated as described in Fig. 3. After 12 days of dendritic cell (DC)–T cell co-culture, T cells were restimulated with anti-CD3 and anti-CD28 overnight. Interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-4 and IL-10 were measured in supernatants by means of multiplex assay. Data represent mean production ± standard error of the mean (n = 9). Statistical analysis was performed by Mann–Whitney U-test. *P < 0·05.
TLR activation by probiotic bacteria
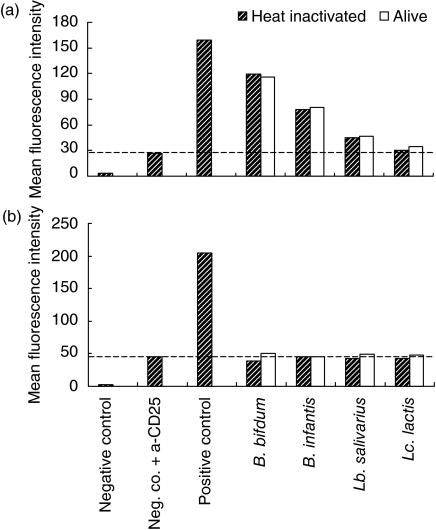
Next, we addressed the question whether cytokine production by probiotic primed DC may indicate the involvement of TLR binding. To this end, CHO-cell lines transfected with human CD14, human TLR-2 and human TLR-4 were incubated with live and heat-inactivated probiotic strains. B. bifidum and B. infantis and, to a lesser extent, Lb. salivarius did activate TLR-2 as shown by increased expression of CD25, in contrast to Lc. lactis(Fig. 5a). The capacity of these strains to activate TLRs was not different when live or heat-inactivated bacteria were used. None of the probiotic strains tested activated TLR4 (Fig. 5b) or CD14 (data not shown).
Fig. 5.
Activation of Toll-like receptors (TLRs) by probiotic bacteria. Chinese hamster ovary (CHO) cell lines transfected with human CD14, TLR-2 and TLR-4 were stimulated with live and heat-inactivated bacteria in a 50 : 1 bacteria : cell ratio. Expression of CD25 on the cell surface induced via a transfected nuclear factor kappa B construct was used via a read-out and determined by flow cytometric analysis. The negative control is the unstimulated transfected CHO-cell line which has been left unstained. Heat-inactivated Neisseria meningitidis was used as positive control in all cell lines. (a) Expression of CD25 indicated as mean fluorescence intensity (MFI) after activation of TLR-2. Negative control is CHO/CD14/TLR-2 cell line. (b) Expression of CD25 indicated as MFI after activation of TLR-4. Negative control is CHO/CD14/TLR-4 cell line. Results are representative of two separate experiments.
Discussion
In the present study, we demonstrate that from the selected strains, B. bifidum had the most consistent effect in modulation of the immune responses of neonatal cells. B. bifidum was most potent to polarize DC to drive Th1 cell responses involving increased IFN-γ producing T cells concomitant with reduction of IL-4-producing T cells. L. lactis was much less effective in this respect. The phenotype of the DC or the cytokine production is not affected directly by any of the tested probiotic bacteria.
Most studies investigating the in-vitro effects of probiotic bacteria on immune competent cells have used either murine models or cells from adult humans. Limited studies have addressed the in-vitro immune responses of newborns to bacteria of the intestinal flora. Results of previous studies indicate that different bacterial species and strains have differential effects on immune responses [14,15,17]. Each strain seems to have its own unique immunomodulatory activity. This may also hold true for their clinical application, and may explain why beneficial effects have been observed with perinatal administration of probiotic bacteria in high-risk children [8] or not [26]. We chose the approach to select specific probiotic strains for in vivo purposes based on their capacity to modulate immune responses. Previously, we showed that B. bifidum, B. infantis and Lc. lactis reduced production of Th2 cytokines and were potent inducers of IL-10 production in PBMC [21]. Subsequently, in this study we investigated the effects of these strains, with Lb. salivarius as control strain, on neonatal cord blood cells. Our results indicate that specific strains of probiotic bacteria are capable of modulating neonatal immune cells and their responses. Furthermore, it supports our approach to make a rational choice from available strains based on immunomodulatory activities [21].
We showed that exposure of cord blood-derived IDC to selected probiotic bacteria lead to a moderate up-regulation of co-stimulatory molecules, but did not induce full maturation independent from the presence of MF. After the addition of MF, the maturation status of probiotic primed DC was not different from DC matured with MF or LPS plus MF. In support of our results, partial maturation of DC upon exposure to probiotic bacteria has been described in human and murine in vitro-cultured DC [14–16,18,27,28]. Full maturation of DC with the tested strains did not affect production of IL-12 and TNF-α by DC, which is in line with previous observations [17]. The presence of B. bifidum during maturation of DC stimulated IL-6 production by DC. IL-6 has been associated with the development of atopy [29,30]. In our experimental set-up, we did not observe Th2 skewing with B. bifidum matured DC. In previous studies, other probiotic bacteria also induced IL-6 production by DC, but in support of our results no Th2 skewing but rather Th1 skewing was observed [17,18]. Previously, it has been shown that CBMC and cord blood-derived monocytes produced IL-6, TNF-α, IL-12 (only by PBMC) as well as IL-10 in response to commensal Gram-positive bacteria [31].
DC-mediated T cell activation is determined by expression of HLA-DR, co-stimulatory signals and cytokine production. In this study, the phenotype of DC as investigated by the expression of these signals and production of cytokines by DC does not seem to be affected by the presence of the tested probiotic bacteria during maturation of the DC. In particular, all the tested bacteria induced approximately the same level of IL-12. Various additional DC-derived molecules, inflammatory chemokines plus other members of the IL-12 family, i.e. IL-23 and IL-27, have the capacity to polarize Th cells [13,28,32]. Because the combination of these factors affects the fate of naive T helper (Th) cells, we investigated the effect of DC matured in the presence of probiotic bacteria on the polarization of naive T cell responses. As a result of the presence of B. bifidum and to a much lesser extent Lc. lactis during maturation of DC, CD4+ T cells were skewed toward a Th1 response, as demonstrated by increased production of IFN-γ and reduced production of IL-4. We therefore speculate that the tested strains indeed affect the phenotype of mature DC, but may involve other DC-derived molecules such as intercellular adhesion molecule 1 (ICAM-1) [13], IL-23 and IL-27 [28], and CXCL9/Mig [29].
These results are, to our best knowledge, the first to show that neonatal naive T cells can be skewed towards a Th1 response upon exposure to probiotic bacteria and are in line with other recent publications [18,20]. In contrast, human DC exposed to strains of lactobacilli used in other studies induced T cell hyporesponsiveness [19] or regulatory T cells [17]. Because B. bifidum-DC polarized CD4+ T cells to produce significantly more IL-10 compared to MF-DC, we speculate that B. bifidum may favour the development of regulatory T cells as well.
The exact mechanisms underlying the beneficial effects of probiotics are not understood completely, but may involve pattern recognition molecules such as TLRs. In our study, we demonstrated that B. bifidum, B. infantis and Lb. salivarius were capable of activating TLR-2. Previous studies have indicated that Gram-positive bacteria, such as bifidobacteria, are ligands for TLR-2 [33]. In support of our results, probiotic strains up-regulated TLR-2 transcripts in dendritic cells, suggesting that TLR signalling could be involved in dendritic cell maturation and activation [18]. In addition, supernatant of B. breve induced DC maturation and activation through a TLR-2-dependent pathway [33].
Because we focus upon the application of probiotic strains in atopic disease, we sought to select strains which reduced neonatal Th2 responses and skewed T cell responses towards Th1 or regulatory T cells. An over-skewing towards Th1 therefore might theoretically pose an enhanced risk for autoimmunity. The hygiene hypothesis also suggests that countries with lower sanitary status have a reduced frequency of autoimmune diseases. Furthermore, the last decades have shown a sharp increase not only in Th2-dominated allergic diseases but a concomitant increase in Th1-mediated autoimmune diseases, such as multiple sclerosis and type I diabetes [34]. These apparent controversies could be resolved if both development of autoimmune as well as allergic diseases could be due to impaired function of regulatory T lymphocytes. Current interpretation of the hygiene hypothesis as well as accumulating experimental data point towards an important role for regulatory T lymphocytes [34].
It has been suggested that newborns who develop atopy in later life show a delayed postnatal maturation of cellular immune functions [35]. High-risk children developing atopic disease in their first year of life developed a Th2 cytokine profile characterized by high levels of IL-4, IL-5 and IL-13 within the first 6 months [36]. Other reports have identified weaker neonatal IFN-γ responses and reduced capacity for production of IFN-γ in infancy as a marker of the atopic phenotype [37,38]. Our results indicate that specific probiotic strains are capable of driving in vitro-cultured neonatal dendritic cells to induce Th1-cell responses. We suggest that selected strains of probiotic bacteria administered orally in the neonatal period and infancy may potentially modulate the immune responses that trigger disorders such as atopic eczema. We are currently investigating this issue in our clinical trial on primary prevention of atopy and allergic diseases by perinatal administration of probiotic bacteria (ISRCTN Register: ClinicalTrials.gov Identifier NCT00200954).
In conclusion, selected strains of Bifidobacteria species prime in vitro-cultured neonatal DC to drive Th1 responses. These strains may be useful candidates to test whether probiotic strains can be applied in the prevention or treatment of atopic disease.
Acknowledgments
We thank Liana Steeghs and Douglas Golenbock for providing the CHO transfected cell lines with CD14, TLR2 and TLR4; and P. Lane and M. Kapsenberg for providing J558 CD40L expressing cell line. We would also like to thank E. Knol for critical reading of the manuscript. This study was funded by the Wilhelmina Children's Hospital. None of the authors had financial relationship with a biotechnology and/or pharmaceutical manufacturer.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner F, Bourdet-Sicard R, Brandtzaeg P, et al. Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol. 2006;3:275–84. doi: 10.1038/ncpgasthep0471. [DOI] [PubMed] [Google Scholar]
- 3.Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3:15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 6.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 7.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 8.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 9.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604–10. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 10.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179–85. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 11.Ogden NS, Bielory L. Probiotics: a complementary approach in the treatment and prevention of pediatric atopic disease. Curr Opin Allergy Clin Immunol. 2005;5:179–84. doi: 10.1097/01.all.0000162312.64308.fc. [DOI] [PubMed] [Google Scholar]
- 12.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 13.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 14.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 15.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;22:2280–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80:1618–25. doi: 10.1093/ajcn/80.6.1618. [DOI] [PubMed] [Google Scholar]
- 20.Pochard P, Hammad H, Ratajczak C, et al. Direct regulatory immune activity of lactic acid bacteria on Der p 1-pulsed dendritic cells from allergic patients. J Allergy Clin Immunol. 2005;116:198–204. doi: 10.1016/j.jaci.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Niers LE, Timmerman HM, Rijkers GT, et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481–9. doi: 10.1111/j.1365-2222.2005.02375.x. [DOI] [PubMed] [Google Scholar]
- 22.de Graaff PM, de Jong EC, van Capel TM, et al. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175:5904–11. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- 23.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–9. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien E, Sellati TJ, Yoshimura A, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–25. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Veckman V, Miettinen M, Pirhonen J, Siren J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–71. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 28.Smits HH, van Beelen AJ, Hessle C, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–80. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 29.Heijink IH, Vellenga E, Borger P, Postma DS, de Monchy JG, Kauffman HF. Interleukin-6 promotes the production of interleukin-4 and interleukin-5 by interleukin-2-dependent and -independent mechanisms in freshly isolated human T cells. Immunology. 2002;107:316–24. doi: 10.1046/j.1365-2567.2002.01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doganci A, Eigenbrod T, Krug N, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–25. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun. 2002;70:6688–96. doi: 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebre MC, Burwell T, Vieira PL, et al. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83:525–35. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J Allergy Clin Immunol. 2006;117:696–702. doi: 10.1016/j.jaci.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 34.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 35.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–6. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 36.van der Velden V, Laan MP, Baert MR, de Waal MR, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997–1006. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 37.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 38.Prescott SL, King B, Strong TL, Holt PG. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy. 2003;58:1187–94. doi: 10.1034/j.1398-9995.2003.00263.x. [DOI] [PubMed] [Google Scholar]