Abstract
Bronchiectasis is characterized by chronic airway infection and damage and remains an important health problem. Recent literature has emphasized the role of host defence and immune deficiency in the pathogenesis of bronchiectasis, but there have been few studies of immune function in adult bronchiectasis. A comprehensive screen of immune function was conducted in 103 adult patients with bronchiectasis, encompassing full blood examinations, immunoglobulins and IgG isotypes, complement levels, lymphocyte subsets and neutrophil function. Full blood examinations were normal in this cohort, as were complement levels. Statistical analysis confirmed that a significant number of subjects had low levels of IgG3 (13 patients), B cell lymphocytes (six patients) and T helper cell lymphocytes (seven patients) when compared with controls (P< 0·05). The most common abnormality was found with testing of the neutrophil oxidative burst. All subjects had a normal neutrophil phagocytic function but 33 of the subjects had an oxidative burst that was below the normal range (P< 0001). Almost half the group (45 subjects) had abnormally low levels of one of these four parameters. The findings of low B cells, Th cells and oxidative burst in bronchiectasis are novel. The results emphasize the importance of immune function assessment for adult bronchiectasis.
Keywords: helper T cells, lung immunology/disease, neutrophils
Introduction
Bronchiectasis is defined as abnormal dilatation of the air-conducting bronchi of the lung and is characterized by chronic respiratory tract infection and sputum production. The bronchi are inflamed and colonized by bacteria. The predominant symptoms are chronic cough and sputum production, shortness of breath, sinusitis and fatigue.
In the pre-antibiotic era bronchiectasis was associated with a poor outcome, and most patients died before the age of 40 years [1]. The introduction of antibiotics markedly improved outcome, and the condition was labelled as an orphan disease in the 1980s [2]. Recent studies have led to an awareness that bronchiectasis still remains a significant health issue. There are more than 100 000 subjects with bronchiectasis in the United States [3]. Chronic obstructive pulmonary disease (COPD) is becoming one of the world’s major health problems, with more than 50 million people having this condition worldwide [4]. The prevalence of bronchiectasis in COPD in two studies was 29%[5] and 50%[6].
The hallmark of bronchiectasis is recurrent infection and its dominant symptom is chronic sputum production. As such bronchiectasis is, with the exception of chronic osteomyelitis, perhaps the only common condition in industrialized countries where there is chronic bacterial infection. In the antibiotic era, most of the subjects with bronchiectasis have had long-standing infective symptoms since childhood and also have recurrent upper respiratory tract symptoms [7,8]. This is suggestive of immune dysfunction. Recent literature has emphasized the role of defects of host defence that predispose to chronic infection. These include cystic fibrosis mutations, mucociliary clearance defects, airway obstruction, allergic bronchopulmonary aspergillosis (ABPA) and hypogammaglobulinaemia.
Despite the crucial role of the immune system in bronchiectasis there has been a lack of studies that assess the immune function in this condition. Perhaps the only recent comprehensive study is that performed by Pasteur et al. [7], published in 2000. This study found that 27% of a cohort of 150 patients had an abnormality of immune function (including ABPA).
The authors undertook a study to extend the current understanding of immune deficiency in bronchiectasis. Complement, lymphocyte subsets and neutrophil responses to bacteria which have not been studied previously in bronchiectasis were emphasized.
Materials and methods
Study populations
The patient group consisted of 103 adult subjects who had bronchiectasis diagnosed on computerized tomography (CT) scanning using standard criteria [9]. Subjects had had a comprehensive assessment of their bronchiectasis (including clinical review, spirometry and sputum microbiological analysis). Subjects had been screened for cystic fibrosis (mutation analysis for 10 most abnormalities in local population), α1 anti-trypsin deficiency and allergic bronchopulmonary aspergillosis (aspergillus precipitins, sputum for analysis, IgE and skin test reactions). The patient group had predominantly idiopathic disease (90 patients); seven patients had post-infectious bronchiectasis, three had ABPA, two had rheumatoid arthritis and one had Young’s syndrome. All patients had had respiratory infections for at least 5 years and most (75) had had symptoms from childhood. The patient characteristics are listed in Table 1.
Table 1.
Characteristics of the 103 subjects undergoing immune function testing
| Age (years) | 61 ± 13 years |
| Sex | |
| Male | 36 male |
| Female | 67 female |
| Symptoms | |
| Chronic productive cough | 102 subjects |
| Daily sputum | 71 subjects |
| Rhinosinusitis | 74 subjects |
| Fatigue | 76 patients |
| Spirometry (% predicted) | |
| FEV1 | 67 ± 19 |
| FVC | 80 ± 21 |
| Sputum microbiology | |
| Haemophilus influenzae | 39 |
| Pseudomonas aeruginosa | 12 |
| Streptococcus pneumoniae | 10 |
| Moxarella catarrhalis | 6 |
| Staphylococcus aureus | 5 |
| Other | 7 |
| Mixed growth | 5 |
| No organism isolated | 30 |
Control values for full blood examinations, complement and immunoglobulin levels were based on 250–1000 tests conducted on healthy controls. Neutrophil function and lymphocyte subsets have been shown to change with age [10,11] and control values for lymphocyte subsets and neutrophil function were based on tests performed on a subgroup of 39 age-matched controls (57 ± 21 years) [mean and standard deviation (s.d.)].
This project was approved by the Monash Medical Centre Ethics Committee and informed consent was obtained from all subjects involved in this study before a peripheral blood sample was taken for analysis of immune parameters. This sample was taken when the patients were clinically stable and had not had an exacerbation for at least 1 month.
Full blood examination/immunoglobulins
Samples were analysed with full blood examination: haemoglobin, platelets and white cell count and differential (neutrophils, lymphocytes, macrophages, eosinophils and basophils), immunoglobulin (IgG, IgM and IgA) and IgG subclasses by standardized nephelometric assays.
Lymphocyte subsets
To measure lymphocyte subsets 100 µl aliquots of lithium heparin whole blood were labelled with combinations of the following fluorochrome [fluorescein isothiocyanate (FITC), phycoerythrin (PE) and Cy-5 conjugated antibodies: CD16, CD20 (Dako, Solna, Denmark); CD4, CD19, CD56 (Exalpha, Boston, MA, USA); and CD3, CD8, CD14, CD45 (in-house preparations]. The red blood cells were then lysed and the sample fixed using the Beckman-Coulter Q-prep machine (Hialeah, FL, USA). The labelled cells were then analysed using a Mo-Flo flow cytometer (Cytomation, Fort Collins, NC, USA).
Neutrophil function: phagocytosis and oxidative burst
The stimuli used most commonly to stimulate oxidative burst are the chemicals phorbol myristate acetate (PMA) and N-formyl-Met-Leu-Phe (fMLP). As bronchiectasis is characterized by recurrent bacterial infection it was decided that the use of a bacterial pathogen to stimulate the oxidative burst would be more appropriate. Several previous studies have used bacterial pathogens including Escherichia coli[12], group B Streptococcus[13] and Staphylococcus aureus[14] to stimulate the neutrophils. The stimulus used was a killed Staph. aureus preparation, pansorbin (Calbiochem, San Diego, CA, USA) labelled with propidium iodide (PI) (Sigma, Melbourne, Australia).
The simultaneous assessment of neutrophil oxidative burst and phagocytic function was performed using a whole blood assay that combined two previous published techniques [15,16]. Briefly, fixed Staph. aureus was incubated with PI at 5% wt/vol and 50 µg/ml, respectively, for 30 min at room temperature. It was then washed twice in Hanks’s balanced salt solution and resuspended at 5% w/v. This was then added to duplicate aliquots of undiluted lithium heparin blood at a final concentration of 0·5% w/v. These were incubated at 37°C for 20 min, at which time the reactive oxygen species (ROS) fluorescent indicator dihydrorhodamine 123 (Molecular Probes, Eugene, OR, USA) was added at a final concentration of 200 ng/ml. The sample was incubated for a further 10 min at 37°C and then a 100 µl sample from each aliquot was Q-prepped and the neutrophil green fluorescence (DHR123) and red fluorescence (PI–Staph. aureus) was measured on the Mo-Flo flow cytometer. The mean intensity (MCF) of both the red and green fluorescence was measured on the neutrophils gated by their forward and 90° light scatter characteristics. The averaged values of the duplicate aliquots were then used as the ROS and phagocytosis index. Samples were all processed on the day of the blood collection.
Statistical analysis
Statistical analysis was performed using either Stata SE version 8·0 (College Station, TX, USA) or Prism (San Diego, CA, USA) software. Comparisons between categorical variables used the Pearson’s χ2 statistic (or Fisher’s exact test for small samples). When comparing continuous outcome variables by categorical predictors, Student’s t-test was used (or its non-parametrical equivalent, Wilcoxon’s rank sum test). The comparison between two continuous variables was performed using Pearson’s correlation coefficient. The significance of the number of subjects below the normal range was obtained by using a goodness-of-fit test against the null hypothesis.
Results
Results obtained from patients were compared with control values and expressed as mean and standard deviation (mean ± s.d.). The control range was taken as the mean ± 2 s.d. (2·5–97·5 percentile) except for neutrophil oxidative burst, where the normal range was expressed as the 10th−90th percentile (as data were not normally distributed).
Full blood examination
Full blood examinations were normal in most subjects. (Table 2) Two subjects had polycythaemia (Hb of 203 and 216 g/l). The white cell count (WCC) was 7·62 ± 3·00 109/l, similar to normal control range. Only six patients had raised WCC numbers (range 11·2–21·2), consistent with the clinically stable condition of subjects in this cohort. The differential WCC parameters were also similar in both groups.
Table 2.
Full blood examinations
| Parameter | Units | Controls | Patients |
|---|---|---|---|
| Haemoglobin | g/l | 135 ± 13 | 140 ± 19 |
| White cell count | × 109/l | 7·5 ± 1·8 | 7·63 ± 3·0 |
| Platelets | × 109/l | 300 ± 85 | 249 ± 97 |
| Neutrophils | × 109/l | 5·5 ± 1·5 | 5·0 ± 3·3 |
| Lymphocytes | × 109/l | 2·10 ± 0·63 | 1·82 ± 0·73 |
| Monocytes | × 109/l | 0·51 ± 0·25 | 0·54 ± 0·17 |
| Eosinophils | × 109/l | 0·23 ± 0·12 | 0·22 ± 0·17 |
| Basophils | × 109/l | 0·15 ± 0·05 | 0·05 ± 0·07 |
Complement
Testing for complement components C3 and C4 showed that the cohort did not have any major abnormalities. Levels of C3 were 1·25 ± 0·23 (g/l) compared to control values of 1·35 ± 0·25. Levels of C4 were 0·36 ± 0·46 (g/l) compared to control values of 0·30 ± 0·10.
Immunoglobulins/immunoglobulin subclasses
Testing for immunoglobulin levels (IgG, IgM and IgA) showed no major abnormalities (Table 3). Of interest was the fact that there were only four subjects had elevated immunoglobulin levels. Elevated immunoglobulin levels are known to be a common accompaniment of inflammation and have been shown to be elevated in cystic fibrosis [17]. The results of few patients having raised immunoglobulins or white cell counts may reflect the clinical stability of this cohort.
Table 3.
Immunoglobulin/immunoglobulin subclass levels
| Parameter | Control (g/l) | Bronchiectasis (g/l) | Below normal range | P |
|---|---|---|---|---|
| IgG | 11·5 ± 2·25 | 12·1 ± 3·2 | 2 patients | 0·749 |
| IgA | 2·4 ± 0·8 | 3·3 ± 1·8 | 0 patients | 0·109 |
| IgM | 1·4 ± 0·5 | 1·5 ± 0·9 | 3 patients | 0·749 |
| IgG1 | 8·32 ± 2·30 | 7·71 ± 0·2.20 | 0 patients | 0·109 |
| IgG2 | 4·2 ± 1·50 | 3·45 ± 1·48 | 4 patients | 0·337 |
| IgG3 | 0·88 ± 0·22 | 0·91 ± 0·56 | 13 patients | < 0·001 |
| IgG4 | 0·60 ± 0·29 | 0·47 ± 0·30 | 0 patients | 0·109 |
Testing for immunoglobulin subclasses (IgG1, IgG2, IgG3 and IgG4) showed that 17 subjects had low levels of one of the IgG subclasses (Table 2). The major finding was that 13 subjects had low levels of IgG3 (ranging from 0·16 to 0·30; P< 0·001). No subject had a complete deficiency. Results for the other three subclasses were similar to control values.
Lymphocyte subsets
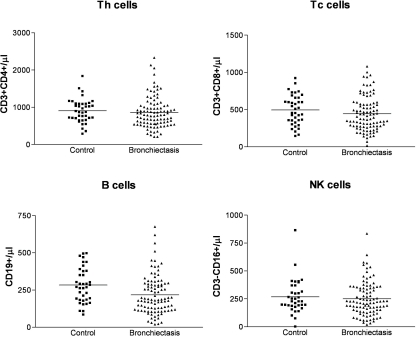
Levels of B cells (CD19+) in the bronchiectasis group were lower than controls: 212 ± 130 (cells/µl) compared with controls 283 ± 116 (P= 0·004). There was also a significant number of six subjects with B cell counts below the normal range (P= 0·025). Levels of T helper (Th) cells (CD3+ CD4+) were similar between controls and patients, but a significant number of seven subjects had a Th cell count below the normal range (P= 0·004). Testing for cytotoxic T (Tc) cells and natural killer (NK) cells showed that both groups were similar. Results of lymphocyte subsets are shown in Table 4 and Fig. 1.
Table 4.
Lymphocyte subsets
| Parameter | Control | Bronchiectasis | P | Below normal range | P |
|---|---|---|---|---|---|
| Th cell | 923 ± 305 | 863 ± 418 | 0·423 | 7 patients | 0·004 |
| Tc cell | 495 ± 200 | 445 ± 230 | 0·221 | 2 patients | 0·749 |
| B cell | 283 ± 116 | 212 ± 130 | 0·008 | 6 patients | 0·025 |
| NK cell | 269 ± 152 | 251 ± 150 | 0·532 | 0 patients | 0·169 |
Cells are numbers per µl. NK cell: natural killer cell.
Fig. 1.
Lymphocyte subsets in control and bronchiectasis groups. Levels of Th cells are similar in both groups but there are seven subjects with low CD4 numbers in the bronchiectasis group (P = 0.004). Levels of B cells are lower in the bronchiectasis group (P = 0.008), with six subjects below the normal range (P = 0.025). Natural killer and Tc cells were similar in both groups.
There did not appear to be any correlation between B cell counts and immunoglobulin/immunoglobulin subclass levels; no subject with low B cell numbers had low IgG3 levels. One subject had a monoclonal B cell population and was diagnosed subsequently as having a B cell lymphoma. All subject with low Th cell numbers had B cell numbers in the normal range. Of the subjects who had low Th cell numbers, six had a Th cell count below 300 cells µl (range 211–292). The subjects with low CD4 counts declined to have formal testing for human immunodeficiency virus, but they did not have any associated risk factors.
Neutrophil phagocytosis and oxidative burst
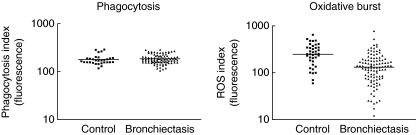
Testing of neutrophil function showed that phagocytosis was the same in both groups (P= 0·309), but oxidative burst was different in the two groups. The oxidative burst as measured by ROS fluorescence production was significantly lower in the bronchiectasis group (150 ± 109) compared with the control group (271 ± 134) (P< 0·001). Approximately one-third (33 subjects) of the bronchiectasis group had an oxidative burst below the control group range). Results of neutrophil function are shown in Table 5 and Fig. 2.
Table 5.
Neutrophil function in controls and patients
| Parameter | Control | Bronchiectasis | P | Below normal range | P |
|---|---|---|---|---|---|
| Phagocytosis | 179 ± 42 | 185 ± 41 | 0·632 | 1 patient | 0·334 |
| Oxidative burst | 271 ± 134 | 150 ± 109 | < 0·001 | 33 patients | < 0·001 |
Fig. 2.
(a) Phagocytosis in the control group and the bronchiectasis group. The neutrophil phagocytosis was very similar in both control and bronchiectasis patient groups. The vertical axis shows the intensity of fluorescence which corresponds to the degree of phagocytosis, and the horizontal axis shows the control and patient groups. (b) Oxidative burst in the control group and the bronchiectasis group. Neutrophil oxidative burst was significantly different in the control and the patient groups (P < 0.001) with 33 bronchiectasis subjects being below the control range (P < 0.001).
Overall summary of results
Four parameters were measured where there was a statistically significant number of subjects below the normal range; these findings are summarized in Table 6.
Table 6.
Immune function in bronchiectasis cohort (103 patients)
| Parameter | Subjects below normal range | P |
|---|---|---|
| Neutrophil oxidative burst | 33 patients | < 0·001 |
| IgG3 levels | 13 patients | < 0·001 |
| Th cells | 7 patients | 0·004 |
| B cells | 6 patients | 0·025 |
Overall, 45 subjects out of the group of 103 had abnormally low results for one of these four parameters. Seven subjects had more than one low level and all these subjects had low IgG3 and low neutrophil oxidative burst. There was no clear connection between any of the abnormally low levels.
Discussion
Bronchiectasis is a heterogeneous disease, with most patients having no clear predisposing condition. Most of the factors that have been shown clearly to be associated with bronchiectasis such as cystic fibrosis, bronchial obstruction and ciliary dyskinesia have a fundamental affect on the lung’s ability to clear pathogens. The resultant chronic infection causes disease, and in one way or another generally reflects compromised immunity. As the knowledge of immunity and human protective immune responses increases, new forms of immune deficiency are being recognized. This study included an assessment of the important relevant areas of the immune response, in particular: testing for complement, lymphocyte subsets and assessment of neutrophil responses to bacteria, which has not been undertaken previously.
The full blood examination of this group showed no major abnormalities. As expected, two patients had polycythaemia. Deficiency of complement function causes recurrent bacterial infection and there is a substantial overlap with the clinical features seen in patients with deficiencies of antibody production. None of the bronchiectasis subjects in this cohort had complement deficiencies, suggesting that complement deficiency is not important in the pathogenesis of adult bronchiectasis.
Hypogammaglobulinaemia has been recognized to be associated with bronchiectasis [18]. Subjects with hypogammaglobinaemia suffer infections from common bacteria such as Haemophilus influenzae, S. pneumoniae and Staph. aureus[19]. No subject had hypogammaglobinaemia. Previous studies have suggested that the incidence of hypogammaglobulinaemia is less than 10%[20], and the functional significance of such an abnormality should be confirmed by antibody production to a stimulus such as Haemophilus influenzae type b (HiB) polysaccharide vaccines. In this group of 103 patients, 14 subjects had low levels of IgG3. The significance of these findings is uncertain. No subject had an absolute deficiency. IgG subclass deficiency has certainly been described previously in the context of bronchiectasis. One study [21] found that almost half a cohort of 65 patients with idiopathic bronchiectasis had IgG subclass deficiencies (mainly IgG2), and this correlated with impaired antibody response to HiB. On the other hand, two recent studies [22,23] found that IgG subclass deficiency was rare in adults with bronchiectasis.
Bronchiectasis is characterized by recurrent infection; the main cell mediating the adaptive immune response is the lymphocyte. Lymphocyte subset analysis showed that there was a significant number of subjects who had B cell and T helper cell levels that were below the normal range. Low levels of B cells were not associated with low immunoglobulin levels. Six of the subjects had a CD4 count below 300 cells/µl and appeared to have idiopathic CD4 lymphocytopaenia, which is associated with opportunistic infections [24].
The major abnormality detected in this group of subjects with predominantly idiopathic bronchiectasis was in their neutrophil oxidative burst. Neutrophil phagocytosis was the same in both control and patient groups. In contrast, the oxidative burst was significantly lower in the bronchiectasis subjects, with a third of the group who were below the normal range. This is a novel finding.
Bronchiectasis is known to be characterized by airway neutrophil inflammation [25] in response to bacterial infection. There has been one previous study by Pasteur et al. that has assessed neutrophil function in bronchiectasis [7]. Pasteur did not find any major changes in neutrophil function when compared with controls. This study stimulated neutrophils with the stimulus fMLP. There are several different ways of stimulating the neutrophil oxidative burst. PMA is a phorbol ester which stimulates the cell directly, particularly by enhancing protein kinase C. fMLP is a potent chemotactic peptide that is produced by enteric flora and acts on a specific receptor to activate the oxidative burst. Pansorbin is killed by whole Staph. aureus. Previous work in older patients has shown that fMLP stimulation produces a normal oxidative burst, but when Staph. aureus was used there was a decrease in oxidative burst [10,14]. Staph. aureus may be a more relevant stimulus in subjects who have chronic bacterial infection.
Whether the difference between the groups was due to a class effect of chronic respiratory infection/inflammation or to a large subgroup with impaired neutrophil function has yet to be determined. Proinflammatory cytokines are able to modify neutrophil function [10], and bacterial products could have a general suppressive effect on neutrophil function. The authors have found previously in a cohort of renal transplant subjects, all of whom were receiving immunosuppressive therapy, that the neutrophil oxidative burst was low in a significant number of subjects [26].
The other alternative explanation considered for the low oxidative burst in a large number of these subjects was whether they had some intrinsic defect of their oxidative burst which predisposed them to recurrent infections. Deficiency of the oxidative burst with recurrent infections from early childhood is the phenotype of chronic granulomatous disease (CGD) arising from a deficiency of one of the four subunits that make up the nicotinamide–dinucleotide–phosphate (NADPH) complex. Classically, CGD has no detectable oxidative burst (rather than a reduced oxidative burst) and in most cases the disease is X-linked and due to a deficiency of the gp91 subunit of the NADPH complex [27]. However, it has become apparent in the last 10 years that CGD is a much more heterogeneous condition than thought originally and may present in adulthood for the first time, often with recurrent respiratory infections [28,29]. Therefore, it is possible that some of the patients may have an atypical form of CGD. Features in this group of patients that were consistent with a clinical phenotype of CGD were the presence of chronic severe sinopulmonary infections originating generally in childhood in subjects who had no clear cause for their disease. However, subjects did not have other features of CGD such as gingivitis, liver granulomas or skin infections; nor was there a strong family history of chronic infections.
There are some factors that need to be considered in the interpretation of the results. The variation in microbial colonization could affect the results, although this was not obvious in the analysis of the data. Thirty-four of the patients were on inhaled corticosteroids and two subjects were taking oral corticosteroids (dose 5 mg). Results from subjects taking corticosteroids were compared with the other patients and no difference was seen. The data would be strengthened by repeated testing, although repeated testing of a subgroup of controls and patient subjects for their neutrophil function showed that results were reproducible. Most causes of bronchiectasis are probably associative and it is very difficult to prove that they are directly causative. Whether the low results for immune parameters are cause or effect has not been established clearly.
In conclusion, testing of the immune function in this cohort of adults with bronchiectasis showed that half the subjects (45 subjects of 103) had an abnormality of four immune parameters: IgG3, Th cell levels, B cell levels and neutrophil oxidative burst. The predominant finding was a deficiency of neutrophil oxidative burst. These findings emphasize the importance of comprehensive immune screening in bronchiectasis.
Acknowledgments
We would like to thank Dr Elmer Villanueva for help with the statistical analysis. This work was supported by a National Health Medical Research Council (NHMRC) of Australia grant (to P. K.)
References
- 1.Perry K, King D. Bronchiectasis: a study of prognosis based on a follow up of 400 patients. Am Rev Tuberc. 1940;41:153–60. [Google Scholar]
- 2.Barker AF, Bardana EJ., Jr Bronchiectasis: update of an orphan disease. Am Rev Respir Dis. 1988;137:969. doi: 10.1164/ajrccm/137.4.969. 2005. [DOI] [PubMed] [Google Scholar]
- 3.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and Economic Burden of Bronchiectasis. Clinical Pulmonary Medicine. 2005;12:205–9. [Google Scholar]
- 4.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 7.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 8.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Gallagher M, Holmes PW. Outcome in adult bronchiectasis. J COPD. 2005;2:27–34. doi: 10.1081/copd-200050685. [DOI] [PubMed] [Google Scholar]
- 9.McGuinness G, Naidich DP. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002;40:1–19. doi: 10.1016/s0033-8389(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 10.Butcher S, Chahel H, Lord JM. Ageing and the neutrophil: no appetite for killing? Immunology. 2000;100:411–6. doi: 10.1046/j.1365-2567.2000.00079.x. [Review article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyere M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13:215–18. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 12.Shalekoff S, Tiemessen CT, Gray CM, Martin DJ. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:41–4. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCloskey PS, Salo RJ. Flow cytometric analysis of group B streptococci phagocytosis and oxidative burst in human neutrophils and monocytes. FEMS Immunol Med Microbiol. 2000;27:59–65. doi: 10.1111/j.1574-695X.2000.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–5. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Perticarari S, Presani G, Banfi E. A new flow cytometric assay for the evaluation of phagocytosis and the oxidative burst in whole blood. J Immunol Meth. 1994;170:117–24. doi: 10.1016/0022-1759(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 16.Emmendorffer A, Hecht M, Lohmann-Matthes ML, Roesler J. A fast and easy method to determine the production of reactive oxygen intermediates by human and murine phagocytes using dihydrorhodamine 123. J Immunol Meth. 1990;131:269–75. doi: 10.1016/0022-1759(90)90198-5. [DOI] [PubMed] [Google Scholar]
- 17.Elborn JS, Cordon SM, Shale DJ. Host inflammatory responses to first isolation of Pseudomonas aeruginosa from sputum in cystic fibrosis. Pediatr Pulmonol. 1993;15:287–91. doi: 10.1002/ppul.1950150505. [DOI] [PubMed] [Google Scholar]
- 18.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 19.Janeway C. Failure of host defence. In: Janeway C, Traver P, Walport M, Schlomchik M, editors. Immunobiology, The immune system in health and disease. Vol. 5. New York: Garland; 2001. pp. 425–50. [Google Scholar]
- 20.Cole PJ. Host–microbial interactions in chronic respiratory disease. In: Reeves D, Geddes G, editors. Recent advances in infection. Edinburgh: Churchill Livingstone; 1989. pp. 141–70. [Google Scholar]
- 21.De Gracia J, Rodrigo MJ, Morell F, et al. IgG subclass deficiencies associated with bronchiectasis. Am J Respir Crit Care Med. 1996;153:650–5. doi: 10.1164/ajrccm.153.2.8564113. [DOI] [PubMed] [Google Scholar]
- 22.Hill SL, Mitchell JL, Burnett D, Stockley RA. IgG subclasses in the serum and sputum from patients with bronchiectasis. Thorax. 1998;53:463–8. doi: 10.1136/thx.53.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooi GC, Khong PL, Chan-Yeung M, et al. High-resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology. 2002;225:663–72. doi: 10.1148/radiol.2253011575. [DOI] [PubMed] [Google Scholar]
- 24.Kanthakumar K, Taylor G, Tsang KW, et al. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect Immun. 1993;61:2848–53. doi: 10.1128/iai.61.7.2848-2853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angrill J, Agusti C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–32. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson P, Chadban SJ, Atkins RC, Holdsworth SR. Laboratory assessment of immune function in renal transplant patients. Nephrol Dial Transplant. 2003;18:983–7. doi: 10.1093/ndt/gfg190. [DOI] [PubMed] [Google Scholar]
- 27.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–14. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 28.Schapiro BL, Newberger PE, Klempner MS, Dinauer MC. Chronic granulomatous disease presenting in a 69-year-old man. N Engl J Med. 1991;325:1786–90. doi: 10.1056/NEJM199112193252506. [DOI] [PubMed] [Google Scholar]
- 29.Liese JG, Jendrossek V, Jansson A, Petropoulou T, Kloos S, Gahr M, Belohradsky BH. Chronic granulomatous disease in adults. Lancet. 1996;347:1048–9. doi: 10.1016/s0140-6736(96)90403-1. [DOI] [PubMed] [Google Scholar]