Abstract
Many patients surviving vasculitis are prone to accelerated atherosclerosis and often have enhanced levels of antibodies to oxidized low-density lipoprotein (oxLDL). To measure anti-oxLDL antibodies, oxidation of LDL is achieved with copper (Cu) or malondialdehyde (MDA). Because, in vivo, LDL may be oxidized with myeloperoxidase (MPO) or its product hypochlorite, we measured anti-hypochlorite LDL antibodies in patients with vasculitis, haemodialysis patients and healthy controls. A newly developed enzyme-linked immunosorbent assay (ELISA) was used to detect antibodies to oxLDL as modified by hypochlorite. Results are compared with data obtained by standard LDL oxidation using MDA–LDL or Cu–LDL as substrate. Results were compared between anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) patients (n = 93), haemodialysis (HD) patients (n = 59) and healthy controls (HC; n = 43). Furthermore, patients with MPO–ANCA-associated vasculitis (n = 47) were compared with patients with proteinase 3 (PR3)–ANCA associated vasculitis (n = 46). Optimal cut-off points were determined by receiver operator characteristic (ROC) curve analysis. Anti-oxLDL antibodies are enhanced in AAV patients (MDA–LDL and hypochlorite–LDL) and in HD patients (hypochlorite–LDL), when compared to HC. Furthermore, patients with MPO–ANCA-associated vasculitis had higher levels of antibodies to hypochlorite–LDL than patients with PR3–ANCA-associated vasculitis. Our newly developed assay, in which hypochlorite–LDL is used as substrate, seems a more sensitive assay than traditional assays to measure oxLDL antibodies. Furthermore, our results suggest that enhanced MPO-mediated LDL oxidation occurs in patients with MPO–ANCA.
Keywords: ANCA-associated vasculitis, glomerulonephritis, hypochlorite, myeloperoxidase, oxidized LDL
Introduction
Small vessel vasculitis, such as Wegener's granulomatosis (WG) and microscopic polyangiitis (MPA) are associated strongly with anti-neutrophil cytoplasmic antibodies (ANCA) which are either directed to proteinase 3 (PR3) or to myeloperoxidase (MPO) [1–3]. ANCA-associated vasculitis (AAV) patients have an increased risk to develop accelerated atherosclerosis [4,5] and to experience subsequent cardiovascular events [6,7]. Atherosclerosis is generally considered an inflammatory disease [8], and chronic inflammation is a promoting factor for the progression of atherosclerosis toward cardiovascular events [9].
Antibodies directed against oxidized low density lipoproteins (oxLDL) can be extracted from human and experimental atherosclerotic lesions [10,11], and circulating anti-oxLDL antibodies can be detected in human individuals [10,12] and animal models of atherosclerosis [10,13]. Both in humans and animals, it has been reported that the level of circulating anti-oxLDL antibodies correlates positively with the severity of atherosclerosis [14–17], although this is not a consistent finding [18–20]. Nevertheless, levels of these antibodies are increased in AAV at diagnosis [21]. Detection of antibodies to oxLDL can be accomplished by oxidation of LDL in vitro by exposing LDL to malondialdehyde (MDA), a reactive aldehyde generated during lipid oxidation, and resulting in adduct formation on apoB100 [22]. Alternatively, LDL can be oxidized in vitro by copper [22,23]. An interesting in vivo pathway for LDL oxidation is catalysed by MPO, a haem enzyme present in neutrophilic granulocytes. MPO catalyses the generation of several reactive oxidants, leading to products which have been found in atherosclerotic lesions [24,25]. It is the only known enzyme capable of producing the highly toxic hypochlorite in vivo in the presence of chloride and hydrogen peroxide (H2O2).
MPO is not only intracellularly active. MPO is also released into the extracellular space after activation of granulocytes. Here, enzyme activity is inhibited by ceruloplasmin, a copper binding protein which attaches to the active site of MPO and hence regulates MPO enzyme activity in the circulation [26]. MPO–ANCA, isolated from AAV patients, is capable of partially reversing this MPO-inactivation [27].
We hypothesize that patients who are prone to accelerated atherosclerosis have antibodies to hypochlorite-modified LDL. Furthermore, we hypothesize that in MPO–AAV patients, but not in PR3–AAV patients, increased circulating MPO activity may result in enhanced LDL modification by hypochlorite and subsequent generation of antibodies specific for hypochlorite-modified LDL epitopes.
To test these hypotheses, we analysed anti-oxLDL antibody levels in 93 consecutive AAV patients with pauci-immune crescentic glomerulonephritis, 59 haemodialysis patients and 43 healthy controls. Forty-seven of the AAV patients had MPO–ANCA and 46 had PR3–ANCA. Anti-oxLDL antibody levels were measured using hypochlorite-modified LDL as well as MDA- and copper-modified LDL.
Methods
Patients
Patients included in this study had biopsy-proven pauci-immune crescentic glomerulonephritis without evidence of systemic lupus erythematosus (SLE), IgA nephropathy, Henoch Schönlein purpura, post-infectious glomerulonephritis (GN), cryoglobulinaemia or anti-glomerular basement membrane (GBM) nephritis [28]. Only patients whose biopsy showed active lesions were included. Serum samples, taken at the time of biopsy (i.e. diagnosis and thus before treatment), were tested for the presence of ANCA. Ninety-three patients with ANCA-associated glomerulonephritis (AAGN) were included in this study, 46 of which had PR3–ANCA and 47 had MPO–ANCA (Table 1); patients with both PR3– and MPO–ANCA and those with both MPO–ANCA and anti-GBM antibodies were excluded. As disease controls we used sera from all patients who are dialysed routinely at the haemodialysis (HD) unit in the University Hospital Maastricht (n = 59). Causes of dialysis are detailed in Table 2; patients with renal failure due to AAV were excluded from this control group. Additionally, 43 healthy controls (HC; laboratory personnel) were tested. Demographics (age and gender) of the three study cohorts are summarized in Table 1. This study was performed in accordance with the 1997 Declaration of Helsinki of the World Medical Association.
Table 1.
Demographics of HC, HD and vasculitis patients.*
| Vasculitis (n = 93) | ||||
|---|---|---|---|---|
| MPO–ANCA n = 47 | PR3–ANCA n = 46 | HD n = 59 | HC n = 43 | |
| Age | 60·0 ± 15·2 | 66·0 ± 11·0 | 67·8 ± 12·6 | 39·7 ± 10·6 |
| Male gender | 30 (64) | 34 (74) | 29 (48) | 22 (51) |
Data are given as mean ± standard deviation or number (%). HC, healthy controls; HD, haemodialysis; ANCA, anti-neutrophil cytoplasmic antibodies; MPO, myeloperoxidase; PR3, proteinase 3.
Table 2.
Causes of dialysis in HD population.
| Cause | Number of patients (%) |
|---|---|
| Vascular nephropathy | 18 (31) |
| Glomerulonephritis (non-ANCA) | 12 (20) |
| Diabetes | 13 (22) |
| Obstructive nephropathy | 6 (10) |
| Polycystic disease | 3 (5) |
| Other | 7 (12) |
HD, haemodialysis; ANCA, anti-neutrophil cytoplasmic antibodies.
LDL isolation and preparation of oxidized LDL
LDL was isolated from plasma of a healthy subject by ultracentrifugation in a KBr discontinuous gradient according to Redgrave et al. [29]. KBr and ethylenediamine tetraacetic acid (EDTA) were removed by rapid filtration through disposable desalting columns (Econo-Pac 10 DG, Bio-Rad, Hercules, CA, USA). The LDL protein content was determined according to Lowry et al. [30]. Native LDL was either freshly used for preparation of oxLDL or stored at 4°C in phosphate-buffered saline (PBS) under N2, with 1 mg/ml EDTA as a preservative.
MDA–LDL was prepared as described by Palinski et al. [22]; in brief, 0·5 M MDA (Merck, Darmstadt, Germany) was added to LDL at a ratio of 100 µl per mg LDL and incubated for 3 h at 37°C. After removal of unbound MDA by exclusion chromatography, MDA–LDL was stored at 4°C in PBS/EDTA under N2 after sterile filtration.
Copper oxidation (Cu-LDL) was performed by incubating a 0·2-mg/ml solution of LDL in PBS with 20 µM CuCl2 for 20 h at 37°C; reaction was stopped by adding 100 µM EDTA.
Hypochlorite modification (hypochlorite–LDL) was performed by coating 100 µl of native LDL, diluted to 100 µg/ml in PBS, in microtitre plates (Nunc MaxiSorp™, Nalge Nunc, Rochester, NY, USA) overnight at 4°C and, after washing three times with PBS, incubating half of each plate for 2 h at 4°C in a solution of 36 µM sodium hypochlorite(Sigma, St Louis, MO, USA) in PBS; the other half of the plate was incubated in PBS. Incubation was followed by a threefold wash with PBS to stop the reaction, and plates were stored in PBS at 4°C for a maximal 2 h before starting the enzyme-linked immunosorbent assay (ELISA). Optimal concentration of hypochlorite was determined using a serial dilution and subsequent testing of antibody reactivity (data not shown).
ELISA for the detection of antibodies against MDA–LDL, Cu–LDL and hypochlorite–LDL
Native LDL and MDA–LDL or Cu–LDL were diluted to 100 µg/ml in PBS, and 100 µl/well was incubated in microtitre plates (Nunc) overnight at 4°C; for detection of antibodies to hypochlorite–LDL, native LDL was coated before hypochlorite modification as described above. Wells were then washed five times with a buffer containing 0·01 M Tris, 0·15 M NaCl and 0·05% Tween 20 (pH 8·0) followed by incubation in triplicate with patient serum in a 1 : 100 dilution, 100 µl/well, in buffer containing 0·1 M Tris, 0·3 M NaCl and 0·05% Tween 20 (pH 8·0) and kept overnight at 4°C; a positive control was used on each plate to test for intra-assay variation. The next day, plates were washed five times with washing buffer and incubated with alkaline phosphatase conjugated goat F(ab′)2 anti-human IgG (γ)-specific conjugate (American Qualex, San Clemente, CA, USA), diluted 1 : 3000 in incubation buffer, for 1 h at 37°C. After washing five times with washing buffer, 100 µl of freshly made substrate containing 1 mg/ml of nitrophenyl phosphate (Sigma) in diethanolamine buffer at pH 9·8 was added to each well. After 30 min on a shaking platform at room temperature, plates were read at 405 nm. Results are expressed as mean anti-oxLDL levels in optical density (OD) from triplicate determinations and were calculated by subtracting binding to native LDL from binding to oxLDL. All results were obtained with native and oxLDL preparations that were stored for less than 2 weeks.
Anti-oxLDL preabsorbtion assay
To test cross-reactivity between MDA– and hypochlorite–LDL, sera of four AAV patients were selected for being positive for both anti-MDA– and anti-hypochlorite–LDL. Sera were diluted 1 : 100 in standard incubation buffer and then incubated in wells coated with either MDA–LDL or native LDL. After an incubation time of > 6 h, sera were transferred to wells with similar coatings. This procedure was repeated until sera had been absorbed six times on MDA–LDL or native LDL. Next, sera were tested as described above on both MDA– and hypochlorite–LDL. OD values obtained after absorption on MDA–LDL were expressed as the percentage of the OD values obtained after absorption on native LDL.
Statistical analyses
All data are presented as median (range) unless stated otherwise. Kruskal–Wallis test was used for comparing values of healthy controls and ANCA patients, and Dunn's post-test was used for testing differences between MPO–ANCA and PR3–ANCA groups. Receiver operator characteristic (ROC) curves were used to calculate appropriate cut-off values. Analyses were performed with spss version 11·0.1 (SPSS Inc., Chicago, IL, USA). A two-sided P-value ≤ 0·05 was considered to indicate statistical significance.
Results
Characterization of the anti-MDA–LDL, anti-Cu–LDL and anti-hypochlorite–LDL antibody assays
To minimize the variation between the different assays, one batch of LDL from one healthy donor was used for all assays. In all assays, intra-assay and interassay variation was evaluated for both native LDL and modified LDL. Intra-assay variation for native LDL varied from 0·1% to 11·8% (median intra-assay variation 2·1%). Inter-assay variation for native LDL varied from 5·5% to 6·9%.
Intra-assay variation for MDA–LDL varied from 0·4% to 5·4%, with an interassay variation of 3·1%. For Cu–LDL, intra-assay variation lay between 0·4% and 11·3% whereas interassay variation was 5·6%. Hypochlorite–LDL produced an intra-assay variation between 1·2% and 3·4% and an interassay variation of 3·9%.
Anti-oxLDL antibody detection in patients with AAV, haemodialysis patients and healthy controls
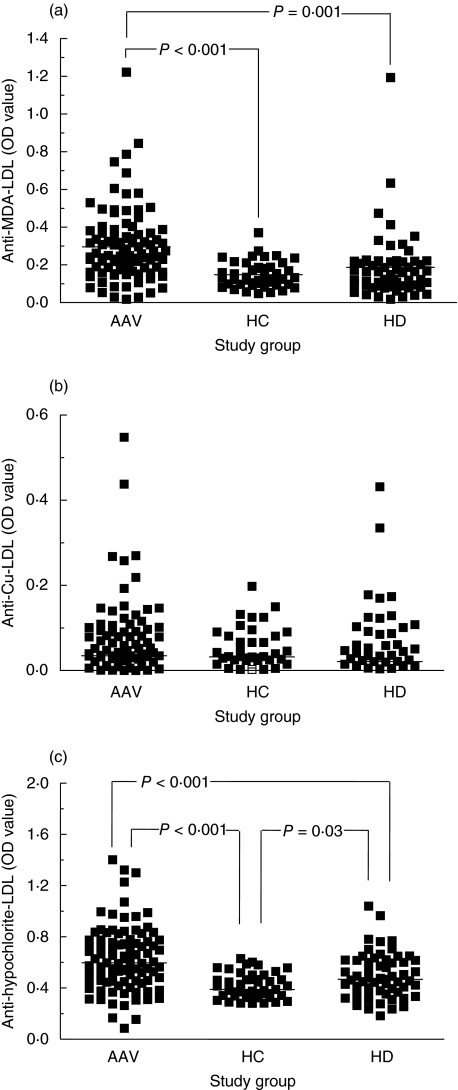
We first confirmed previous findings that antibody levels to oxLDL are increased in AAV patients by using MDA–LDL as antigen [21]. Anti-MDA–LDL antibody levels are higher in AAV patients than in HC (0·250 versus 0·131; P < 0·001) and/or HD patients (0·250 versus 0·164; P = 0·001; Fig. 1a). Antibody levels in HD patients were not significantly higher than in HC.
Fig. 1.
Anti-oxidized low-density lipoprotein (anti-oxLDL) reactivity in patients with anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) compared to healthy controls (HC) and chronic haemodialysis patients (HD). LDL was oxidized by malondialdehyde (MDA) (a), copper (Cu) (b) and hypochlorite (c). Lines represent median values.
Next, we evaluated a second commonly used LDL oxidation procedure, i.e. copper oxidation. This did not reveal a significant difference in anti-Cu–LDL antibody levels between AAV patients (0·035), HD patients (0·021) or HC (0·032; Fig. 1b).
Thirdly, we evaluated a new method for oxidation of LDL by using hypochlorite. The difference in anti-hypochlorite–LDL antibody levels between AAV patients (0·598) and HC (0·389) was statistically highly significant (P < 0·001; Fig. 1c). As for MDA–LDL, antibody levels against hypochlorite–LDL were significantly higher in AAV patients compared to HD patients (0·598 and 0·468, respectively, P < 0·001). Interestingly, detection of antibody levels against hypochlorite–LDL also enabled discrimination between HD patients and HC (0·468 and 0·389, respectively, P = 0·03).
In our study cohort, there was a clear difference in age of the HC with both AAV patients and HD patients (Table 1). Because an influence of age on the prevalence of anti-oxLDL antibodies has been suggested [31], we investigated the relation between age and anti-oxLDL antibody levels. No correlation (for all: R2 < 0·05) was observed between age and the levels of the various anti-oxLDL antibodies within each study cohort. Therefore, correction for age was considered not relevant.
Finally, we determined the discriminating performance of the respective assays for AAV patients, HD patients and HC using ROC curve analysis (Fig. 2). The ROC curves of hypochlorite– and MDA–LDL were almost identical and revealed, in contrast to the ROC curve of Cu–LDL, the ability to discriminate between the different study populations. For comparison we analysed the number of positive samples (sensitivity) from AAV patients and HD patients at a cut-off linked to an arbitrarily chosen specificity of 88·4% (five HC positive for anti-oxLDL; Table 3). With this given specificity,sensitivity is highest using hypochlorite–LDL (53 of 93 AAV patients positive (57·0%; OR 10·1, 95% CI 3·6–27·9, P < 0·001). Additionally, 21 of 59 HD patients were positive (35·6%; OR 4·2, 95% CI 1·4–12·3, P = 0·006). With MDA–LDL, 51 AAV patients were positive (54·8%; OR 9·2, 95% CI 3·3–25·5, P < 0·001) and nine HD patients were positive [15·3%; not significant (n.s.)]. Finally, while using Cu–LDL we found only 17 AAV patients (18·3%) and nine HD patients (15·3%) positive (Table 3).
Fig. 2.
Receiver operating characteristic (ROC) curves of anti-neutrophil cytoplasmic antibodies (ANCA) positive vasculitis patients versus healthy controls. Assay sensitivity in comparing ANCA-associated vasculitis (AAV) patients to healthy controls is higher using hypochlorite modified low density lipoprotein (LDL) (grey solid line) and malondialdehyde (MDA) modified LDL (black dotted line) than for copper-modified LDL (black solid line). The latter does not even discriminate between ANCA patients and healthy controls (cf. grey dotted reference line).
Table 3.
Sensitivity of the three anti-oxLDL assays at a given specificity.
| AAV versus HC | Cut-off OD value | % specificity | % sensitivity |
|---|---|---|---|
| Antigen | |||
| MDA-LDL | 0·242 | 88·4 | 54·8 |
| Cu-LDL | 0·108 | 88·4 | 18·3 |
| Hypochlorite-LDL | 0·559 | 88·4 | 57·0 |
AAV, ANCA-associated vasculitis; HC, healthy controls.
AAV patients with MPO–ANCA have higher antibody levels to hypochlorite–LDL than patients with PR3–ANCA
Antibody levels to the different oxLDL antigens were compared between patients with MPO–ANCA and PR3–ANCA. Anti-MDA–LDL antibody levels were not different in MPO–AAV patients (0·250) in comparison with PR3–AAV patients (0·256; data not shown). Using cut-off values as presented in Table 3, 25 of 46 PR3–AAV patients (54%) were positive compared to 26 of 47 MPO-AAV patients (55%; n.s.). Using Cu–LDL as an antigen, a significant difference was found between MPO–AAV patients (0·052) and PR3–AAV patients (0·025; P = 0·03; data not shown). However, given the low net OD values for anti-Cu–LDL antibodies, in combination with the fact that Cu–LDL did not differentiate between disease groups and healthy controls, we consider this difference not relevant. The use of hypochlorite–LDL resulted in significantly increased antibody levels in MPO–AAV patients (0·697) versus PR3–AAV patients (0·507; P < 0·001; Fig. 3). MPO–AAV patients more often had anti-hypochlorite–LDL antibodies (34 of 47, 72%) compared to PR3–AAV patients (19 of 46, 41%; OR 3·7, 95% CI 1·6–8·8, P = 0·003).
Fig. 3.
Anti-oxidized low-density lipoprotein (anti-oxLDL) reactivity in patients with myeloperoxidase–anti-neutrophil cytoplasmic antibodies (MPO–ANCA) compared to patients with proteinase 3 (PR3)–ANCA. LDL was oxidized by hypochlorite. Lines represent median values.
In conclusion, modification of LDL with hypochlorite performs best when differentiating between both groups of AAV patients.
Pre-absorption with MDA–LDL does not affect the level of antibodies to hypochlorite–LDL
In order to demonstrate the presence of hypochlorite–LDL-specific antibodies, sera of four AAV patients were preabsorbed repeatedly with either MDA–LDL or native LDL. OD values obtained after absorption with MDA–LDL were expressed as the percentage of OD values obtained after absorption with native LDL, the latter serving as a control for loss of antibody activity due to nonspecific binding. This procedure clearly diminished, although not completely removed, the reactivity for MDA–LDL (15–35%; Fig. 4). However, OD values for antibodies to hypochlorite–LDL were not affected by preabsorption with MDA–LDL (0–3%; Fig. 4). These data indicate that antibodies detected in the hypochlorite–LDL ELISA are truly specific for this antigen.
Fig. 4.
Anti-hypochlorite–low density lipoprotein (LDL) antibodies do not cross-react with malondialdehyde (MDA)–LDL. While preabsorption with MDA–LDL clearly diminishes the reactivity in an MDA–LDL enzyme-linked immunosorbent assay (ELISA) (grey bars), this is not the case for the reactivity in a hypochlorite–LDL ELISA (black bars). OD values for MDA– and hypochlorite–LDL, obtained after absorption on MDA–LDL, were expressed as the percentage of the OD values for MDA– and hypochlorite–LDL, respectively, obtained after absorption on native LDL.
Discussion
Our results show that antibody levels to hypochlorite–LDL are significantly increased when comparing AAV patients and HD patients to healthy controls and that these antibodies are more increased in patients with MPO–AAV than in patients with PR3–AAV. When comparing our new method for oxidation of LDL with previously established methods, we found that hypochlorite–LDL and MDA–LDL are equally sensitive for detection of anti-oxLDL antibodies in AAV patients, while hypochlorite–LDL, but not MDA–LDL, enabled detection of anti-oxLDL antibodies in HD patients. Cu–LDL lacked sensitivity in both patient populations. The increase in anti-oxLDL antibodies, as detected by hypochlorite–LDL, was most apparent in AAV patients with MPO–ANCA compared to AAV patients with PR3–ANCA.
These results are in accordance with our hypothesis that the MPO-catalysed pathway of LDL oxidation is increased in patients with accelerated atherosclerosis. Several pathways for MPO-catalysed modification of LDL have been described indicating that both the protein and lipid parts are affected, which processes seem to interact. Oxidation of the apoB100 part of LDL activates lipid peroxidation [32,33] while the latter can lead to protein alterations [32,34]. In the presence of NO, MPO generates reactive nitrogen species initiating lipid peroxidation [35,36], and in the presence of chloride and H2O2, MPO catalyses chlorination of unsaturated fatty acids and phospholipids [37,38] and conversion of LDL-cholesterol into chlorinated sterols [24]. However, protein modification seems to play a more pronounced role. Podrez [36] reported tyrosine nitration, while tyrosine chlorination was described by Hazen [24], in turn causing formation of dityrosyl in the apoB100 part of LDL [39–41]. Cysteine, methionine, lysine and tryptophan are also targets of MPO-catalysed modification [42–44]. Another effect of tyrosyl radical formation is the promotion of peroxidation of the lipid part of LDL [33].
MPO-derived epitopes on LDL are, at least in part, different from those generated by copper- and MDA-treatment; this is reflected in the above study, where we showed that the use of hypochlorite–LDL to detect autoantibodies results in a more sensitive assay to differentiate between different groups of patients. Furthermore, preabsorbion on MDA–LDL did not effect the level of antibodies reactive with hypochlorite–LDL. However, as preabsorbion on MDA–LDL could only partially remove the anti-MDA–LDL reactivity, cross-reactivity of anti-oxLDL antibodies with differing oxidized substrates remains a matter of discussion. In atherosclerosis patients, anti-oxLDL antibody levels are commonly tested using MDA– or Cu–LDL as the antigen of choice. Indeed, in HD patients, antibodies against both antigens are increased [45,46] and these antibodies are thought to mirror the presence of atherosclerosis [46], a well-known complication in these patients [47,48]. In our study, anti-hypochlorite–LDL antibody levels were increased in HD patients, whereas anti-MDA–LDL and anti-Cu–LDL antibody levels were not, indicating that our hypochlorite–LDL method may be a more sensitive test for atherosclerosis. Further studies are needed to see whether the use of hypochlorite–LDL is a useful tool for this purpose.
While the presence of antibodies against oxLDL has been associated repeatedly with the presence and severity of atherosclerosis [15,49], the role of these antibodies is still largely unknown. Circulating IgG anti-oxLDL antibodies, as detected in the present study, might have a pro-atherogenic effect, as they form immune complexes with oxLDL that can bind to Fc receptors present on macrophages in the lesions [50]. IgM anti-oxLDL antibodies, on the other hand, have been reported to be anti-atherogenic [51], due possibly to the inhibition of oxLDL uptake by macrophages in the lesions, thereby preventing the foam cell formation. Obviously, discrimination of these isotypes might further clarify the potentially pathogenic role of the anti-oxLDL antibodies in future studies.
Previously, we demonstrated enhanced levels of antibodies to MDA–LDL in vasculitis patients compared to healthy controls [21]. In the present study, we confirm these findings in a much larger cohort of patients. Furthermore, we found that patients with MPO–ANCA have higher levels of anti-oxLDL antibodies than patients with PR3–ANCA, when using hypochlorite–LDL as an antigen. Patients with PR3–ANCA and necrotizing crescentic glomerulonephritis (NCGN) generally have a more dramatic deterioration of their renal function compared with MPO–ANCA-positive AAV patients who exhibit a more indolent course of renal function loss [52]. Therefore, more chronic oxidation of LDL may have occurred in MPO–ANCA-positive AAV patients. Otherwise, an alternative hypothesis to explain the difference in anti-oxLDL levels between patients with MPO– and PR3–ANCA could be postulated. The presence of MPO–ANCA seems to influence the formation of antibodies to hypochlorite–LDL. These anti-MPO antibodies are thought to reverse inactivation of MPO by ceruloplasmin resulting in persistent MPO activity in the circulation, which might result in oxidation of LDL in blood vessels [27,53]. In HD patients, MPO activity increases during dialysis treatment, as we have shown previously [54]; this may have similar consequences to the presence of MPO–ANCA.
In conclusion, the use of hypochlorite–LDL has advantage over other modifications of LDL when detecting antibodies to oxLDL. Overall, our newly developed assay proved to be a more sensitive assay to measure these antibodies than the traditional assays in which LDL is modified by MDA or copper. By using our new assay, we found enhanced levels of autoantibodies to oxLDL in MPO–AAV patients when compared to PR3–AAV patients, suggesting that MPO–ANCA may interfere with MPO inhibition by ceruloplasmin. Our study also suggests that measuring antibodies to hypochlorite–LDL may be a useful addition for testing anti-oxLDL antibodies in patients who are suspected of accelerated atherosclerosis.
Acknowledgments
We would like to thank Professor Dr P. J. C. van Breda Vriesman, H. van Rie and P. Heerings, Clinical and Experimental Immunology, University Hospital Maastricht, for the collection of study material and all participating physicians and patients for their contribution (including the following physicians: Dr F. de Heer and Dr G. H. Verseput, Maasland Ziekenhuis, Sittard; Dr W. Grave, Dr J. Wirtz and Dr S. Boorsma, St Laurentius Ziekenhuis, Roermond; Dr J. Wolters and Dr L. A. M. Frenken, Atrium Medisch Centrum, Heerlen; Dr E. Zeppenfelt, Landgraaf; Professor Dr K. M. L. Leunissen, University Hospital Maastricht, Maastricht). Ms Marjan Slot is supported by a grant from ZonMW.
References
- 1.Cohen Tervaert JW, Goldschmeding R, Elema JD, et al. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990;37:799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 3.Velosa JA, Homburger HA, Holley KE. Prospective study of anti-neutrophil cytoplasmic autoantibody tests in the diagnosis of idiopathic necrotizing-crescentic glomerulonephritis and renal vasculitis. Mayo Clin Proc. 1993;68:561–5. doi: 10.1016/s0025-6196(12)60370-x. [DOI] [PubMed] [Google Scholar]
- 4.Conn DL. Update on systemic necrotizing vasculitis. Mayo Clin Proc. 1989;64:535–43. doi: 10.1016/s0025-6196(12)65558-x. [DOI] [PubMed] [Google Scholar]
- 5.Juvonen T, Juvonen J, Savolainen MJ. Is vasculitis a significant component of atherosclerosis? Curr Opin Rheumatol. 1999;11:3–10. doi: 10.1097/00002281-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 6.McLaren JS, Salisbury EM, Jayne DRW, Luqmani RA. Cardiovascular disease incidence and risk factors in ANCA-associated primary systemic vasculitis (AASV) Kidney Blood Press Res. 2003;26:282. [Google Scholar]
- 7.Slot MC, Cohen Tervaert JW, Franssen CFM, Stegeman CA. Renal survival and prognostic factors in patients with PR3–ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63:670–7. doi: 10.1046/j.1523-1755.2003.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis − an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 10.Palinski W, Rosenfeld ME, Yla-Herttuala S, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–6. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto M, Shoji T, Emoto M, Kawagishi T, Okuno Y, Nishizawa Y. Antibodies against oxidized LDL and carotid artery intima-media thickness in a healthy population. Arterioscler Thromb Vasc Biol. 2000;20:703–7. doi: 10.1161/01.atv.20.3.703. [DOI] [PubMed] [Google Scholar]
- 13.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–16. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 14.Bergmark C, Wu R, de Faire U, Lefvert AK, Swedenborg J. Patients with early-onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:441–5. doi: 10.1161/01.atv.15.4.441. [DOI] [PubMed] [Google Scholar]
- 15.Salonen JT, Yla-Herttuala S, Yamamoto R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 16.Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1569–76. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 18.Schumacher M, Eber B, Tatzber F, et al. Transient reduction of autoantibodies against oxidized LDL in patients with acute myocardial infarction. Free Radic Biol Med. 1995;18:1087–91. doi: 10.1016/0891-5849(94)00216-7. [DOI] [PubMed] [Google Scholar]
- 19.Virella G, Virella I, Leman RB, Pryor MB, Lopes-Virella MF. Anti-oxidized low-density lipoprotein antibodies in patients with coronary heart disease and normal healthy volunteers. Int J Clin Laboratory Res. 1993;23:95–101. doi: 10.1007/BF02592290. [DOI] [PubMed] [Google Scholar]
- 20.Tsimikas S, Brilakis ES, Lennon RJ, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–33. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Swets BP, Brouwer DAJ, Cohen Tervaert JW. Patients with systemic vasculitis have increased levels of autoantibodies against oxidized LDL. Clin Exp Immunol. 2001;124:163–7. doi: 10.1046/j.1365-2249.2001.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palinski W, Yla-Herttuala S, Rosenfeld ME, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–35. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 23.Reardon CA, Miller ER, Blachowicz L, et al. Autoantibodies to OxLDL fail to alter the clearance of injected OxLDL in apolipoprotein E-deficient mice. J Lipid Res. 2004;45:1347–54. doi: 10.1194/jlr.M400075-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–81. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinecke JW. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr Opin Lipidol. 1997;8:268–74. doi: 10.1097/00041433-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Park YS, Suzuki K, Mumby S, Taniguchi N, Gutteridge JM. Antioxidant binding of caeruloplasmin to myeloperoxidase: myeloperoxidase is inhibited, but oxidase, peroxidase and immunoreactive properties of caeruloplasmin remain intact. Free Radic Res. 2000;33:261–5. doi: 10.1080/10715760000301421. [DOI] [PubMed] [Google Scholar]
- 27.Griffin SV, Chapman PT, Lianos EA, Lockwood CM. The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies. Kidney Int. 1999;55:917–25. doi: 10.1046/j.1523-1755.1999.055003917.x. [DOI] [PubMed] [Google Scholar]
- 28.van Paassen P, van Breda Vriesman PJ, van Rie H, Cohen Tervaert JW. Signs and symptoms of thin basement membrane nephropathy: a prospective regional study on primary glomerular disease − the Limburg Renal Registry. Kidney Int. 2004;66:909–13. doi: 10.1111/j.1523-1755.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 29.Redgrave TG, Roberts DC, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–9. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin fenol agent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 31.Tinahones FJ, Gomez-Zumaquero JM, Garrido-Sanchez L, et al. Influence of age and sex on levels of anti-oxidized LDL antibodies and anti-LDL immune complexes in the general population. J Lipid Res. 2005;46:452–7. doi: 10.1194/jlr.M400290-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–400. [PubMed] [Google Scholar]
- 34.Chen Q, Esterbauer H, Jurgens G. Studies on epitopes on low-density lipoprotein modified by 4-hydroxynonenal. Biochemical characterization and determination. Biochem J. 1992;288:249–54. doi: 10.1042/bj2880249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostyuk VA, Kraemer T, Sies H, Schewe T. Myeloperoxidase/nitrite-mediated lipid peroxidation of low-density lipoprotein as modulated by flavonoids. FEBS Lett. 2003;537:146–50. doi: 10.1016/s0014-5793(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 36.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–60. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winterbourn CC, van den Berg JJ, Roitman E, Kuypers FA. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys. 1992;296:547–55. doi: 10.1016/0003-9861(92)90609-z. [DOI] [PubMed] [Google Scholar]
- 38.Thukkani AK, Albert CJ, Wildsmith KR, et al. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem. 2003;278:36365–72. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 39.Francis GA, Mendez AJ, Bierman EL, Heinecke JW. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc Natl Acad Sci USA. 1993;90:6631–5. doi: 10.1073/pnas.90.14.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–72. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinecke JW, Li W, Daehnke HL, III, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993;268:4069–77. [PubMed] [Google Scholar]
- 42.Jerlich A, Fritz G, Kharrazi H, et al. Comparison of HOCl traps with myeloperoxidase inhibitors in prevention of low density lipoprotein oxidation. Biochim Biophys Acta. 2000;1481:109–18. doi: 10.1016/s0167-4838(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 43.Yang CY, Gu ZW, Yang HX, Yang M, Gotto AM, Jr, Smith CV. Oxidative modifications of apoB-100 by exposure of low density lipoproteins to HOCL in vitro. Free Radic Biol Med. 1997;23:82–9. doi: 10.1016/s0891-5849(96)00624-7. [DOI] [PubMed] [Google Scholar]
- 44.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maggi E, Bellazzi R, Gazo A, Seccia M, Bellomo G. Autoantibodies against oxidatively-modified LDL in uremic patients undergoing dialysis. Kidney Int. 1994;46:869–76. doi: 10.1038/ki.1994.344. [DOI] [PubMed] [Google Scholar]
- 46.Van Tits L, De Graaf J, Hak-Lemmers H, et al. Increased levels of low-density lipoprotein oxidation in patients with familial hypercholesterolemia and in end-stage renal disease patients on hemodialysis. Lab Invest. 2003;83:13–21. doi: 10.1097/01.lab.0000048633.76607.e0. [DOI] [PubMed] [Google Scholar]
- 47.Huysmans K, Lins RL, Daelemans R, Zachee P, De Broe ME. Hypertension and accelerated atherosclerosis in endstage renal disease. J Nephrol. 1998;11:185–95. [PubMed] [Google Scholar]
- 48.Ritz E. Atherosclerosis in dialyzed patients. Blood Purif. 2004;22:28–37. doi: 10.1159/000074921. [DOI] [PubMed] [Google Scholar]
- 49.Yla-Herttuala S. Is oxidized low-density lipoprotein present in vivo? Curr Opin Lipidol. 1998;9:337–44. doi: 10.1097/00041433-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Kiener PA, Rankin BM, Davis PM, Yocum SA, Warr GA, Grove RI. Immune complexes of LDL induce atherogenic responses in human monocytic cells. Arterioscler Thromb Vasc Biol. 1995;15:990–9. doi: 10.1161/01.atv.15.7.990. [DOI] [PubMed] [Google Scholar]
- 51.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–12. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 52.Franssen CF, Stegeman CA, Kallenberg CG, et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 53.Yang JJ, Preston GA, Pendergraft WF, et al. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am J Pathol. 2001;158:581–92. doi: 10.1016/S0002-9440(10)64000-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutgers A, Heeringa P, Kooman JP, van der Sande FM, Cohen Tervaert JW. Peripheral blood myeloperoxidase activity increases during hemodialysis. Kidney Int. 2003;64:760–2. doi: 10.1046/j.1523-1755.2003.00139.x. [DOI] [PubMed] [Google Scholar]