Abstract
The guinea pig model of low-dose pulmonary tuberculosis has been used to study the pathogenesis of infection as well as the mechanisms of bacille Calmette–Guérin (BCG) vaccine-induced resistance. We investigated the function of lung cells from naive and BCG-vaccinated guinea pigs after enzymatic digestion of lung tissue with collagenase and DNase I. The total lung digest cells proliferated poorly to purified protein derivative (PPD) but comparatively better to ConA as assessed by [3H]-thymidine uptake. However, the non-adherent population obtained after plastic adherence of lung digests showed an enhanced response to concanavalin A (ConA) and PPD. Therefore, proliferation to ConA and PPD of nylon wool-purified T cells co-cultured with peritoneal (PMøs), alveolar (AMøs) or lung macrophages (LMøs) was assessed. Co-cultures of lung T cells and PMøs showed maximum proliferation to PPD, whereas proliferation was suppressed significantly by the addition of AMøs or LMøs. The response of T cells to ConA was unaffected in co-cultures. Incubation of co-cultures with recombinant guinea pig interferon-γ (rgpIFN-γ) did not reverse the suppression. In contrast, rgpIFN-γ-treated plastic adherent LMøs that were non-specific esterase-positive were capable of reducing the intracellular growth of Mycobacterium tuberculosis. Similarly, total, non-adherent and adherent lung digest cells from BCG-vaccinated guinea pigs showed IFN-γ and tumour necrosis factor (TNF)-α mRNA expression in response to ConA, lipopolysaccharide or PPD by reverse transcription–polymerase chain reaction followed by release of TNF protein but not IFN. These studies indicate that rgp-IFN-γ-treated lung tissue macrophages from BCG-vaccinated guinea pigs are defective for inducing antigen-specific proliferation in T cells, but control the intracellular accumulation of virulent M. tuberculosis.
Keywords: BCG, cytokine mRNA, guinea pig lung digest cells, rgpIFN-γ, T cell proliferation
Introduction
Tuberculosis is a global health problem and it is estimated that one-third of the world's population is infected with Mycobacterium tuberculosis[1]. It is also known that ∼10% of infected individuals develop active disease after M. tuberculosis infection, resulting in the death of 2 million cases annually [2]. The spread of HIV/AIDS infection as well as the emergence of multi-drug-resistant strains of M. tuberculosis have increased the risk of tuberculosis [3]. The disease is transmitted by inhalation of droplet nuclei carrying M. tuberculosis where the lung is the primary target of infection [4]. The tubercle bacilli that reach the alveoli are engulfed by alveolar macrophages and those that survive multiply within the macrophages and are carried into the parenchyma of the lung and, ultimately, to the lymph nodes that drain the site of infection [5]. M. bovis BCG is the only vaccine currently available for the prevention of tuberculosis. BCG has been shown to induce protective immunity against infectious challenge in many animal models of tuberculosis [6], and protects against tuberculosis in children [7]. However, the efficacy of BCG vaccine in adults has been highly variable [8] in clinical trials.
Effector mechanisms in anti-mycobacterial immunity involve macrophages and lymphocytes that act in concert with the help of co-stimulatory molecules and molecular mediators such as cytokines and chemokines [9]. Macrophages act as the primary effector cells for the early clearance of mycobacteria. Both CD4+ and CD8+ T cells are known to mediate the immune response against M. tuberculosis but other cell types, including natural killer (NK) cells, are also involved [10–12]. Both interferon (IFN)-γ and tumour necrosis factor (TNF)-α have been identified as important cytokines for the effector functions against mycobacteria [13,14]. The formation and maintenance of the granuloma, a defensive reaction on the part of the host, is mediated by TNF-α which, along with IFN-γ, activates macrophages to produce reactive oxygen and nitrogen intermediates [14,15]. Several other cytokines such as interleukin (IL)-12 and IL-23 contribute to the host-response to mycobacteria by enhancing the development of T helper 1 (Th1) immunity [16,17]. The importance of IFN-γ as a potent activator of macrophages in the effective growth restriction and clearance of mycobacteria in vitro has been well documented [18]. Treatment of mice with anti-IFN-γ antibody or disruption of the mouse IFN-γ or the IFN-γ receptor gene resulted in an exacerbation of disease after M. tuberculosis or M. bovis infection [13,19]. Furthermore, mutations in the IFN-γ receptor or IFN-γ receptor signal-transducing chain predisposed humans to develop disseminated mycobacterial infections [20].
Pulmonary T cell responses are regulated by alveolar macrophages [21], which have the capacity to suppress T cell responses in vitro in humans and in rodents [22]. T cell proliferation in peripheral blood mononuclear cells cultured with concalavalin A (ConA), house-dust mite or tetanus toxoid was inhibited in the presence of alveolar macrophages [23]. These studies also demonstrated that early steps in T cell activation, such as CD3 down-modulation, up-regulation of IL-2 receptor expression and IL-2 production were unaffected by human, rat and mouse alveolar macrophages. In other reports, the immunosuppressive activity of alveolar macrophages was inhibited by pretreatment with granulocyte–macrophage colony-stimulating factor (GM-CSF) [24]. Similarly, immunosuppression by alveolar macrophages of ConA-induced T cell proliferation has been documented in murine models [25,26]. Guinea pig alveolar macrophages significantly suppressed the lymphoproliferation of splenic T cells to ConA [27].
Earlier studies from our laboratory focused on the cellular and molecular responses induced by BCG vaccination in phagocytic cells from various anatomical sites exposed to attenuated and virulent strains of M. tuberculosis in vitro[28–33]. Recently, we have also purified and characterized the biological activities of several recombinant guinea pig cytokines [34–37]. Guinea pig recombinant IFN-γ (rgpIFN-γ) was found to up-regulate major histocompatibility complex (MHC) class II expression, and to activate macrophages to produce H2O2 and restrict the intracellular growth of M. tuberculosis[34]. However, the cellular responses in the lungs of guinea pigs, the primary site of M. tuberculosis infection, are not well understood. Thus, the purpose of the present study was to characterize the effect of vaccination on lung digest cells by assessing the functional activity of T cells and macrophages obtained after enzyme digestion.
Materials and methods
Animals and BCG vaccination
Random-bred Hartley strain guinea pigs weighing 200–300 g were obtained from Charles River Breeding Laboratories, Inc. (Wilmington, MA, USA). The animals were housed individually in polycarbonate cages in a temperature- and humidity-controlled environment; ambient lighting was controlled automatically to provide 12-h light−12-h dark cycles. Animals were given commercial chow (Ralston Purina, St Louis, MO, USA) and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee. Guinea pigs were vaccinated once intradermally with 0·1 ml (103 viable units) of M. bovis BCG (Danish 1331 strain; Statens Seruminstitut, Copenhagen, Denmark) in the left and right inguinal regions. The lyophilized vaccine was reconstituted with Sauton's medium (Statens Seruminstitut) just before injection.
Preparation of lung digest cells
The lung digest cells were prepared using the protocols modified from those published earlier for mice [38,39]. The naive and BCG-vaccinated guinea pigs at 6–8 weeks after vaccination were euthanized by the injection of 3 ml sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc.) either intraperitoneally or intramuscularly. All the lung lobes were collected either before or after bronchoalveolar lavage (BAL). The lung tissue was cut into small pieces with scissors in a Petri dish and dispensed in 15 ml RPMI-1640 (Irvine Scientific, Santa Ana, CA, USA) medium supplemented with 2 µM glutamine (Irvine Scientific), 0·01 mM 2-mercaptoethanol (2-ME; Sigma, St Louis, MO, USA), 100 U/ml of penicillin (Irvine Scientific), 100 µg/ml of streptomycin (Irvine Scientific) and 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA, USA) containing freshly made collagenase (150 U/ml, Sigma) and DNAse I (100 U/ml, Sigma). Another 30 ml of complete medium was added to this preparation and incubated for 2 h at 37°C. Single cell suspensions were obtained by vigorous pipetting and passing the homogenate first through a 100-µm nylon Falcon cell strainer (BD Biosciences, Bedford, MA, USA) to remove large pieces of tissue, and then through a 40-µm cell strainer (BD Biosciences) to remove smaller debris. The volume was brought to 50 ml in complete RPMI-1640 medium and the cells were centrifuged at 440 g for 10 min. The pellet was resuspended in ammonium chloride potassium bicarbonate (ACK) lysis buffer [0·14 M NH4Cl, 1·0 mM KHCO3, 0·1 mM Na2 ethylenediamine tetraacetic acid (EDTA) (pH 7·2–7·4)], washed three times in RPMI-1640 medium by centrifuging for 10 min at 320 g, and viable cells were counted by the trypan blue exclusion method. The viability of lung digest cells was more than 95% as determined by the trypan blue staining method.
Preparation of lung mononuclear cells for flow cytometry
Lung mononuclear cells obtained by digesting the lung tissue were stained with monoclonal antibodies (mAb) against guinea pig MHC class II cells, pan T (CT5), CD4 (CT7) and CD8– T cell (CT6) phenotypic markers (Biosource International, Camarillo, CA, USA). For each mAb or control, 5–10 × 105 cells were incubated with 10 µl of (1 mg/ml) normal goat IgG (Sigma) for 10 min to block FcR binding. This was followed by the addition of 50 µl of mouse anti-guinea pig MHC class II (1 : 10) antibody, anti-guinea pig T cell antibody (1 : 500), anti-CD4 (1 : 500) or anti-CD8 antibody (1 : 1000) and incubating the cells in ice on a shaker. After washing, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated AffiniPure goat anti-mouse IgG (H + L) (Jackson ImmunoResearch Laboratories, Inc., West Grove, CA, USA) and fixed in Hank's balanced salt solution (HBSS) containing 1% paraformaldehyde. The proportions of positive cells were determined with a fluorescence activated cell sorter (FACS)Calibur flow cytometer and CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Cytospin preparations of the lung digests were also performed and the cells were stained by the Diff-Quik staining method (American Scientific Products, McGraw Park, IL, USA).
Harvesting of resident peritoneal, alveolar and lung macrophages
The resident peritoneal and alveolar macrophages from naive and BCG-vaccinated guinea pigs were harvested using methods published earlier [33,40]. Following euthanasia, the cells from the peritoneal cavity were harvested by flushing the cavity three times with 20 ml of cold RPMI-1640 (Irvine Scientific). ACK lysing buffer was used to deplete the erythrocytes. The cells were washed in RPMI-1640 medium and the viable cells were counted by the trypan blue exclusion method. The cells were suspended at 5 × 106 cells/ml in RPMI-1640 medium supplemented with glutamine, 2-ME, penicillin/streptomycin and 10% heat-inactivated FBS (Atlanta Biologicals). Peritoneal, alveolar and lung cells (2 × 106/ml) were incubated in 96-well microtitre plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA) for 2–3 h, and non-adherent cells were removed. The monolayers were comprised predominantly of macrophages (> 95%) as determined by non-specific esterase staining [41].
The resident alveolar macrophages were isolated by BAL by cannulating the trachea and washing the lungs three to four times with 10 ml RPMI-1640 containing 2% FBS and 12 mM lidocaine. The cells were then centrifuged at 4°C for 10 min at 400 g in complete RPMI-1640 medium containing glutamine, 2-ME, penicillin/streptomycin and 10% FBS. The cells were washed three more times in the same medium after lysing the red blood cells, if any, with ACK lysis buffer. The cells were counted and 2 × 106 cells/ml were plated in 96-well microtitre plates. The non-adherent cells were removed after 2 h of incubation at 37°C and the monolayers were used for different assays.
The macrophages from lung digest cells were enriched by adherence for 2 h on either 24-well or 96-well plates as described above. At the end of the incubation, the non-adherent cells were removed by washing three times with warm HBSS (with Ca2+ and Mg2+) containing 25 mM HEPES, 2 mM EDTA (disodium) and 2% FBS without the antibiotics. The adherent cells were detached by adding warm phosphate-buffered saline (PBS) (without Ca and Mg) containing only 10 mM HEPES, 2 mM EDTA and no antibiotics and incubating the plate at 37°C for 30 min. The cells were detached by vigorous pipetting and then collected in tubes kept on ice. The cells were centrifuged (200 g for 8 min at 4°C) and the cell pellet suspended in RPMI-1640 containing 2% FBS, 10 mM HEPES, 2 µM glutamine and 0·01 mM 2-mercaptoethanol without antibiotics. The cells were counted and cytospins were prepared and stained for non-specific esterase according to the manufacturer's protocol [41].
Purification of T cells
The non-adherent cells from the digested lung tissue of BCG-vaccinated guinea pigs were enriched for T lymphocytes on nylon wool columns using our standard protocol [31]. The column was prepared by packing 0·5 g, scrubbed and combed ready for use, nylon wool fibre (Polysciences, Inc., Warrington, PA, USA) into a 10-ml syringe barrel and autoclaving for 15 min. The column was washed with RPMI-1640 containing 10% FCS and left wet with no air bubbles. The prepared column was then incubated at 37°C for 1 h and loaded with 1–2 × 108 viable cells in a volume of 2 ml. The loaded column was incubated for 1 h at 37°C and the non-adherent cells were collected using 50 ml warm RPMI-1640 medium. The collected cells were centrifuged at 320 g for 10 min and the cell pellet was resuspended in RPMI-1640 medium containing 10% FCS and counted by the trypan blue exclusion method.
Proliferation of total lung digest cells and co-cultures of lung T cells and macrophages
The ability of lung digest cells to proliferate in response to a mitogen or specific antigen was assessed in total lung cells, nylon wool-purified T cells, or in co-cultures of nylon wool-purified T cells and macrophages obtained from various anatomical sites. The total lung digest cells (4 × 105/well) were plated onto 96-well plates with or without concanavalin A (ConA, 10 µg/ml; Sigma) or purified protein derivative (PPD, 12·5 and 25 µg/ml; Statens Seruminstitut) and cultured for 4 days using our standard protocols [42]. For the final 6 h of culture, [3H]-thymidine was added at a concentration of 1 µCi/well. The cells were harvested using a FilterMate harvester, and the [3H]-thymidine uptake was measured in a scintillation counter (Beckman LS-1801). The stimulation index (SI) was calculated by dividing the counts per minute (cpm) of stimulated cells by the cpm of unstimulated cells.
For the co-culture experiments, only BCG-vaccinated animals were used. The T cells from lung digests obtained after nylon wool purification were mixed at a ratio of 3 : 1 with macrophages obtained from lung digests, peritoneal or alveolar lavages. The co-cultures of T cells (3 × 105) and macrophages (1 × 105) were plated onto 96-well plates and stimulated with either ConA or PPD for 96 h using the protocols described above. Nylon wool-purified T cells from the lung digests (4 × 106/ml) were also cultured in the same manner. At the initiation of the culture period, some cells were stimulated with 200 or 500 ng/ml rgpIFN-γ, doses which have been shown to up-regulate MHC class II expression, enhance H2O2 production and reduce the intracellular growth of M. tuberculosis in peritoneal macrophages [34].
Effect of rgpIFN-γ on intracellular growth of mycobacteria in lung macrophages
The ability of macrophages obtained from lung digests to control the intracellular growth of mycobacteria was determined by the incorporation of [3H]-uracil [40]. Lung digest macrophages from BCG-vaccinated guinea pigs were plated (3 × 105 cells/well) in 96-well microtitre plates in complete RPMI-1640 medium containing 10% FBS and the non-adherent cells were removed after 3 h of incubation at 37°C. The cells were treated with varying concentrations of rgpIFN-γ (50–1000 ng/ml) for 24 h, at the end of which the medium was removed and the cells washed once in antibiotic-free RPMI-1640 medium. Macrophages were then infected with M. tuberculosis H37Rv (multiplicity of infection [MOI] 1 : 1) for 3 h and the extracellular bacteria were removed by washing two times with antibiotic-free medium. In some experiments, rgpIFN-γ was added both before and after phagocytosis and, in the latter experiments, recombinant protein was present throughout the 7-day culture period. Infected macrophages were then incubated in medium containing gentamycin (50 µg/ml) to inhibit the extracellular mycobacteria for 7 days at 37°C. The macrophages were pulsed with 1 µCi of [3H]-uracil for 24 h before harvesting. The mycobacteria were killed by incubating the plate at 80°C for 30 min, harvested using a FilterMate harvester, and the [3H]-uracil uptake was measured in a scintillation counter (Beckman LS-1801); the results are expressed as cpm.
Total RNA isolation and real-time polymerase chain reaction (PCR)
Total unseparated lung digest cells, adherent or non-adherent subpopulations from BCG-vaccinated guinea pigs were stimulated with ConA (10 µg/ml), lipopolysaccharide (LPS) (1 µg/ml) or PPD (25 µg/ml) for 3, 12 or 24 h. At the end of the incubation period, the supernatant was removed by centrifugation and the cells were lysed with RNA lysis buffer (RLT) buffer (Qiagen, Valencia, CA, USA); the lysates were kept frozen at −80°C until RNA extraction. Total RNA was isolated using the RNeasy columns (Qiagen). Reverse transcription was performed with TaqMan reverse transcription reagents and real-time PCR using SYBR Green I double-stranded DNA binding dye (Applied Biosystems, Foster City, CA, USA) and the ABI Prism 7700 sequence detector according to the protocols published from our laboratory [2,11]. Real-time primers for IFN-γ, gpTNF-α and HPRT were designed with Primer Express software (Biosystems) based upon sequences that we have published previously [2,11]. Fold induction of mRNA was calculated from the threshold cycle values (Ct) normalized to HPRT Ct values and then to unstimulated cultures.
Bioassay of TNF-α
The bioassay for TNF was carried out using the protocol published previously from our laboratory [35,40]. The TNF activity in the culture supernatants was assayed by measuring their cytotoxicity on L929 cells (American Type Culture Collection).
Bioassay for IFN-γ
Concentrations of bioactive IFN in the culture supernatants were determined using a viral protection assay involving encephalomyocarditis virus (EMCV) in the guinea pig fibroblast cell line, 104C1, as described previously by us [34,43].
Statistics
The data are expressed as means ± standard errors (s.e.). The differences between naive and BCG-vaccinated groups were analysed by Student's t-test and values of P < 0·05 were considered statistically significant. The effects of treatments were assessed by analysis of variance (anova) and the differences between means were analysed by the Tukey post hoc test. There were three to 10 guinea pigs per group and each experiment was performed at least three times.
Results
Phenotypic analysis of lung digest cells
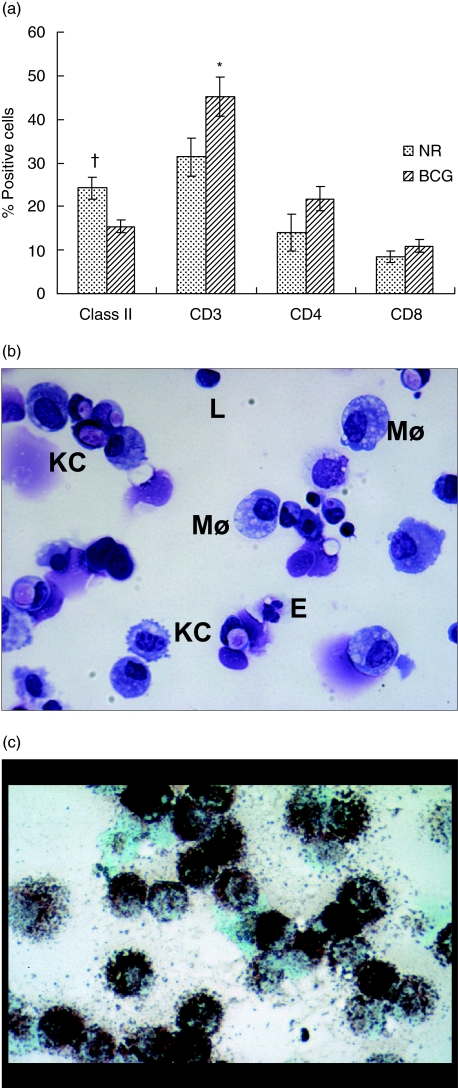
In order to characterize the cell populations in the lung digests, lung tissue from both naive and BCG-vaccinated guinea pigs was digested with collagenase and DNase. Figure 1 shows the phenotypic analysis of lung digest cells by flow cytometry (Fig. 1a) and by Diff-Quik staining (Fig. 1b). There was a significant reduction (P < 0·04) in the proportions of MHC class II+ cells in the lung digests of BCG-vaccinated animals compared to the naive animals. In contrast, the proportions of T cells in the BCG-vaccinated group were increased significantly (P < 0·007) compared to the naive group; however, no significant changes in the proportions of T cell subsets in these two groups were observed (Fig. 1a). Lung digests from both naive and BCG-vaccinated guinea pigs contained approximately 8–29% MHC class II positive cells, 28–73% CD3+ T cells, 8–48% CD4+ T cells and approximately 6–21% CD8+ T cells.
Fig. 1.
Phenotypic analysis of guinea pig lung digest cells. Lung digest cells obtained by enzymatic digestion from naive and bacille Calmette–Guérin (BCG)-vaccinated guinea pigs was characterized by flow cytometry (a), by Diff-quik staining (b) and by non-specific esterase staining (c). Proportions of major histocompatibility complex (MHC) class II+ cells, T cells and their subsets were determined by fluorescence activated cell sorter (FACS) analysis after staining the cells with monoclonal antibodies (mAb) directed against the surface markers of MHC class II+ cells, T cells (CT5), CD4+ T cells (CT7) and CD8+ T cells (CT6). Results are expressed as percentage of positive cells. †P < 0·04 and *P < 0·007 compared with naive group as determined by Student's t-test. The Diff-Quik staining of the cytospins of total lung digest cells from BCG-vaccinated guinea pigs (b) show abundant macrophages (Møs) and Kurloff cells (KC) with occasional lymphocytes (L), neutrophils and eosinophils (b). (c) Lung macrophages obtained after plastic adherence by non-specific esterase staining. Cells from naive animals show similar staining properties.
The cytospin preparations of lung digests were stained using the Diff-Quik method and the differential counts revealed significant differences in the number of cells between unvaccinated and BCG-vaccinated groups (Fig. 1b and Table 1). Macrophages were seen in abundance in the lung digests of both naive and BCG-vaccinated guinea pigs (Fig. 1b); however, there was a significant reduction in the percentage of cells (P < 0·01) in the latter. Eosinophils and neutrophils were seen frequently in the cytospin preparations but they were not clearly distinguishable from each other and, thus, these two types of granulocytes were enumerated together. In the BCG-vaccinated guinea pigs, the proportion of these cells increased significantly (P < 0·0001). Similarly, there were numerous Kurloff cells, mononuclear cells with a large cytoplasmic inclusion body that are uniquely present in guinea pig tissues, in the lung digests. BCG vaccination induced a significant increase (P < 0·03) in the proportion of Kurloff cells in the lung. The percentage of lymphocytes in the lung digests of unvaccinated and BCG vaccinated guinea pigs, however, remained similar. Identification of certain cell types in the digests was confounded by the fact that the integrity and anatomical location of these cells in the tissue was lost due to the nature of isolation procedures after enzymatic digestion. Figure 1c indicates that lung digest cells yielded mainly macrophages after plastic-adherence as assessed by non-specific esterase staining. Thus, BCG vaccination resulted in a decrease in the proportions of MHC class II+ cells accompanied by a reduction in the percentage of macrophages. In contrast, the proportions of CD3+ T cells increased in the lung and the percentage of neutrophils/eosinophils and Kurloff cells increased after BCG vaccination.
Table 1.
Differential counts of guinea pig lung digest cells (mean percentage ± s.e.m.).
| Groups | Lymphocytes | Macrophages | Neutrophils/eosinophils | Kurloff cells |
|---|---|---|---|---|
| Unvaccinated | 23·4 ± 1·3 | 43·3 ± 2·0 | 13·6 ± 1·2 | 19·8 ± 1·4 |
| BCG-vaccinated | 21·3 ± 1·6 | 34·9 ± 2·4† | 22·6 ± 1·7* | 24·0 ± 1·6† |
P < 0·003–0·01
P < 0·0001 compared to the unvaccinated group as determined by Student's t-test; BCG: bacille Calmette–Guérin; s.e.m.: standard error of the mean.
Lymphoproliferation of lung digest cells
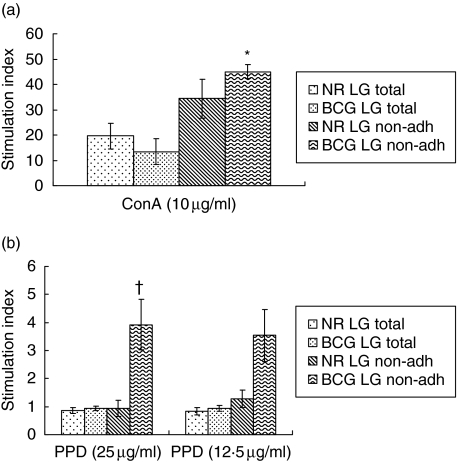
In order to assess the function of lung digest cells, the proliferative capacity of total lung cells from naive and BCG-vaccinated guinea pigs to ConA and PPD was examined. Total lung digest cells proliferated well to ConA but poorly in response to PPD (Fig. 2). Therefore, non-adherent cells were obtained by incubating the lung digest cells in 100-mm-diameter Petri dishes for 2 h at 37°C. As is clear from Fig. 2, the ConA-induced proliferative capacity of the non-adherent population from lung digests from both naive and BCG-vaccinated guinea pigs was significantly enhanced after removing the adherent population. As expected, proliferation to PPD of non-adherent cells from naive guinea pigs remained low, while that of the BCG-vaccinated group was increased significantly (P < 0·01) after removing the adherent cells. These results indicated that the adherent population in the lung digests suppressed the proliferative capacity of lung T cells to ConA and PPD.
Fig. 2.
Proliferation of total and non-adherent cells from guinea pig lung digests. Lung digest cells from naive and bacille Calmette–Guérin (BCG)-vaccinated guinea pig cells (2 × 106/ml) were cultured for 4 days in the presence of concanavalin A (ConA) (10 µg/ml) and purified protein derivative (PPD) (12·5 and 25 µg/ml). The cells were harvested after the addition of [3H]-thymidine (1 µg/well) 6 h earlier. The results are expressed as stimulation index calculated by dividing the counts per minute (cpm) of stimulated cells by the cpm of unstimulated cells. The results are the mean and standard error of the mean from three experiments. There were five animals per group. *P < 0·005 in comparisons between groups by Student's t-test.
Lymphoproliferation in the co-cultures
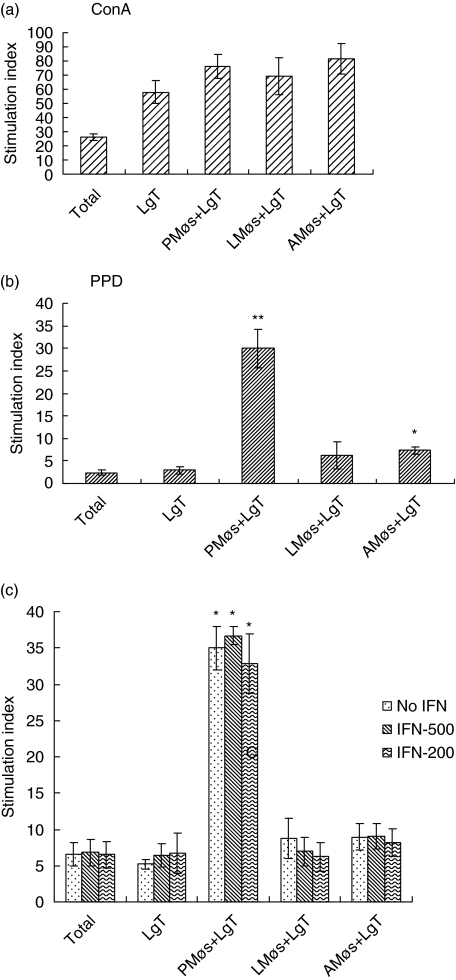
Because the proliferation of non-adherent cells from lung digests in response to ConA and PPD was affected by the adherent population, we examined whether T cells obtained after nylon wool purification would act differently when co-cultured with macrophages obtained from different anatomical sites. Nylon wool-purified T cells from the lung digests of BCG-vaccinated guinea pigs were co-cultured with macrophages obtained from BAL (alveolar macrophages, AMøs), peritoneal macrophages (PMøs) or lung parenchymal macrophages (LMøs) at a ratio of 3 : 1. This ratio of lymphocytes to macrophages was used based on our previous work with spleen T cells and peritoneal macrophages [31] and also because our preliminary experiments showed no change in the proliferation trend with other combinations (data not shown). The co-cultures were stimulated for 4 days in the presence of ConA or PPD and the proliferation was assessed by tritiated thymidine uptake. Results from Fig. 3 indicate that total unseparated cells or T cells alone from the lung digests proliferated well to ConA (Fig. 3a). Similarly, T cells co-cultured in the presence of all macrophage populations showed an enhanced response. The proliferative response to PPD was lower than the response to ConA. Total lung digest cells as well as purified T cells responded poorly to PPD when cultured alone; however, in the co-cultures the PPD-induced proliferation of lung T cells was significantly higher in the presence of PMøs (Fig. 3b). In contrast, the proliferative capacity of lung T cells to PPD in the presence of AMøs and LMøs was significantly suppressed, although the response was greater than that observed with either total digest cells or T cells alone (Fig. 3b). Therefore, AMøs and LMøs inhibited T cell proliferation to PPD.
Fig. 3.
Proliferation in co-cultures of guinea pig lung T cells and macrophages from different anatomical sites. Total and lung T (LgT) cell proliferation in the presence of macrophages from peritoneal (PMøs), lung (LMøs) and alveolar (AMøs) sites. Lung T cells (3 × 105/well) purified on nylon wool columns from bacille Calmette–Guérin (BCG)-vaccinated guinea pigs were co-cultured with peritoneal, lung or alveolar macrophages (1 × 105/well) in the presence of concanavalin A (ConA) (a) or purified protein derivative (PPD) (b) for 4 days and the [3H]-thymidine uptake was assessed. The cell populations were also treated with recombinant guinea pig interferon-γ (rgpIFN-γ) (200 and 500 ng/ml) at the beginning of the culture period and these results are shown in (c). The results represent mean ± standard error of the mean (s.e.m.) from five to 10 animals. Results are expressed as stimulation index. *P < 0·01 and **P < 0·005 when compared with total cells as determined by analysis of variance.
We then determined whether addition of rgpIFN-γ would rescue the ability of LMøs to induce proliferation of T cells to PPD. Therefore, total lung digest cells, purified T cells and co-cultures of T cells and macrophages from different anatomical sites were treated with the rgpIFN-γ at the beginning of the culture period. As is clear from Fig. 3c, the addition of 200 ng/ml or 500 ng/ml rgpIFN-γ to any of these cell cultures did not influence proliferation.
Intracellular growth of M. tuberculosis in lung macrophages
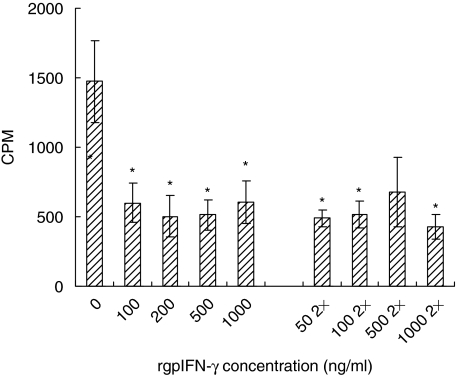
Because LMøs suppressed the proliferation of lung T cells compared to peritoneal macrophages, we wanted to determine whether these cells retained other functions, such as restricting the intracellular growth of M. tuberculosis after rgpIFN-γ treatment. LMøs from BCG-vaccinated guinea pigs were cultured with different doses of rgpIFN-γ for 24 h. The medium was removed, fresh medium without antibiotics was added, and the cultures were infected with M. tuberculosis and incubated for 7 days. In some cultures, IFN-γ was added both before and after infection (2×). Figure 4 shows the [3H]-uracil uptake by M. tuberculosis in LMøs. rgpIFN-γ significantly (P < 0·02–0·0004) reduced the intracellular growth of bacteria in LMøs at doses ranging from 100 to 1000 ng/ml compared to the untreated LMøs. No additional effect was seen when the cytokine was added again after infection (Fig. 4). It is clear from these experiments that although LMøs were lymphocytostatic, they were capable of restricting the intracellular growth of mycobacteria after activation with rgpIFN-γ.
Fig. 4.
Effect of recombinant guinea pig interferon-γ (rgpIFN-γ) on intracellular growth of Mycobacterium tuberculosis in lung macrophages. LMøs from bacille Calmette–Guérin (BCG)-vaccinated guinea pigs were treated with varying doses (50–1000 ng/ml) of rgpIFN-γ for 24 h. The RPMI-1640 medium was removed and fresh medium without antibiotics was added and the cultures were infected with M. tuberculosis (multiplicity of infection 1 : 1) for 3 h. The extracellular bacteria were removed and some cultures were treated with 50–1000 ng/ml also after infection. The [3H]-uracil uptake by viable M. tuberculosis was measured on day 7 and is expressed as counts per minute (cpm). The results represent mean ± standard error of the mean (s.e.m.) from five experiments. The differences between unstimulated and stimulated cultures were examined by a Student's t-test. *P < 0·005 when compared with the untreated cultures.
Cytokine mRNA expression and protein production
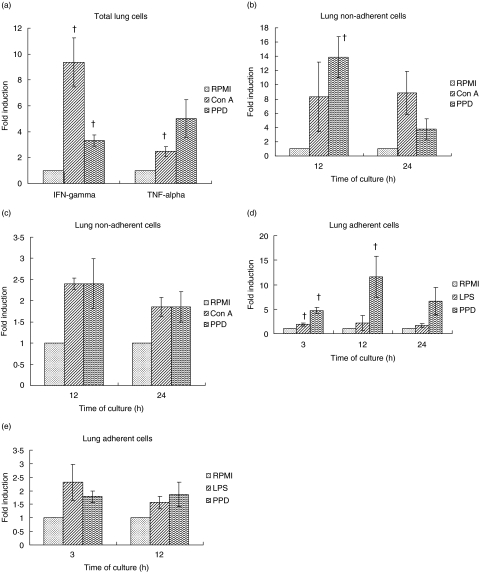
The expression of TNF-α and IFN-γ mRNA was examined by real-time PCR in the total, non-adherent and adherent populations of lung digest cells stimulated with ConA, LPS or PPD for 3–24 h. The results are illustrated in Fig. 5. Total lung digest cells showed a significant increase in IFN-γ mRNA expression after stimulation with ConA or PPD for 24 h, although the fold induction was much higher with ConA. TNF-α mRNA was also increased significantly in total lung digests stimulated for 24 h with ConA but not with PPD (Fig. 5a). Similarly, the non-adherent cells also showed a significant increase in IFN-γ mRNA expression after PPD stimulation at 12 h and the mRNA level was reduced at 24 h (Fig. 5b). The TNF-α mRNA expression was increased modestly in the lung non-adherent cells after stimulation with both ConA and PPD at 12 and 24 h; however, the levels did not attain statistical significance (Fig. 5c). Surprisingly, IFN-γ mRNA expression was increased significantly in the lung adherent cells after LPS stimulation at 3 h and after PPD stimulation at 3 and 12 h (Fig. 5d). In contrast, stimulation of lung adherent cells with either LPS or PPD had no significant effect on TNF-α mRNA expression (Fig. 5e). Thus, the level of IFN-γ mRNA was higher than TNF-α mRNA in all the lung cell types stimulated with various stimulants.
Fig. 5.
Cytokine mRNA expression in guinea pig lung digest cells. Total (a), non-adherent (b, c) and adherent lung digest cells (d, e) from bacille Calmette–Guérin (BCG)-vaccinated guinea pigs were stimulated with concanavalin A (ConA) (10 µg/ml), lipopolysaccharide (LPS) (1 µg/ml) or purified protein derivative (PPD) (25 µg/ml) for 3, 12 or 24 h. Interferon (IFN)-γ (b, d) and tumour necrosis factor (TNF)-α mRNA (c, e) expression was quantified using real-time polymerase chain reaction (PCR). Fold induction of mRNA was calculated from the threshold cycle values (Ct) normalized to hypoxanthine-quanine phosphoribosyl transferase (HPRT) Ct values and then to unstimulated cultures. The results are expressed as mean ± standard errors of means (s. e.m.) from four to six animals. The differences in the fold induction between unstimulated and stimulated cultures were determined by a Student's t-test or by analysis of variance. †P < 0·05–0·01.
The bioactive IFN protein levels were measured in the culture supernatants of lung digest cells stimulated with ConA, LPS or PPD based on their ability to reduce the cytopathic effect of EMCV in guinea pig fibroblast cell line. IFN protein was not detected in the culture supernatants from total or non-adherent cells stimulated with ConA or PPD. The supernatants from adherent lung cells stimulated with LPS or PPD showed low levels of IFN protein (data not shown). Thus, IFN-γ mRNA expression in the lung digest cells stimulated with various antigens was not associated with the release of IFN protein.
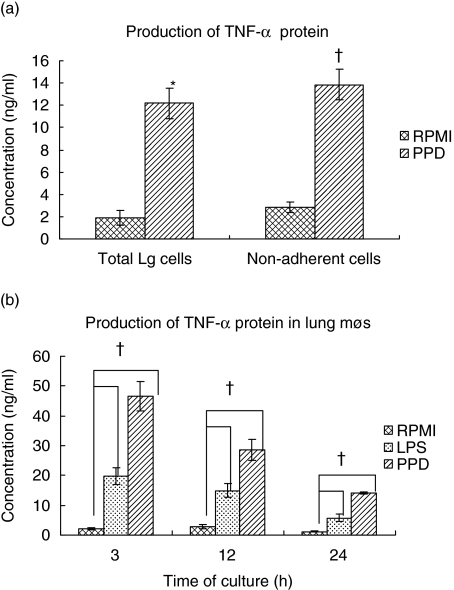
Bioactive TNF-α levels were assessed by the L929 cytotoxicity assay in the culture supernatants from total, non-adherent and adherent lung cells stimulated with LPS or PPD. Both total and non-adherent cells produced significant levels of TNF-α protein in response to PPD 24 h after stimulation when compared to the unstimulated cultures (Fig. 6a). Similarly, the adherent lung cells stimulated for 3, 12, and 24 h with LPS or PPD secreted copious amounts of TNF-α protein compared to the unstimulated cultures. The maximum induction of protein was observed at 3 h after LPS or PPD stimulation and the levels receded by 12–24 h (Fig. 6b). The protein levels in the lung adherent population were much higher after PPD stimulation than with LPS.
Fig. 6.
Production of tumour necrosis factor (TNF)-α protein in guinea pig lung digest cells. Total and non-adherent lung digest cells (a) were stimulated with purified protein derivative (PPD) (25 µg/ml) for 24 h and the adherent population (b) was stimulated with lipopolysaccharide (LPS) (1 µg/ml) or PPD for 3, 12 or 24 h. The culture supernatants were collected and assayed for the presence of TNF-α protein production by the L929 cytotoxicity assay. The results are expressed as concentration in ng/ml and denote mean and standard errors of means from four animals. *P < 0·006 and †P < 0·05 when compared to the response in untreated cultures determined by a Student's t-test [results in a)] or by analysis of variance [results in (b)].
Discussion
Despite our knowledge of the functions of immune cells isolated from peripheral lymphoid organs such as spleen or lymph nodes, or from the peritoneal cavity or BAL fluid of guinea pigs [28–33,37], little is known about the local cellular responses that occur in the lung after BCG vaccination. Our study provides the first evidence that functional lymphocytes and macrophages can be isolated from the lungs of guinea pigs. The lung digest cells were prepared by the enzymatic digestion of collagenase and DNase using modified mouse protocols in order to allow for the processing of a larger amount of tissue. Although initial characterization of cell types was carried out in naive and BCG-vaccinated guinea pigs, the functional assays for macrophages and lymphocytes were performed only in BCG-vaccinated guinea pigs because the assays required antigen-specific T cell reactivity. Flow cytometric analysis indicated that the BCG-vaccinated group had significantly lower proportions of MHC class II+ cells but higher proportions of CD3+ cells in the lung digests in comparison with the unvaccinated animals (Fig. 1a). There were no changes in the proportions of T cell subsets in the lung after BCG vaccination, although our previous findings indicated that CD4+ T cells increased in the spleens of BCG vaccinated guinea pigs [31]. The significant decrease in the proportions of MHC class II+ cells in the lung after vaccination is due clearly to the reduction in the number of macrophages after BCG vaccination, as revealed by the differential counts. It is quite probable that macrophages migrate from one location to the other after bacterial encounter.
The differential counts of lung digest cells from the naive guinea pigs revealed that macrophages were seen abundantly while lymphocytes, granulocytes and Kurloff cells were comparatively fewer (Table 1). However, after BCG vaccination, the lung digest cells showed a reduction in the percentage of macrophages but an increase in the number of granulocytes and Kurloff cells. These macrophages are apparently replaced with granulocytes and Kurloff cells. It is known that neutrophils [44,45] and eosinophils [46] are recruited to the site of M. tuberculosis infection, and are found in the granulomas of human, mice and guinea pigs. The unique presence of Kurloff cells in the guinea pig lung is noteworthy, as these cells are found only in tissue preparations including spleen homogenates [32] and are not seen in the resident cells collected from peritoneal or alveolar space (data not shown). It has been reported that Kurloff cells are NK-like cells mediating TNF-dependent cytotoxic activity [47] and also cytolytic activity on a guinea pig leukaemic cell line [10]. Its cytotoxic activity has been demonstrated using 51Cr release assay with human and murine targets. The increased influx of Kurloff cells in the lung following BCG vaccination suggests that they may be components of a positive innate immune response against mycobacteria. Identification of other cells types such as epithelial cells, fibroblasts or mast cells was confounded because the integrity of normal cellular network was lost due to the nature of isolation procedures.
In co-cultures, the proliferation of T cells to ConA was similar in the presence of macrophages from various anatomical sites (Fig. 3a). However, lung T cells proliferated well to PPD only in the presence of PMøs and not when co-cultured with AMøs or LMøs (Fig. 3b). Previous research from our laboratory demonstrated that spleen T cells from guinea pig cells proliferated poorly to ConA in the presence of AMøs and the effect was not due to nitric oxide (NO), prostaglandin E2 (PGE2) or H2O2 production by macrophages. Those studies indicated that cell-to-cell contact was necessary for suppression to occur and the suppression was mediated by soluble factors released in the co-cultures [27].
Similarly, AMøs from human and other mammalian species are known to suppress proliferation of T cells [21,48]. AMøs from humans and rodents selectively inhibited T cell proliferation, while other indices of T cell activation (e.g. CD3-down-modulation, IL-2R expression, IL-2 secretion) proceeded normally [23,49]. The suppression of T cell proliferation by AMøs is thought to represent a protective immunoregulatory mechanism within the lung [21], which is challenged constantly with both pathogenic and non-pathogenic organisms. Therefore, it is important to maintain the balance between appropriately effective responses against infectious organisms and unnecessary or excessive responses to non-pathogenic stimuli [23]. In our studies, the suppression in T cell proliferation by AMøs was not abrogated by the stimulation of cultures with rgpIFN-γ (Fig. 3c). In some studies using mouse and human alveolar macrophages, the addition of cytokines such as TNF-α or GM-CSF bypassed the suppression caused by pulmonary alveolar macrophages [50]. Furthermore, LMøs behaved in a similar manner to the resident AMøs in suppressing T cell proliferation. Our previous studies indicated that AMøs produce lower levels of cytokine mRNA and protein when cultured in the presence of whole mycobacteria or PPD [29]. Lung digest cells, when stimulated with ConA, LPS or PPD, showed enhanced mRNA expression for IFN-γ and TNF-α, although the levels of IFN-γ mRNA were significantly higher than TNF-α mRNA in total, non-adherent or adherent populations (Fig. 5). However, a higher expression of IFN-γ mRNA was not accompanied by the production of IFN protein. Expression of TNF-α mRNA in the total, non-adherent or adherent lung populations was associated with the release of large quantities of TNF-α protein. These results indicate a dissociation between IFN-γ mRNA expression and protein production, which has been reported previously for both TNF-α and IFN-γ mRNAs in the rectal samples of Shigella-infected patients [51]. It is clear that the frequency of cytokine mRNA-expressing cells and the corresponding protein-synthesizing cells are highly variable. Alternatively, limitations of the protein assay may also contribute to this phenomenon.
Surprisingly, adherent population from the lung digest cells that were mainly macrophages as assessed by non-specific esterase staining showed significant induction of IFN-γ mRNA. Stimulation with LPS induced IFN-γ mRNA expression in murine macrophages [52]. Similarly, mouse pulmonary macrophages isolated from BCG-vaccinated mice produced IFN-γ protein when cultured in vitro with LPS or PPD [53]. These studies also demonstrated that IFN-γ was released in the culture supernatants of freshly isolated lung macrophages from naive mice after stimulation with LPS and IL-12 or BCG and IL-12 [53]. Human AMøs, when stimulated with virulent or attenuated M. tuberculosis for 24–48 h, showed induction of IFN-γ mRNA and release of IFN-γ protein [54]. Thus, it is clear that multiple cytokine signalling may be required for the release of IFN-γ protein by macrophages and that macrophage activation occurs in an autocrine fashion by responding to, as well as producing, IFN-γ.
We have reported recently that rgpIFN-γ up-regulated MHC class II expression, enhanced H2O2 production and restricted the intracellular growth of virulent M. tuberculosis in guinea pig PMøs [34]. Although LMøs were lymphocytostatic, they restricted the intracellular replication of virulent M. tuberculosis when activated with rgpIFN-γ (Fig. 4). It is quite possible that, similar to AMøs, the suppression of T cell proliferation by LMøs might also be an important immunoregulatory mechanism in preserving lung function.
Our studies indicate clearly that functionally active lymphocytes and macrophages can be purified from guinea pig lung digests after enzymatic digestion. We have demonstrated that LMøs, like AMøs, suppress T cell proliferation compared to peritoneal macrophages in co-cultures. Furthermore, treatment of LMøs with rgpIFN-γ induced a reduction in intracellular accumulation of virulent M. tuberculosis. These experimental approaches will enhance significantly our understanding of the mechanisms of vaccine-induced resistance, as well as the cellular and molecular events that occur at the site of M. tuberculosis infection in the guinea pig model.
Acknowledgments
This work was supported by USPHS, NIH grant no. RO1 AI 15495 to D. N. M and travel grants from CRDF 2412 and ISTC 1879 for K. M. We are grateful to Jane Miller for her help and expertise in FACS analysis and to Drs Rajesh Miranda and Karen Russell for helping us to identify the cell types from lung digest.
References
- 1.Kaufmann S. Towards new leprosy and tuberculosis vaccines. Microbiol Sci. 1987;4:324–8. [PubMed] [Google Scholar]
- 2.Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 3.Young LS, Inderlied B, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986;8:1024–33. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- 4.Canetti G. The tubercle bacillus in the pulmonary lesion in man. New York: Springer Publishing Co.; 1955. [Google Scholar]
- 5.Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Harding G, Chan J, et al. Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea-pigs. J Biol Stand. 1979;7:179–97. doi: 10.1016/s0092-1157(79)80021-9. [DOI] [PubMed] [Google Scholar]
- 7.Udani PM. BCG vaccination in India and tuberculosis in children: newer facets. Indian J Pediatr. 1994;61:451–62. doi: 10.1007/BF02751703. [DOI] [PubMed] [Google Scholar]
- 8.Roche PW, Triccas JA, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145:149–54. [PubMed] [Google Scholar]
- 10.Debout C, Quillec M, Izard J. New data on the cytolytic effects of natural killer cells (Kurloff cells) on a leukemic cell line (guinea pig L2C) Leuk Res. 1999;23:137–47. doi: 10.1016/s0145-2126(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 11.Mackaness GB. The immunology of antituberculous immunity. Am Rev Respir Dis. 1969;97:337–44. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- 12.Orme IM, Miller ES, Roberts AD, et al. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–96. [PubMed] [Google Scholar]
- 13.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holscher C, Atkinson RA, Arendse B, et al. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 17.Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flesch IE, Kaufmann SH. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–7. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemijo R, Le J, Shapiro D, et al. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with bacillus Calmette–Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–40. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–9. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt PG. Regulation of antigen presenting cell(s) in lung and airway tissues. Eur Respir J. 1993;6:120–9. [PubMed] [Google Scholar]
- 22.McCombs CC, Michalski JP, Westerfield BT, Light RW. Human alveolar macrophages suppress the proliferative response of peripheral blood lymphocytes. Chest. 1982;82:266–71. doi: 10.1378/chest.82.3.266. [DOI] [PubMed] [Google Scholar]
- 23.Upham JW, Strickland DH, Bilyk N, Robinson BW, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T cell proliferation but permit T cell activation and cytokine secretion. Immunology. 1995;84:142–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–7. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt PG. Alveolar macrophages. I. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979;37:429–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Holt PG. Alveolar macrophages. IV. Interspecies differences in activity in proliferating lymphocyte cultures. Cell Immunol. 1980;50:210–5. doi: 10.1016/0008-8749(80)90020-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, McMurray DN. Suppression of lymphoproliferation by alveolar macrophages in the guinea pig. Tuber Lung Dis. 1998;79:119–26. doi: 10.1054/tuld.1998.0014. [DOI] [PubMed] [Google Scholar]
- 28.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71:4271–7. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H, Lasco TM, Sedberry S, Yoshimura T, McMurray DN. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect Immun. 2005;73:1367–76. doi: 10.1128/IAI.73.3.1367-1376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeevan A, Yoshimura T, Foster G, McMurray DN. Effect of BCG infection on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect Immun. 2002;70:1245–53. doi: 10.1128/IAI.70.3.1245-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeevan A, Yoshimura T, Lee KE, McMurray DN. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect Immun. 2003;71:354–64. doi: 10.1128/IAI.71.1.354-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasco TM, Yamamoto T, Yoshimura T, Allen SS, Cassone L, McMurray DN. Effect of Mycobacterium bovis BCG vaccination on Mycobacterium-specific cellular proliferation and tumor necrosis factor alpha production from distinct guinea pig leukocyte populations. Infect Immun. 2003;71:7035–42. doi: 10.1128/IAI.71.12.7035-7042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyons MJ, Yoshimura T, McMurray DN. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect Immun. 2002;70:5471–8. doi: 10.1128/IAI.70.10.5471-5478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeevan A, McFarland CT, Yoshimura T, et al. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect Immun. 2006;74:213–24. doi: 10.1128/IAI.74.1.213-224.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasco TM, Cassone L, Kamohara H, Yoshimura T, McMurray DN. Evaluating the role of tumor necrosis factor-alpha in experimental pulmonary tuberculosis in the guinea pig. Tuberculosis. 2005;85:245–58. doi: 10.1016/j.tube.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Lyons MJ, Yoshimura T, McMurray DN. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis. 2004;84:283–92. doi: 10.1016/j.tube.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Skwor TA, Cho H, Cassidy C, Yoshimura T, McMurray DN. Recombinant guinea pig CCL5 (RANTES) differentially modulates cytokine production in alveolar and peritoneal macrophages. J Leukoc Biol. 2004;7:1229–39. doi: 10.1189/jlb.0704414. [DOI] [PubMed] [Google Scholar]
- 38.Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:2666–74. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyadova IV, Eruslanov EB, Khaidukov SV, et al. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J Immunol. 2000;165(10):5921–31. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 40.Cho H, McMurray DN. Neutralization of tumor necrosis factor alpha suppresses antigen-specific type 1 cytokine responses and reverses the inhibition of mycobacterial survival in cocultures of immune guinea pig T lymphocytes and infected macrophages. Infect Immun. 2005;73:8437–41. doi: 10.1128/IAI.73.12.8437-8441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971;55:283–90. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MK, Bartow RA, Mintzer CL, McMurray DN. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect Immun. 1987;55:314–9. doi: 10.1128/iai.55.2.314-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto T, Jeevan A, Ohishi K, et al. A new assay system for guinea pig interferon biological activity. J Interferon Cytokine Res. 2002;22:793–7. doi: 10.1089/107999002320271387. [DOI] [PubMed] [Google Scholar]
- 44.Antony VB, Sahn SA, Harada RN, Repine JE. Lung repair and granuloma formation. Tubercle bacilli stimulated neutrophils release chemotactic factors for monocytes. Chest. 1983;83:S95–6. doi: 10.1378/chest.83.5.95s. [DOI] [PubMed] [Google Scholar]
- 45.Appleberg R, Silva MT. T cell dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989;78:478–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Lasco TM, Turner OC, Cassone L, et al. Rapid accumulation of eosinophils in lung lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect Immun. 2004;72:1147–9. doi: 10.1128/IAI.72.2.1147-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pouliot N, Maghni K, Sirois P, Rola-Pleszczynski M. Guinea pig Kurloff (NK-like) cells mediate TNF-dependent cytotoxic activity: analogy with NC effector cells. Inflammation. 1996;20:263–80. doi: 10.1007/BF01488203. [DOI] [PubMed] [Google Scholar]
- 48.Holt PG. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986;63:261–70. [PMC free article] [PubMed] [Google Scholar]
- 49.Upham JW, Strickland DH, Robinson BW, Holt PG. Selective inhibition of T cell proliferation but not expression of effector function by human alveolar macrophages. Thorax. 1997;52:786–95. doi: 10.1136/thx.52.9.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilyk N, Holt PG. Cytokine modulation of the immunosuppressive phenotype of pulmonary alveolar macrophage populations. Immunology. 1995;86:31–237. [PMC free article] [PubMed] [Google Scholar]
- 51.Raqib R, Ljungdahl A, Lindberg AA, Wretlind B, Andersson U, Andersson J. Dissociation between cytokine mRNA expression and protein production in shigellosis. Eur J Immunol. 1996;26:1130–8. doi: 10.1002/eji.1830260526. [DOI] [PubMed] [Google Scholar]
- 52.Fultz MJ, Barber SA, Dieffenbach CW, Vogel SN, Bloom BR. Induction of IFN-γ in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–92. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–56. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]