Abstract
Dendritic cell (DC) maturation is required for efficient presentation of autoantigens leading to autoimmunity. In this report, we have examined whether release of tissue antigens from necrotic thyroid epithelial cells can trigger DC maturation and initiation of a primary antiself response. DC were cocultured with either viable (VT/DC) or necrotic (NT/DC) thyrocytes, and their phenotypic and functional maturation as well as immunopathogenic potential were assessed. Significant up-regulation of surface MHC class II and costimulatory molecule expression was observed in NT/DC but not in VT/DC. This was correlated with a functional maturation of NT/DC, determined by IL-12 secretion. Challenge of CBA/J mice with NT/DC, but not with VT/DC, elicited thyroglobulin (Tg)-specific IgG as well as Tg-specific CD4+ T-cell responses and led to development of experimental autoimmune thyroiditis. These results support the view that thyroid epithelial cell necrosis may cause autoimmune thyroiditis via maturation of intrathyroidal DC.
Keywords: autoimmunity, dendritic cell, necrosis, thyroiditis
Introduction
Dendritic cells (DC), loaded with self proteins or peptides, have been well known to provoke organ-specific autoimmune diseases [1–3]. This was first observed in experimental autoimmune thyroiditis (EAT) with thyroglobulin (Tg)-pulsed DC [4] and was later confirmed in the same [5] or other animal models [6,7]. These observations, the unrivalled capacity of DC to activate naïve T cells, and the detection of DC in lesions associated with numerous autoimmune diseases [3], including thyroiditis [8–11], have strongly argued for DC involvement in the initiation of autoimmunity. The maturation stage of DC seems to play a pivotal role in this process: under homeostatic conditions, immature DC are believed to continually transport autoantigens to draining lymph nodes, process and present them to cognate T cells in a substimulatory context, leading to T-cell tolerance [3,12–14]. Under the influence of endogenous ‘danger signals’ released by tissues undergoing stress, damage or abnormal death, or exogenous danger signals elaborated by pathogens, DC undergo maturation [15]. During this terminal differentiation stage, DC generate high levels of peptide-MHC class II complexes on their surface and up-regulate costimulatory molecule expression, emerging as ideal antigen presenting cells (APC) for naïve T-cells.
Necrotic cells, i.e. disintegrated cells which have released their cell contents prior to their ingestion by phagocytes, can cause mouse and human DC maturation in vitro[16–18]. However, the implications of these findings in the induction of organ-specific autoimmunity have not been adequately explored. Primary necrosis is triggered by noxious stimuli such as toxins, hypoxia and extremes of temperature [19], whereas secondary necrosis refers to the eventual disintegration of cells that have initially undergone apoptosis but have not been captured by phagocytes. To the extent that chronic dietary iodine excess is known to have toxic effects on thyrocytes of animals prone to autoimmunity [20–22], we sought in this study to test:
whether necrotic primary thyrocytes can mediate maturation of DC;
whether DC that have ingested syngeneic necrotic thyrocytes can elicit EAT or thyroid antigen-specific responses, following their adoptive transfer in CBA/J hosts.
LPS-matured DC loaded with Tg, the major thyroid antigen, were used as controls in the study to ensure the efficiency of T cell priming by DC.
Materials and methods
Animals, antigens and antibodies
Female CBA/J mice, 6–8 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All experimental procedures were reviewed and approved by the Animal Care Committee at Memorial University of Newfoundland. Tg was purified from frozen thyroid glands of outbred ICR mice (Bioproducts for Science, Indianapolis, IN, USA) by passing thyroid homogenates through a Sepharose CL-4B column [23]. PE-labelled hamster anti-CD11c (clone HL3), FITC-labelled rat mAbs specific for I-Ak (clone 10–3·6), CD80 (clone 16–10A1), CD86 (clone GL1) and CD40 (clone 3/23), and appropriately labelled isotype-matched control mAbs were purchased from BD Biosciences (Bedford, MA, USA).
Preparation of mouse thyrocytes and induction of thyrocyte necrosis
Fresh thyrocytes were prepared from CBA/J mouse thyroids according to a protocol described previously [24,25]. Briefly, thyroid lobes were fragmented and incubated for 30 min in 250 µl of digestion medium, consisting of 112 units/ml of Type I collagenase (Sigma, St. Louis, MO, USA) and 1·2 units/ml of dispase (Roche Diagnostics Corp, Indianapolis, IN, USA) in DMEM medium. After digestion, thyroid follicles were collected and cultured in Petri dishes or 6-well plates in complete F-12 medium, i.e. F-12 supplemented with 25% Nu-Serum IV (BD Biosciences), 10 ng/ml somatostatin and 2 ng/ml glycyl-L-histidyl-L-lysine acetate (both from Sigma). One week after culture, this procedure yielded approximately 105 thyrocytes from one thyroid gland. Primary necrosis of thyrocytes was achieved by four freeze (−80 °C)–thaw (room temperature) cycles leading to complete disruption of the cell membrane.
Immuofluorescent labelling of Tg in primary thyrocytes
Thyrocytes were grown in 8-well chamber slides (Nalge Nunc International, Naperville, IL, USA) for one week. The cells were then fixed in acetone at −20 °C for 5 min, and incubated in the presence of rabbit polyclonal antibodies specific for human Tg but cross reactive to murine Tg (DAKO, Carpinteria, CA, USA). After one hour, the cells were washed, and a secondary FITC-conjugated, goat anti-rabbit antibody (Sigma), was added for 30 min. Thyrocytes cultured only with the secondary antibody were used as control. Slides were mounted in 50% glycerol and sealed for immunofluorescence imaging under an Olympus Fluoview 300 Laser scanning confocal microscope.
Generation, antigen treatment and phenotypic analysis of DC
DC were derived from bone marrow precursors according to a protocol developed by Inaba et al.[26] and modified by Lutz et al.[27]. As previously described [28], bone marrow was collected from femurs and tibias from female CBA/J mice, depleted of red blood cells by treatment with NH4CL and plated at 2 × 106 cells per 100 mm Petri dish in complete RPMI 1640 medium supplemented with 10% supernatant from the murine- GM-CSF-secreting X63Ag8 cell line [29] (kindly provided by B. Stockinger, National Institute of Medical Research, London, UK). Eight days later, non adherent DC were gently dislodged and cultured overnight at 2 × 107 cells per 10 ml in the presence of 200 µg/ml Tg, or control antigen (200 µg/ml ovalbumin, OVA), followed by treatment with lipopolysaccharide (LPS, 1 µg/ml, 6 h). Similar numbers of DC were also cocultured with approximately 2 × 106 necrotic or viable thyrocytes for 24 h. Following exposure to antigen or thyrocytes, DC were analysed by immunofluorescence for cell-surface expression of CD11c, I-Ak, CD80, CD86 and CD40 markers. Detection of IL-12 secretion in culture supernatants was performed by sandwich ELISA using the BD OptEIA mouse ELISA kit, according to the manufacturer’s protocol (BD Biosciences).
Induction and histological assessment of EAT
DC incubated with soluble antigens or thyrocytes were washed twice with PBS and 2 × 106 cells per 0·2 ml PBS were injected i.p. into syngeneic recipient mice. Two weeks later, the mice were boosted with same number of similarly treated DC. Thyroid glands were removed 2 weeks after boosting, and were sectioned and stained with haematoxylin and eosin (H&E) for histological examination. The degree of mononuclear cell infiltration was scored as follows: 0 = no infiltration, 1 = small interstitial accumulation distributed between two or more follicles, 2 = one or two foci of inflammatory cells more than the size of one follicle, 3 = diffuse infiltration of 10–40% of total area.
T-cell proliferation assay and cytokine detection by sandwich ELISA
At the time of thyroid gland removal, spleens were collected for detection of antigen-specific T cells. After lysis of red blood cells by NH4Cl, single cell suspensions of splenocytes were prepared in complete DMEM medium supplemented with 10% fetal bovine serum (Cansera, Ontario, Canada), 20 mM HEPES buffer, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (all from Life Technologies, Rockville, MD, USA) and 5 × 10−5 M 2-ME (Sigma), and CD4+ T-cells were isolated using MACs separation according to the manufacturer’s instructions (Miltenyi Biotec Inc., Auburn, CA, USA). In a 96-well plate, splenic CD4+ T cells at 2 × 105 cells/well were cocultured with 2 × 105 cells/well of mitomycin C-treated DC for 3 days in the presence of titrated antigens. During the last 18 h, 1 µCi [3H]-thymidine was added to each well in 25 µl of culture medium. Cells were harvested and incorporated radioactivity was measured. Stimulation index (S.I) is defined as (cpm in the presence of antigen/cpm in the absence of antigen). After a 48-h stimulation, the IL-2, IL-4, IL-10, and IFN-γ cytokine content of culture supernatants was measured by sandwich ELISA using the BD OptEIA ELISA kit (BD Biosciences).
Detection of Tg-specific IgG by ELISA
The presence of Tg-specific IgG antibodies in serum was determined by ELISA as previously described [30]. Briefly, in each experimental group, pooled sera (8 samples per group) were initially diluted at 1 : 30 in PBS/Tween/0·1% BSA and added to Tg or OVA-coated plates at two-fold serial dilutions at room temperature for 1 h. The binding of serum IgG to the coated antigens was detected with alkaline phosphatase-conjugated goat antimouse IgG antibodies (Sigma). The light absorption of the p-nitrophenolate product at 405 nm was determined using a Vmax plate reader (Molecular Devices, Sunnyvale, CA, USA).
Results
Generation of Tg-secreting thyrocytes
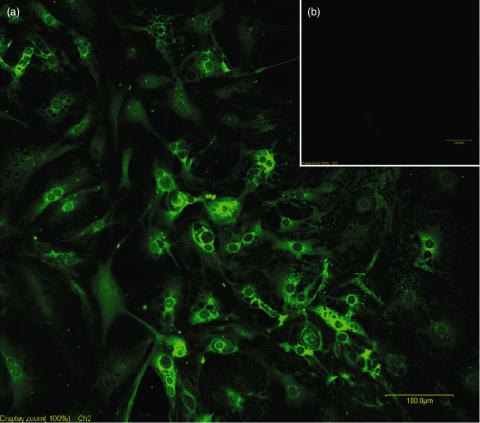
Primary thyrocytes were freshly prepared from CBA/J mouse thyroids, and cultured in 8-well chamber slide in complete F-12 medium. After one week, thyrocytes were washed in PBS and treated with rabbit anti-Tg antibodies (primary antibody) and FITC-labelled goat anti-rabbit antibodies (secondary antibody). Thyrocytes directly labelled with secondary antibodies were used as control. As shown in Fig. 1, almost 100% of the cultured cells showed Tg labelling in their cytoplasm, confirming their thyrocyte origin.
Fig. 1.
Immunofluorescent labelling of intracellular Tg in primary thyrocytes. (a) Primary thyrocytes were isolated from CBA/J mice, cultured for 7 days, and stained with rabbit anti-Tg antibody followed by FITC-goat antirabbit antibodies. (b) Control thyrocytes were stained with second antibody only.
Necrotic thyrocytes stimulate DC maturation
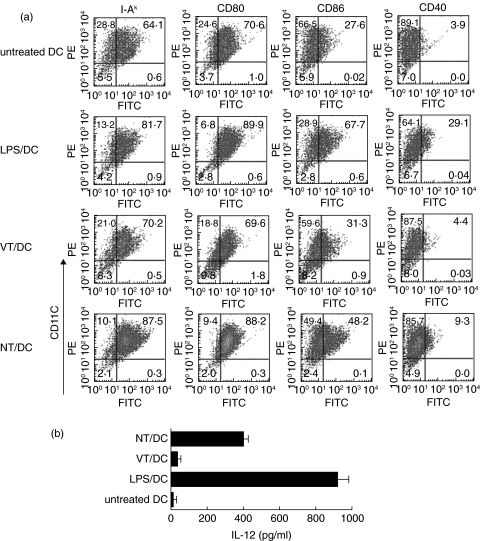
To test whether exposure to necrotic thyrocytes triggers DC maturation, phenotype markers of DC, cocultured with necrotic (NT/DC) or viable thyrocytes (VT/DC), were assessed by flow cytometry. DC treated with LPS (LPS/DC) or untreated DC were used as controls. The expression of MHC class II, CD80, CD86, and CD40 markers in VT/DC (70%, 70%, 31% and 4%, respectively), was similar to those in untreated DC (Fig. 2a). In contrast, these markers were significantly up-regulated in NT/DC (87%, 88%, 48% and 9%, respectively) (Fig. 2a), as well as in LPS/DC with the exception of CD86 and CD40 which were higher in LPS/DC (68% and 29%, respectively). IL-12 secretion, a critical parameter of DC functional maturation [28,30,31], was also monitored by sandwich ELISA in culture supernatants. As shown in Fig. 2b, necrotic, but not viable, thyrocytes activated DC to release significantly higher amounts of IL-12 (approximately 400 pg/ml) than those found in cultures of untreated DCs (< 20 pg/ml). These data clearly demonstrated that exposure to necrotic thyrocytes can trigger both phenotypic and functional DC maturation. Tg or OVA antigen alone did not mediate phenotypic maturation of DC (data not shown).
Fig. 2.
(a) Phenotypic analysis of DC after exposure to various stimuli by double labelling with PE-anti-CD11c mAb, and FITC-conjugated mAbs against MHC class II, CD80, CD86 or CD40. (b) Functional analysis of DC exposed to the same stimuli as in (a), as determined by IL-12 secretion in the culture supernatants. The results are expressed as means ± S.D. of triplicate wells. Data are representative of two independent experiments in (a) and (b).
DC exposed to necrotic thyrocytes elicit Tg-specific T- and B-cell responses
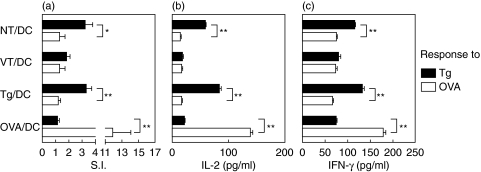
To test the immunogenic potential of DC exposed to necrotic or viable thyrocytes, CBA/J mice (8 mice per group) were i.p. challenged twice (on day 1, day 15) with 2 × 106 NT/DC or VT/DC. Additional groups of mice received the same number of Tg- or OVA- loaded, LPS-matured DC (Tg/DC, OVA/DC). It was observed that splenic CD4+ T cells exhibited low but significant proliferative responses to Tg following priming with Tg/DC or NT/DC (S.I. = 3·3 ± 0·4 or 3·2 ± 0·6, respectively) (Fig. 3a). In contrast, Tg-specific proliferative responses were undetectable in mice challenged with OVA/DC or VT/DC. Significant amounts of IL-2 and IFN-γ were detected in culture supernatants of Tg/DC- and NT/DC-activated CD4+ T cells (Fig. 3b,c), whereas IL-4 or IL-10 were undetectable (data not shown). As expected, challenge with OVA/DC elicited strong OVA-specific proliferative T-cell responses and IL-2 or IFN-γ release. Interestingly, mice challenged with NT/DC mounted strong Tg-specific IgG responses which were significantly higher than those detected in the sera of Tg/DC-challenged mice (mean OD405 nm = 1·195 versus 0·548, P < 0·001) (Table 1). These results demonstrated that NT/DC were strongly immunogenic, as they could initiate both B- and T-cell responses against Tg, the most abundant thyroid antigen.
Fig. 3.
NT/DC induce Tg-specific Th1 responses. (a) On d1 and d15, CBA/J mice received an i.p.injection of 2 × 106 DCs exposed to the stimuli shown. Two weeks after the last challenge, purified splenic CD4+ T cells were cocultured with syngeneic mitomycin C-treated DCs (APC) to test recall proliferative responses to Tg or OVA. Data represent the mean S.I. values of triplicate wells, obtained at antigen concentration of 100 µg/ml. Background cpm ranged from 1600 to 2200. (b,c) Cytokine determination by sandwich ELISA in 48-h supernatants of the corresponding cultures is shown in (a). Results are representative of two separate experiments. Statistical significance was determined by the t-test (*P < 0·05, **P < 0·01).
Table 1.
EAT and IgG responses induced by DC exposed to various stimuli.
| Induction of EAT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum IgG response (OD 405 nm) against | ||||||||
| Infiltration Index (I.I) | ||||||||
| 0 | 1 | 2 | 3 | Mean ± SD | No. of mice with EAT | Tg | OVA | |
| NT/DC | 4 | 2 | 1 | 1 | 0·88 ± 1·13 | 4/8 | 1·195 ± 0·010 | 0·193 ± 0·006 |
| VT/DC | 8 | 0 | 0 | 0 | 0·00 | 0/8 | 0·285 ± 0·021 | 0·176 ± 0·005 |
| Tg/DC | 3 | 1 | 1 | 3 | 1·50 ± 1·41 | 5/8 | 0·548 ± 0·001 | 0·197 ± 0·006 |
| OVA/DC | 8 | 0 | 0 | 0 | 0·00 | 0/8 | 0·192 ± 0·004 | 1·615 ± 0·010 |
CBA/J mice (8 mice per group) were i.p. challenged with 2 × 106 of the indicated DC and boosted, 2 weeks later, with the same number of DC. Thyroids were removed 28 days after the initial challenge for EAT assessment. At the same time, immune sera were collected, pooled and diluted at 1 : 30 in PBS/Tween/0·1% BSA for IgG determination. ELISA results are expressed as mean OD values of duplicate wells ± SD.
DC exposed to necrotic thyrocytes mediate EAT development
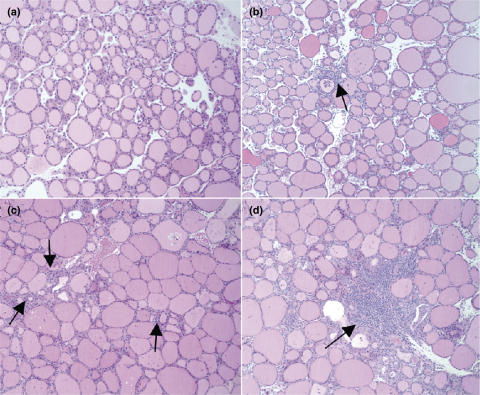
To assess the pathogenicity of NT/DC, the thyroids of CBA/J mice (8 mice per group) treated as described above, were obtained on day 28 for histological assessment. As shown in Table 1, four out of eight mice receiving NT/DC developed EAT (mean I.I. = 0·9 ± 1·1) with thyroid infiltration indices ranging from 1 to 3 (Fig. 4). The EAT incidence was similar to that of the group challenged with Tg/DC, since five out of eight mice presented with thyroiditis, albeit of higher severity (mean I.I. = 1·5 ± 1·4). Mononuclear cell infiltration of the thyroid was undetectable in mice challenged with either VT/DC or OVA/DC. These data directly support the notion that DC which engulf necrotic thyroid epithelial cells can initiate an autoimmune response leading to development of thyroiditis.
Fig. 4.
Histological appearance of EAT elicited by NT/DC. On d1 and d15, CBA/J mice were i.p. challenged with 2 × 106 indicated NT/DC. Two weeks after boosting, the mice were sacrificed and the thyroid glands were removed for EAT assessment. (a) Normal gland, I.I. = 0; (b) Interstitial accumulation of inflammatory cells (arrows), I.I. = 1; (c) One or two foci of inflammatory cells (arrows), I.I. = 2; (d) Diffuse infiltration, 10–40% of total area (arrows), I.I. = 3. Magnification: ×100.
Discussion
Our present findings show that DC which have captured necrotic thyrocytes can undergo maturation, and enable the immunogenic presentation of thyroid antigen(s), such as Tg, on their surface, leading to development of EAT. To our knowledge, this is the first report showing that DC can precipitate an organ-specific autoimmune disease following ingestion of necrotic epithelial tissue. Analogous findings have been recently reported for a model of a systemic autoimmune disease in which DC ingesting necrotic splenocytes induced strong anti-dsDNA antibody responses and accelerated disease progression in lupus-prone mice [32]. Excess iodide is well known to bring about thyrocyte necrosis both in vitro[33,34] and in experimental animals prone to thyroiditis such as Obese Strain chickens [20], BB/W rats [21] and NOD mice [10]. In humans, administration of the potent anti-arrhythmia drug amiodarone, which is very rich in iodine, has been linked to the pathogenesis of thyroiditis, possibly through the release of autoantigens from the injured thyroid [35]. Factors that promote enhanced apoptosis of thyrocytes [36] may also contribute in these processes since apoptotic cells may undergo secondary necrosis if not removed in time [37].
The maturation stimuli released by the disintegrated necrotic thyrocytes are unknown but may include genomic DNA [38], heat shock proteins [39], the high-mobility group B1 protein [40] or uric acid [41]. Under the conditions described herein (i.e. culturing of necrotic thyrocytes with DC at a 1 : 10 ratio), it is evident that enough antigenic material can be released to induce Tg-specific Th1 responses which were equivalent in magnitude to those obtained by DC loaded with purified Tg. Intrathyroidal DC are prime candidates for antigen capture in vivo and a small increase in the number of DC, clustering in the thyroidal interstitium, is one of the first signs of developing autoimmunity [8]. Thyroid tissue, obtained predominantly from patients with Graves’ disease, shows the presence of perifollicular immature DC at the basal surface of thyrocytes, with long cytoplasmic protrusions which penetrate the tight junctions between adjacent thyrocytes [42]. In addition, mature DC have been observed within lymphoid – like clusters in close proximity to CD4+ T cells [43] in agreement with the concept that mature DC that fail to migrate to lymph nodes may serve as nucleation sites for chronic inflammatory reaction [44]. According to current theory, however, DC ingesting necrotic thyrocytes are expected to reach the draining lymph nodes and activate thyroid antigen-specific naïve T cells there.
Tg/DC induced EAT with equivalent incidence but higher severity than that elicited by NT/DC. These findings confirm earlier observations that Tg-pulsed DC can initiate EAT [4,5]. It is quite likely, however, that NT/DC display on their cell surface T-cell epitopes of other thyroid antigens such as thyroid peroxidase; but the extent of other antigen participation, or the involvement of antigenic competition in this process, remains unknown. The capacity of Tg/DC or NT/DC to elicit significant Tg-specific IgG responses is also in agreement with the finding that DC can interact directly with naïve B cells to transfer unprocessed antigen and initiate class switching [45]. It is not clear why NT/DC induce stronger anti Tg IgG responses than Tg/DC since maturation of DC was mediated by distinct stimuli, in each case. In addition, it is not known whether Tg freshly released from necrotic cells may have minor conformational differences from lyophilized Tg preparations which could be detected by B cells. Cleavage of autoantigens during necrotic cell death seems to differ from the caspase-dependent proteolysis observed in apoptosis [46,47]. An intriguing possibility is that Tg processing in NT/DC may take place in a different manner than in Tg/DC, revealing the generation of cryptic determinants to which tolerance has not been established [47]. This is a testable hypothesis given the large number of pathogenic but cryptic Tg determinants already mapped [48]. A similar hypothesis can be made in iodide-induced thyrocyte necrosis since we have observed that the processing of highly iodinated Tg can promote the generation of a cryptic pathogenic determinant [49]. Lastly, it will be interesting to test whether use of Tg- pulsed tolerogenic DC may prevent EAT induction by NT/DC, as has been recently observed for EAT elicited by Tg in adjuvant [28], or whether apoptotic thyrocytes may be needed to generate tolerogenic DC for this purpose.
Acknowledgments
This study was supported by a grant from the Canadian Institutes of Health Research. We thank Dr B. Stockinger for providing the X63Ag8 cell line.
References
- 1.Drakesmith H, Chain B, Beverley P. How can dendritic cells cause autoimmune disease? Immunol Today. 2000;21:214–7. doi: 10.1016/s0167-5699(00)01610-8. [DOI] [PubMed] [Google Scholar]
- 2.Ludewig B, Odermatt B, Ochsenbein AF, Zinkernagel RM, Hengartner H. Role of dendritic cells in the induction and maintenance of autoimmune diseases. Immunol Rev. 1999;169:45–54. doi: 10.1111/j.1600-065x.1999.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 3.Turley SJ. Dendritic cells: inciting and inhibiting autoimmunity. Curr Opin Immunol. 2002;14:765–70. doi: 10.1016/s0952-7915(02)00399-0. [DOI] [PubMed] [Google Scholar]
- 4.Knight SC, Farrant J, Chan J, Bryant A, Bedford PA, Bateman C. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1988;48:277–89. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Inaba M, Adachi Y, et al. Experimental autoimmune thyroiditis induced by thyroglobulin-pulsed dendritic cells. Autoimmunity. 1999;31:273–82. doi: 10.3109/08916939908994073. [DOI] [PubMed] [Google Scholar]
- 6.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–9. [PubMed] [Google Scholar]
- 7.Weir CR, Nicolson K, Backstrom BT. Experimental autoimmune encephalomyelitis induction in naive mice by dendritic cells presenting a self-peptide. Immunol Cell Biol. 2002;80:14–20. doi: 10.1046/j.1440-1711.2002.01056.x. [DOI] [PubMed] [Google Scholar]
- 8.Canning MO, Ruwhof C, Drexhage HA. Aberrancies in antigen-presenting cells and T cells in autoimmune thyroid disease. A role in faulty tolerance induction. Autoimmunity. 2003;36:429–42. doi: 10.1080/0891630310001602984. [DOI] [PubMed] [Google Scholar]
- 9.Hala K, Malin G, Dietrich H, et al. Analysis of the initiation period of spontaneous autoimmune thyroiditis (SAT) in obese strain (OS) of chickens. J Autoimmun. 1996;9:129–38. doi: 10.1006/jaut.1996.0016. [DOI] [PubMed] [Google Scholar]
- 10.Many MC, Maniratunga S, Varis I, Dardenne M, Drexhage HA, Denef JF. Two-step development of Hashimoto-like thyroiditis in genetically autoimmune prone non-obese diabetic mice: effects of iodine-induced cell necrosis. J Endocrinol. 1995;147:311–20. doi: 10.1677/joe.0.1470311. [DOI] [PubMed] [Google Scholar]
- 11.Voorby HA, Kabel PJ, de Haan M, et al. Dendritic cells and class II MHC expression on thyrocytes during the autoimmune thyroid disease of the BB rat. Clin Immunol Immunopathol. 1990;55:9–22. doi: 10.1016/0090-1229(90)90065-x. [DOI] [PubMed] [Google Scholar]
- 12.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz M, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 14.Veeraswamy RK, Cella M, Colonna M, Unanue ER. Dendritic cells process and present antigens across a range of maturation states. J Immunol. 2003;170:5367–72. doi: 10.4049/jimmunol.170.11.5367. [DOI] [PubMed] [Google Scholar]
- 15.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 16.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 17.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 18.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 20.Bagchi N, Brown TR, Sundick RS. Thyroid cell injury is an initial event in the induction of autoimmune thyroiditis by iodine in obese strain chickens. Endocrinology. 1995;136:5054–60. doi: 10.1210/endo.136.11.7588241. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Boyages SC. Iodide induced lymphocytic thyroiditis in the BB/W rat: evidence of direct toxic effects of iodide on thyroid subcellular structure. Autoimmunity. 1994;18:31–40. doi: 10.3109/08916939409014677. [DOI] [PubMed] [Google Scholar]
- 22.Ruwhof C, Drexhage HA. Iodine and thyroid autoimmune disease in animal models. Thyroid. 2001;11:427–36. doi: 10.1089/105072501300176381. [DOI] [PubMed] [Google Scholar]
- 23.Chronopoulou E, Carayanniotis G. Identification of a thyroiditogenic sequence within the thyroglobulin molecule. J Immunol. 1992;149:1039–44. [PubMed] [Google Scholar]
- 24.Caturegli P, Rose NR, Kimura M, Kimura H, Tzou SC. Studies on murine thyroiditis: new insights from organ flow cytometry. Thyroid. 2003;13:419–26. doi: 10.1089/105072503322021070. [DOI] [PubMed] [Google Scholar]
- 25.Jeker LT, Hejazi M, Burek CL, Rose NR, Caturegli P. Mouse thyroid primary culture. Biochem Biophys Res Commun. 1999;257:511–5. doi: 10.1006/bbrc.1999.0468. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/ macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol. 2005;174:7433–9. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 29.Zal T, Volkmann A, Stockinger D. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–99. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verginis P, Stanford MM, Carayanniotis G. Delineation of five thyroglobulin T cell epitopes with pathogenic potential in experimental autoimmune thyroiditis. J Immunol. 2002;169:5332–7. doi: 10.4049/jimmunol.169.9.5332. [DOI] [PubMed] [Google Scholar]
- 31.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 32.Ma L, Chan KW, Trendell-Smith NJ, et al. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–75. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 33.Golstein J, Dumont JE. Cytotoxic effects of iodide on thyroid cells: difference between rat thyroid FRTL-5 cell and primary dog thyrocyte responsiveness. J Endocrinol Invest. 1996;19:119–26. doi: 10.1007/BF03349847. [DOI] [PubMed] [Google Scholar]
- 34.Many MC, Mestdagh C, van den Hove MF, Denef JF. In vitro study of acute toxic effects of high iodide doses in human thyroid follicles. Endocrinology. 1992;131:621–30. doi: 10.1210/endo.131.2.1639011. [DOI] [PubMed] [Google Scholar]
- 35.Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118:706–14. doi: 10.1016/j.amjmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Arscott PL, Baker JR., Jr Apoptosis and thyroiditis. Clin Immunol Immunopathol. 1998;87:207–17. doi: 10.1006/clin.1998.4526. [DOI] [PubMed] [Google Scholar]
- 37.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 38.Ishii KJ, Suzuki K, Coban C, et al. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–7. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 40.Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 42.Molne J, Jansson S, Ericson LE, Nilsson M. Adherence of RFD-1 positive dendritic cells to the basal surface of thyroid follicular cells in Graves’ disease. Autoimmunity. 1994;17:59–71. doi: 10.3109/08916939409014659. [DOI] [PubMed] [Google Scholar]
- 43.Quadbeck B, Eckstein AK, Tews S, et al. Maturation of thyroidal dendritic cells in Graves’ disease. Scand J Immunol. 2002;55:612–20. doi: 10.1046/j.1365-3083.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–4. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–9. [PubMed] [Google Scholar]
- 46.Casiano CA, Ochs RL, Tan EM. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 1998;5:183–90. doi: 10.1038/sj.cdd.4400336. [DOI] [PubMed] [Google Scholar]
- 47.Rodenburg RJ, Raats JM, Pruijn GJ, van Venrooij WJ. Cell death. a trigger of autoimmunity? Bioessays. 2000;22:627–36. doi: 10.1002/1521-1878(200007)22:7<627::AID-BIES5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.Carayanniotis G. The cryptic self in thyroid autoimmunity. the paradigm of thyroglobulin. Autoimmunity. 2003;36:423–8. doi: 10.1080/08916930310001602975. [DOI] [PubMed] [Google Scholar]
- 49.Dai YD, Rao VP, Carayanniotis G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol. 2002;168:5907–11. doi: 10.4049/jimmunol.168.11.5907. [DOI] [PubMed] [Google Scholar]