Abstract
The expression of intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) on human gingival fibroblasts (HGF) may be important for migration and retention of inflammatory cells in periodontally diseased tissue. This study aimed to assess which cytokines regulate ICAM-1 and VCAM-1 expression on HGF. Tumour necrosis factor (TNF)-α and interferon (IFN)-γ enhanced both ICAM-1 and VCAM-1 expression on HGF. Interleukin (IL)-1β mainly up-regulated ICAM-1 expression. On the other hand, IL-4 and IL-13 enhanced only VCAM-1 expression on HGF. IL-10 did not modulate both ICAM-1 and VCAM-1 expression. Transforming growth factor (TGF)-β1 enhanced ICAM-1 expression. However, TGF-β1 inhibited the VCAM-1 expression induced by TNF-α or IL-4. Both ICAM-1 and VCAM-1 expression by HGF was inhibited by nuclear factor-kappaB (NF-κB) activation inhibitor (MG-132). Mitogen-activated protein kinases (MAPK) inhibitors did not influence ICAM-1 expression induced by TNF-α. Interestingly, VCAM-1 expression was enhanced by MEK inhibitor (PD98059) and c-Jun NH2-terminal kinase (JNK) inhibitor (SP600125). These results mean that the balance of cytokines in periodontally diseased tissue may be essential for control of ICAM-1 and VCAM-1 expression on HGF, and the balance of ICAM-1 and VCAM-1 expression might be important for regulation of leucocytes infiltration and retention in periodontally diseased tissue.
Keywords: ICAM-1, VCAM-1, gingival fibroblasts
Introduction
Periodontal disease is characterized as chronic inflammation associated with Gram-negative bacteria in the oral cavity [1,2], resulting in soft tissue destruction and periodontal bone resorption. Host immune response to these bacteria has been suggested to be associated with alteration or even progression of disease; and inflammatory cells in periodontally diseased tissue are related to disease progression [3]. The recruitment and retention of these cells are mediated by a family of cell surface receptors known as the cell adhesion molecules [4]. Adhesion molecules are now believed to play a crucial role in the pathogenesis of inflammatory disease such as arthritis due to their up-regulation in response to certain pro-inflammatory cytokines and their ability to act as costimulatory receptors in the activation of inflammatory cells [5].
ICAM-1 and VCAM-1 belong to the Ig superfamily [6]. ICAM-1 is widely distributed on leucocytes, endothelial cells, fibroblasts and epithelial cells [7]. VCAM-1 is expressed on monocytes, endothelial cells and synovial cells [8]. In immunological and inflammatory reactions ICAM-1 and VCAM-1 are surface glycoproteins that promote adhesion and subsequent recruitment of leucocytes. Adhesion molecules have been implicated in the pathogenesis of rheumatoid arthritis [6]. The levels of circulating ICAM-1 and VCAM-1 in plasma and synovial fluid both were significantly increased in rheumatoid arthritis patients compared to normal controls [9].
It has been reported that ICAM-1 [10] and VCAM-1 [11] expressed in periodontally diseased tissue. It is known that both ICAM-1 and VCAM-1 mRNA express by nonstimulated HGF [12], ICAM-1 is induced by proinflammatory cytokines (IL-1β, TNF-α, IFN-γ and IL-2) [13] and Escherichia coli LPS [12], and VCAM-1 is induced by IL-1β[14]. These results indicate that ICAM-1 and VCAM-1 on HGF may be related to progression of periodontitis.
There are at least three distinct and parallel MAPK pathways that have been characterized, which include extracellular signal-regulated kinases (ERK) [15], p38 MAPK [16] and JNK [17]. Activation of MAPKs exerts distinct cellular responses mediated by phosphorylation of specific target proteins [18]. Although cytokines such as IL-1β and TNF-α are reported to activate all of these MAPKs [19,20], the relationship between the activation of these pathways and expression of adhesion molecules or other genes has been controversial. In addition, it is interest that many of the genes regulated by MAPKs are dependent on NF-κB for transcription. NF-κB has also been shown to be involved in the expression of adhesion molecules at the transcriptional level in various cell types [21,22]. However, it is uncertain which MAPK pathway is involved in adhesion molecule expression.
It is reported that various cytokines including Th1 type cytokines, Th2 type cytokines, proinflammatory cytokines and growth factors exist in periodontal diseased tissue [23–25]. These cytokines may complexly regulate ICAM-1 and VCAM-1 expression on HGF in periodontal disease. Therefore, we investigated that the effects of proinflammatory cytokines (IL-1β and TNF-α), Th1 type cytokine (IFN-γ), Th2 type cytokines (IL-4, IL-10 and IL-13) and growth factor (TGF-β1) on the expression of ICAM-1 and VCAM-1 on HGF in vitro. Moreover, we investigated the roles of ERK, p38 MAPK, JNK and NF-κB in cytokine-induced ICAM-1 and VCAM-1 expression by HGF.
Materials and methods
Reagents
Recombinant human IL-1β, TNF-α, IFN-γ, IL-4, IL-10, IL-13 and TGF-β1 were purchased from Peprotech (Rocky Hill, NJ, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin-streptomycin and Trypsin-EDTA were obtained from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from JRH Bioscience (Lenexa, KA, USA). Bovine serum albumin (BSA), mouse anti-human ICAM-1 antibody (clone: 8·4A6) and SP600125 were purchased from Sigma (St. Louis, MO, USA). Mouse anti-human VCAM-1 antibody (clone: 1.G11B1) was obtained from Cymbus Biotechnology (Hants, UK). FITC-conjugated rabbit anti-mouse F (ab’) 2 fragment were purchased from DAKO (Kyoto, Japan). SB203580 was obtained from Santacruz (Santa Cruz, CA, USA). PD98059 and MG-132 were purchased from Calbiochem (La Jolla, CA, USA). RNeasy total RNA isolation Kit and Hot star Taq DNA polymerase were obtained from Qiagen (Hilden, Germany). Primer oligo(dT)12−18 and superscriptII reverse transcriptase were purchased from Invitrogen (Carlsbad, CA, USA).
Collection of samples
All subjects were submitted to clinical, periodontal and radiographic examination. Prior to the beginning of the study, all subjects received supragingival prophylaxis to remove gross calculus and allow probing access. All teeth were scored for probing depth and clinical attachment level, at six sites per tooth. The patients were categorized according to the classification of the American Academy of Periodontology into healthy control or chronic periodontitis. The patients were systemically healthy with no evidence of known systemic modifiers of periodontal disease (type 1 and 2 diabetes mellitus, osteoporosis, and medications known to influence periodontal tissues). Exclusion criteria included those patients who had taken systemic antibiotic, anti-inflammatory, hormonal or other assisted drug therapy in the last 6 months prior to the study, or who had received previous periodontal therapy in the last 2 years. Smokers were not specifically excluded. Chronic periodontitis patients had moderate to advanced periodontal disease (at least one tooth per sextant with probing depth > 4 mm, attachment loss > 3 mm, extensive radiographic bone loss and sulcular bleeding on probing). In this experiment, healthy gingival tissues from 3 healthy control subjects (1 male and 2 females, aged 26–40 years old) were used to prepare HGF. Informed consent was obtained from all subjects participating in this study. The study was performed with the approval and compliance of the Tokushima University Ethical Committee.
Cells and culture condition
HGF were prepared from the explants of normal gingival from patients with informed consent. Explants were cut into pieces and cultured in 100-mm diameter tissue culture dishes in DMEM supplemented with 10% FBS, penicillin 50 IU/ml and streptomycin 50 µg/ml with a medium change every 3 days for 10–15 days until confluent cell monolayers were formed. The cells were detached with 0·25% trypsin-EDTA, washed with PBS and subcultured in plastic flasks. After three to four subcultures by trypsinization, homogeneous, slim spindle-shaped cells grown in characteristic swirls were obtained. The cells were used as confluent monolayers at subculture levels 5 to 15. HGF were stimulated with IL-1β (0·1–100 ng/ml), TNF-α (0·1–100 ng/ml), IFN-γ (0·1–100 ng/ml), IL-4 (0·1–100 ng/ml), IL-13 (0·1–100 ng/ml) or TGF-β1 (0·1–100 ng/ml) for 24 h. HGF were cultured for 1 h in the presence or in the absence of SB203580 (0·2–20 µM), PD98059 (0·2–20 µM), SP600125 (0·2–20 µM), MG-132 (0·5–50 µM), prior to incubation with the various stimuli.
RNA extraction and reverse transcriptional-PCR (RT-PCR) analysis
Total RNA was prepared from HGF using RNeasy total RNA isolation Kit. Single-strand cDNA for a PCR template was synthesized from 48 ng of total RNA using primer oligo(dT)12−18 and the superscriptII reverse transcriptase under the condition indicated by the manufacture. Specific primers were designed from cDNA sequence for ICAM-1, VCAM-1 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Each cDNA was amplified by PCR using Hot star Taq DNA polymerase. The sequences of the primers were as follows: ICAM-1-F (5′-CGT GCC GCA CTG AAC TGG AC-3′), ICAM-1-R (5′-CCT CAC ACT TCA CTG TCA CCT-3′), VCAM-1-F (5′-ATT GGG AAA AAC AGA AAA GAG-3′), VCAM-1-R (5′-GGC AAC ATT GAC ATA AAG T-3′), GAPDH-F (5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′), and GAPDH-R (5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′). The conditions for PCR were 1× (95°C, 15min), 35× (94°C, 40 s; 55°C, 40 s; 72°C, 1min) and 1× (72°C, 10min). The products were analysed on a 2·0% agarose gel containing ethidium bromide.
Flow cytometric analyses
Following the required time in culture, cells were washed twice with ice-cold PBS. HGF were harvested by incubation with PBS-4 mmol/l EDTA. Most of cells rounded up following this treatment and could be removed by gentle agitation. Any cells that failed to detach were removed with gentle scraping. Cells were washed twice with ice-cold PBS and incubated (20 min on ice) in PBS-1%BSA. Cells were incubated with mouse anti-human ICAM-1 antibody, mouse anti-human VCAM-1 antibody or isotype control antibody on ice for 30 min. After washing three times with PBS-1% BSA, the cells were incubated with the FITC-conjugated rabbit anti-mouse F (ab’) 2 fragments for 30 min on ice. After three times wash with PBS-1% BSA, cells were immediately analysed by flow cytometry (Epics XL-MCL; Coulter, Hialeah, FL, USA). Cells were gated using forward versus side scatter to remove any dead cells and cellular debris and thus give a uniform population of HGF. For each sample 10000 cells were analysed. Results were expressed as the corrected mean fluorescence intensity (MFI) or percentage of positive cells by using nonspecific fluorescence of isotype control.
Statistical analysis
Statistical significance was analysed by Student’s t-test. P-values < 0·05 were considered significant. We show representative data of experiments using HGF from three different donors.
Results
Cytokines differently induced ICAM-1 and VCAM-1 mRNA by HGF
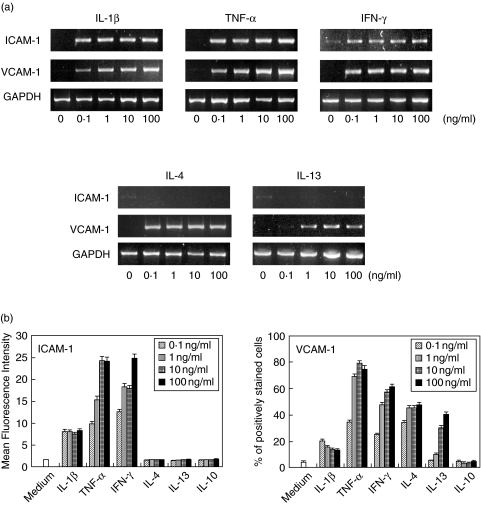
At first, we carried out immunohistochemical staining to investigate the expression of ICAM-1 and VCAM-1 in periodontally diseased tissue. Both ICAM-1 positive cells and VCAM-1 positive cells were present in the same area of periodontally diseased tissue. Morphologically, HGF and endothelial cells mainly expressed ICAM-1 and VCAM-1 (data not shown). We examined ICAM-1 and VCAM-1 expression in normal gingival tissues. We detected both ICAM-1 and VCAM-1 expression in normal gingival tissues. However, the expression was weak compared with periodontally diseased tissue (data not shown). Next, we examined ICAM-1 and VCAM-1 mRNA expression by HGF. As shown Fig. 1a, IL-1β, TNF-α and IFN-γ induced ICAM-1 mRNA by HGF in a dosed dependent fashion. However, IL-4 and IL-13 did not induce ICAM-1 mRNA expression in HGF. On the other hand, VCAM-1 mRNA was enhanced by IL-1β, TNF-α, IFN-γ, IL-4 and IL-13 stimulation by HGF.
Fig. 1.
Induction of ICAM-1 and VCAM-1 by cytokines. (a)HGF were treated without or with IL-1β (0·1–100 ng/ml), TNF-α (0·1–100 ng/ml), IFN-γ (0·1–100 ng/ml), IL-4 (0·1–100 ng/ml) and IL-13 (0·1–100 ng/ml) for 4 h. Total RNA was isolated, and RT-PCR analysis was carried out for ICAM-1, VCAM-1 and GAPDH. Similar results were obtained in three repeated experiments. (b) Cells were stimulated with IL-1β (0·1–100 ng/ml), TNF-α (0·1–100 ng/ml), IFN-γ (0·1–100 ng/ml), IL-4 (0·1–100 ng/ml), IL-13 (0·1–100 ng/ml) and IL-10 (0·1–100 ng/ml) for 24 h. Cell surface ICAM-1 and VCAM-1 expression was analysed by flow cytometry. These results expressed mean fluorescence intensity (ICAM-1) or percentage of positively stained cells (VCAM-1). Data are presented as the mean ± SD of three experiments.
Expression of ICAM-1 and VCAM-1 on HGF
Next, we examined the effects of cytokines on ICAM-1 and VCAM-1 protein expression by HGF. Figure 1b shows that nonstimulated HGF expressed ICAM-1 constitutively, and that ICAM-1 expression was enhanced by IL-1β, TNF-α and IFN-γ in a dose dependent manner (Fig. 1b). However, IL-4, IL-13 and IL-10 did not modulate ICAM-1 expression by HGF. On the other hand, VCAM-1 expression was increased by not only IL-1β, TNF-α and IFN-γ but also IL-4 and IL-13 in a dose dependent fashion (Fig. 1b). Escherichia coli LPS, Porphyromonas gingivalis LPS and Staphylococcus aureus peptidoglycan induced both ICAM-1 and VCAM-1 expression on HGF in a dose dependent manner (data not shown).
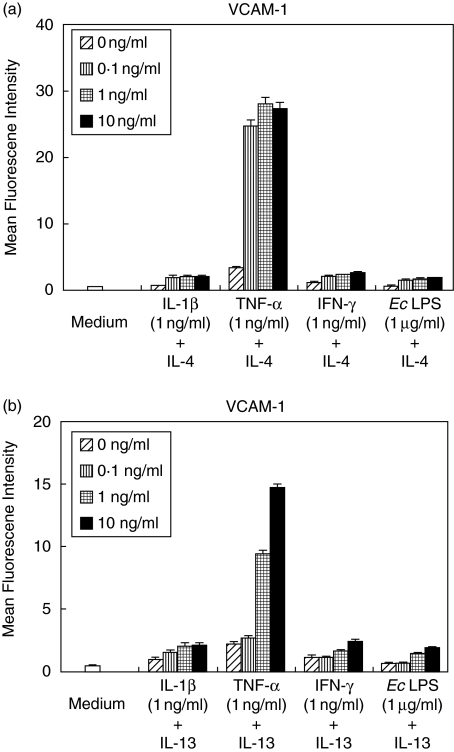
IL-4 and IL-13 synergistically augments TNF-α induced VCAM-1 expression by HGF
Figure 1 explained IL-4 and IL-13 enhanced VCAM-1 expression by HGF. Next, we examined the effects of IL-4 and IL-13 stimulation on proinflammatory cytokines-induced VCAM-1 expression. Figure 2 shows that IL-4 and IL-13 had a little effect on VCAM-1 expression induced by IL-1β and IFN-γ. Meanwhile, IL-4 and IL-13 showed a synergistic effect on the TNF-α-induced VCAM-1 expression on HGF in a concentration-dependent manner (Fig. 2).
Fig. 2.
IL-4 and IL-13 synergizes with TNF-α for VCAM-1. Cells were stimulated with IL-1 β (1 ng/ml), TNF-α (1 ng/ml), IFN-γ (1 ng/ml) or E.coli LPS (1 µg/ml) and 0, 0·1, 1 or 10 ng/ml of (a) IL-4 or (b) IL-13 for 24 h. Cell surface VCAM-1 expression was analysed by flow cytometry. These results express percentage of positively stained cells. Data are presented as the mean ± SD of three experiments. *P < 0·05 (compared with medium-only control).
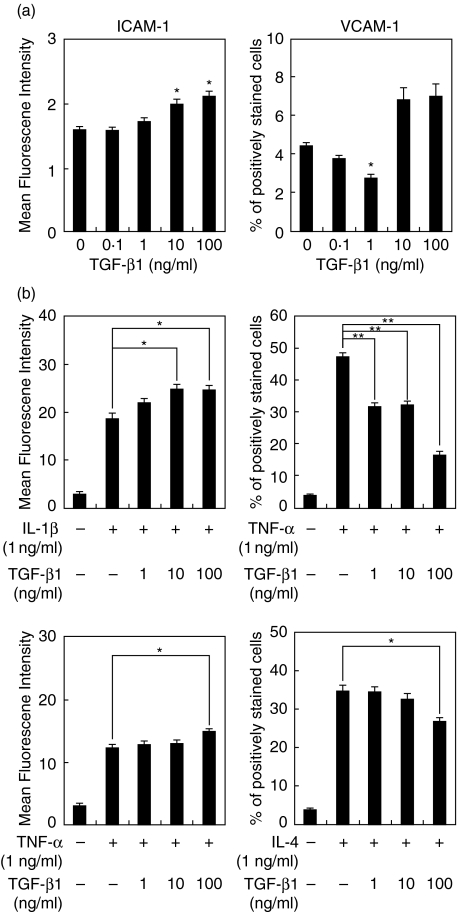
Effect of TGF-β1 on ICAM-1 and VCAM-1 expression by HGF
Figure 3a shows that TGF-β1 enhanced ICAM-1 expression in a dose-dependent manner. VCAM-1 expression was slightly inhibited by TGF-β1 (1 ng/ml) treatment. We investigated the influence of TGF-β1 on ICAM-1 and VCAM-1 expression induced by cytokines. TGF-β1 enhanced ICAM-1 expression induced by IL-1β or TNF-α on HGF in a concentration-dependent manner. On the other hand, TGF-β1 inhibited VCAM-1 expression induced by TNF-α or IL-4 in a dose-dependent fashion (Fig. 3b).
Fig. 3.
TGF-β1 differently modulates ICAM-1 and VCAM-1 expression on HGF. (a) HGF were stimulated with TGF-β1 (0·1–100 ng/ml) for 24 h. (b) HGF were stimulated with indicated concentrations of TGF-β1 in combination with IL-1 β (1 ng/ml), TNF-α (1 ng/ml) or IL-4 (1 ng/ml) for 24 h. ICAM-1 and VCAM-1 expression on HGF was analysed by flow cytometry. These results expressed mean fluorescence intensity (ICAM-1) or percentage of positively stained cells (VCAM-1). Data are presented as the mean ± SD of three independent experiments. *P < 0·05; **P < 0·01 (compared with medium-only control or between two groups).
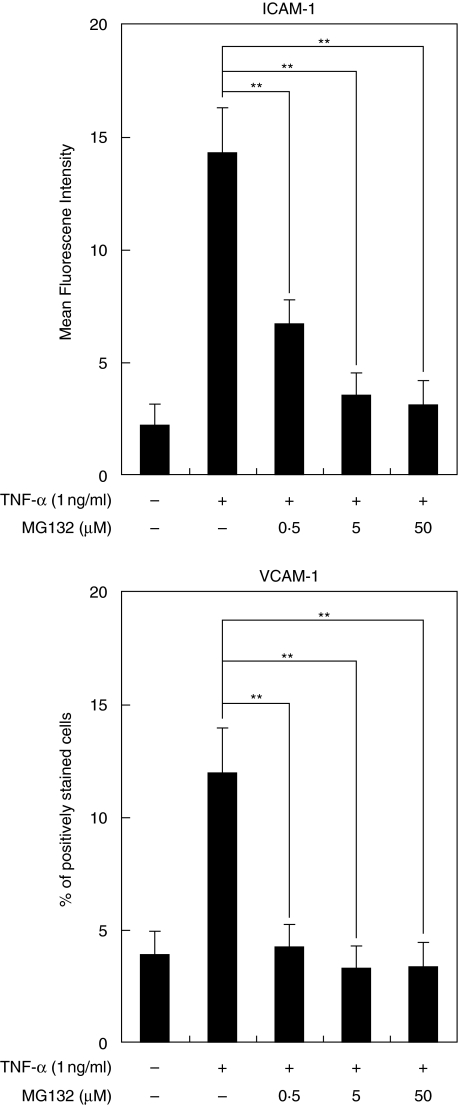
NF-κB inhibitor suppressed ICAM-1 and VCAM-1 expression by HGF
To determine whether NF-κB is required for ICAM-1 and VCAM-1 expression by HGF, the effect of NF-κB activation inhibitor (MG-132) on ICAM-1 and VCAM-1 expression by HGF was examined. MG-132 inhibited both TNF-α induced ICAM-1 expression and VCAM-1 expression by HGF in a dose-dependent fashion. 50 µM of MG-132 completely suppressed both ICAM-1 and VCAM-1 expression on HGF (Fig. 4).
Fig. 4.
Effects of NF-κB inhibitor on TNF-α stimulated ICAM-1 and VCAM-1 expression by HGF. Cells were preincubaled with MG-132 (0·5–50 µM) for 1 h and then incubated with TNF-α (1 ng/ml). After a 24 h incubation, ICAM-1 and VCAM-1 expression was analysed by flow cytometry. These results expressed mean fluorescence intensity (ICAM-1) or percentage of positively stained cells (VCAM-1). Data are presented as the mean ± SD of three independent experiments. **P < 0·01 (between two groups).
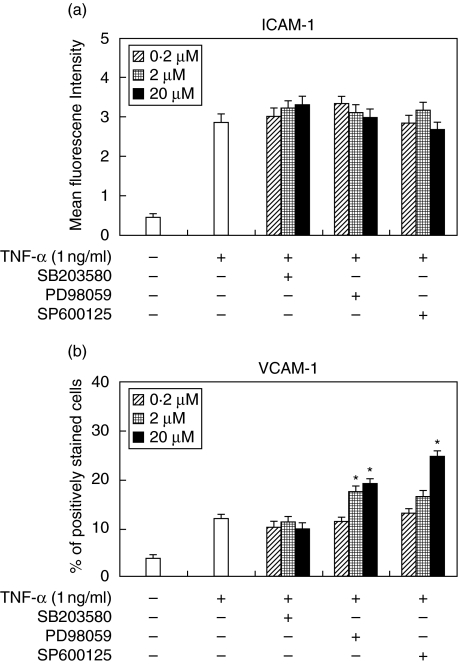
Effects of MAPK inhibitors on ICAM-1 and VCAM-1 expression by HGF
To investigate whether MAPKs are necessary for ICAM-1 and VCAM-1 expression by HGF, we used p38 MAPK inhibitor (SB203580), MEK inhibitor (PD98059) and JNK inhibitor (SP600125). MAPK inhibitors used in this study did not effect on ICAM-1 expression induced by TNF-α (1 ng/ml) (Fig. 5a). Meanwhile, TNF-α-induced VCAM-1 expression was enhanced by MEK inhibitor and JNK inhibitor in a concentration-dependent fashion. P38 MAPK inhibitor did not have an effect on VCAM-1 expression induced by TNF-α (Fig. 5b).
Fig. 5.
Effects of MAPKs inhibitors on TNF-α stimulated ICAM-1 and VCAM-1 expression by HGF. Cells were preincubated with SB203580 (0·2–20 µM), PD98052 (0·2–20 µM) or SP600125 (0·2–20 µM) for 1 h and then incubated with TNF-α (1 ng/ml). After a 24 h incubation, (a) ICAM-1 and (b) VCAM-1 expression was analysed by flow cytometry. These results expressed mean fluorescence intensity (ICAM-1) or percentage of positively stained cells (VCAM-1). Data are presented as the mean ± SD of three independent experiments. *P < 0·05 (compared with TNF-α stimulated HGF without MAPKs inhibitor).
Discussion
Up-regulation of adhesion molecules on the surface of HGF may play a key role in infiltration and retention of inflammatory cells at sites of periodontally diseased tissue. Proinflammatory cytokines (IL-1β and TNF-α) are also related to the progression of periodontal disease [26]. In this experiments, IL-1β mainly induced ICAM-1 on HGF and TNF-α induced both ICAM-1 and VCAM-1 on HGF. TNF-α induced much more ICAM-1 expression on HGF compared to IL-1β. It is reported that IL-1β and TNF-α could induce both ICAM-1 and VCAM-1 on lung fibroblasts and IL-1β much more induce ICAM-1 expression compared to TNF-α[27]. The pattern of ICAM-1 and VCAM-1 expression induced by IL-1β or TNF-α will be dependent on the source of fibroblasts.
IFN-γ induced both ICAM-1 and VCAM-1 expression on HGF. On the other hand, IL-4 and IL-13 induced only VCAM-1 expression. Recently, it has been described that ICAM-1 and VCAM-1 are involved in Th1/Th2 response. It is described that in vitro differentiated Th2 cells but not Th1 cells were capable of sustaining high–affinity adhesive interaction with VCAM-1 [28]. Moreover, it is reported that ICAM-1 and lymphocyte function associated antigen (LFA)-1 (the ligand of ICAM-1) ligation favours human Th1 development [29]. These reports mean that ICAM-1 may be related with Th1-type cells infiltration and VCAM-1 may be involved in Th2-type cells migration. In our experiments, Th2 type cytokines (IL-4 and IL-13) induced only VCAM-1 expression on HGF. These results mean that ICAM-1 and VCAM-1 on HGF may be involved in Th1/Th2 balance in periodontal diseased tissue.
We revealed that a synergistic increase of VCAM-1 expression on HGF treated with TNF-α in combination with IL-4 or IL-13. It is reported synergic effects of VCAM-1 expression on synoviocytes was obtained by combining either IL-4 or IL-13 with TNF-α, which results in a high elevated but also sustained expression of VCAM-1 [30]. These results mean that the existence of both TNF-α and IL-4 or IL-13 may be related to the exacerbation of not only arthritis but also periodontal disease.
It is known that IL-10 is an immunosuppressive cytokine. It is described that IL-10 inhibits P. gingivalis LPS-stimulated HGF production of IL-6 [31]. However, IL-10 did not change ICAM-1 and VCAM-1 expression on nonstimulated HGF and did not inhibit ICAM-1 and VCAM-1 expression induced by TNF-α (data not shown). It has been reported that IL-10 inhibit ICAM-1 expression on human langerhans cells but not dermal keratinocytes or fibroblasts [32]. Our results about ICAM-1 agree with theirs. It was shown for the first time on human fibroblasts that VCAM-1 expression was not modified by IL-10. The anti-inflammatory role of IL-10 on HGF may be inhibition of proinflammatory cytokines such as IL-6, and IL-10 does not have a role of modification of adhesion molecules expression on HGF.
TGF-β1 induced ICAM-1 expression and down-regulated VCAM-1 expression on HGF. It has been reported that TGF-β1 did not influence ICAM-1 expression, but down-regulated VCAM-1 expression on lung fibroblasts [27]. The influence of TGF-β1 may be dependent on source of fibroblasts. TGF-β1 is known to act primarily on fibroblasts. The changes of adhesion molecules expression might be an effect of a changing phenotype of fibroblasts after TGF-β1 stimulation.
ICAM-1 expression on HGF induced by TNF-α was inhibited by MG-132. On the other hand, MAPK inhibitors did not influence ICAM-1 expression by HGF. It is reported that TNF-α has been shown to induce ICAM-1 expression mediated through activation of NF-κB, but not p38 MAPK, ERK and JNK in A549 epithelial cells [33]. Our results agree with theirs.
NF-κB activation is required for TNF-α induced VCAM-1 expression by HGF. However, activation of p38 MAPK is not involved in VCAM-1 expression induced by TNF-α. Moreover, activation of ERK and JNK is related to inhibition of TNF-α-induced VCAM-1 expression on HGF induced by TNF-α. It is reported that activation of p38 MAPK induced VCAM-1 expression and activation of JNK inhibit VCAM-1 expression induced by TNF-α in chondrosarcoma cells [34]. It is described that p38 MAPK inhibitor did not affect TNF-α-induced VCAM-1 expression in sertoli cells [35]. Wang et al. [36] reported activation of ERK, p38 MAPK, JNK and NF-κB pathways is essential for IL-1β-induced VCAM-1 expression in human tracheal smooth muscle cells. The discrepancies in these previous reports imply that there are divergent pathways leading to VCAM-1 expression, depending on the nature of stimuli and cell types.
It is known that tobacco smoking affects ICAM-1 expression in periodontal tissues. The proportion of the total number of vessels expressing ICAM-1 in noninflamed sites was greater in nonsmoker compared with smokers [37]. It has been reported that the concentration of soluble ICAM-1 in the gingival crevicular fluid was significantly lower in the smokers compared with nonsmokers [38]. However, we don’t exclude smokers in this report. Further investigation will be necessary for tobacco smoking related with adherent molecules expression on HGF.
It has been reported ICAM-1 expression on HGF was induced by IL-1β, TNF-α or IFN-γ[12, 13, 14]. Their reports agree with ours. However, Joe et al. [14] reported that the level of VCAM-1 was not statistically different from HGF treated with IL-1β compared to nonstimulated HGF. We demonstrated here that IL-1β up-regulated VCAM-1 expression on HGF. In their experiments, they detected VCAM-1 expression on half of the HGF samples they used. The discrepancies in this previous report might be dependent on the source of HGF. Further investigation will be necessary.
This study shows that proinflammatory cytokines (IL-1β, TNF-α) and Th1 type cytokine (IFN-γ) induced both ICAM-1 and VCAM-1 expression on HGF and Th2 type cytokines (IL-4 and IL-13) induced only VCAM-1 expression. TGF-β1 increased ICAM-1 expression and decreased VCAM-1 expression on HGF. The selective increase of VCAM-1 on HGF by IL-4 and IL-13 may contribute to selective Th2 type cells retention in periodontally diseased tissues. On the other hand, inhibition of VCAM-1 expression and up-regulation of ICAM-1 expression by TGF-β1 may be related to selective Th1 type cells infiltration in periodontally diseased tissue. Moreover, our results show that ERK and JNK may be involved in inhibition of VCAM-1 expression by HGF in periodontally diseased tissue and activation of NF-κB is related to both ICAM-1 and VCAM-1 expression on HGF. These results show that HGF may be related to the control of Th1/Th2 cells infiltration in periodontally diseased tissue. These results may explain that HGF might be a target for periodontal therapy.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (16591915) from the Japan Society for the Promotion of Science.
References
- 1.Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:648–59. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Taubman MA, Eastcott JW, Shimauchi H, Takeichi O, Smith DJ. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco RJ, Hamada S, Mergenhagen SE, et al., editors. Molecular pathogenesis of periodontal disease. Washington, DC: American. Society for Microbiology; 1994. p. 14757. [Google Scholar]
- 4.Haskard DO. Cell adhesion molecules in rheumatoid arthritis. Curr Opin Rheumatol. 1995;7:229–34. doi: 10.1097/00002281-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Liao HX, Haynes BF. Role of adhesion molecules in the pathogenesis of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:715–40. [PubMed] [Google Scholar]
- 6.Mojcik CF, Shevach EM. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997;40:991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- 7.Buck CA. Immunoglobulin superfamily: structure, function and relationship to other receptor molecules. Semin Cell Biol. 1992;3:179–88. doi: 10.1016/s1043-4682(10)80014-5. [DOI] [PubMed] [Google Scholar]
- 8.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 9.Mason JC, Kapahi P, Haskard DO. Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosus. Lack of correlation with levels of circulating vascular cell adhesion molecule 1. Arthritis Rheum. 1993;36:519–27. doi: 10.1002/art.1780360412. [DOI] [PubMed] [Google Scholar]
- 10.Gemmell E, Walsh LJ, Savage NW, Seymour GJ. Adhesion molecule expression in chronic inflammatory periodontal disease tissue. J Periodontal Res. 1994;29:46–53. doi: 10.1111/j.1600-0765.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 11.Del Castillo LF, Schlegel Gomez R, Pelka M, Hornstein OP, Johannessen AC, von den Driesch P. Immunohistochemical localization of very late activation integrins in healthy and diseased human gingiva. J Periodontal Res. 1996;31:36–42. doi: 10.1111/j.1600-0765.1996.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi J, Saito I, Ishikawa I, Miyasaka N. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–12. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozawa A, Tada H, Tamai R, et al. Expression of IL-2 receptor beta and gamma chains by human gingival fibroblasts and up-regulation of adhesion to neutrophils in response to IL-2. J Leukoc Biol. 2003;74:352–9. doi: 10.1189/jlb.0103044. [DOI] [PubMed] [Google Scholar]
- 14.Joe BH, Borke JL, Keskintepe M, Hanes PJ, Mailhot JM, Singh BB. Interleukin-1beta regulation of adhesion molecules on human gingival and periodontal ligament fibroblasts. J Periodontol. 2001;72:865–70. doi: 10.1902/jop.2001.72.7.865. [DOI] [PubMed] [Google Scholar]
- 15.Boulton TG, Yancopoulos GD, Gregory JS, Slaughter C, Moomaw C, Hsu J, Cobb MH. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–7. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–60. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 18.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–9. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 20.Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000;275:14838–45. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, Chen JJ, Chou CY. Protein kinase calpha but not p44/42 mitogen-activated protein kinase, p38, or c-Jun NH (2) -terminal kinase is required for intercellular adhesion molecule-1 expression mediated by interleukin-1beta. involvement of sequential activation of tyrosine kinase, nuclear factor-kappaB-inducing kinase, and IkappaB kinase 2. Mol Pharmacol. 2000;58:1479–89. doi: 10.1124/mol.58.6.1479. [DOI] [PubMed] [Google Scholar]
- 22.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 23.Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004;35:21–41. doi: 10.1111/j.0906-6713.2004.003557.x. [DOI] [PubMed] [Google Scholar]
- 24.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Medical. 2001;12:125–35. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 25.Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol 2000. 2003;31:125–34. doi: 10.1034/j.1600-0757.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- 26.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 27.Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Interferon-gamma and interleukin-4 differentially regulate ICAM-1 and VCAM-1 expression on human lung fibroblasts. Eur Respir J. 1999;14:759–66. doi: 10.1034/j.1399-3003.1999.14d06.x. [DOI] [PubMed] [Google Scholar]
- 28.Lim YC, Wakelin MW, Henault L, et al. Alpha4beta1-integrin activation is necessary for high-efficiency T–cell subset interactions with VCAM-1 under flow. Microcirculation. 2000;7:201–14. [PubMed] [Google Scholar]
- 29.Smits HH, de Jong EC, Schuitemaker JH, Geijtenbeek TB, van Kooyk Y, Kapsenberg ML, Wierenga EA. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J Immunol. 2002;168:1710–6. doi: 10.4049/jimmunol.168.4.1710. [DOI] [PubMed] [Google Scholar]
- 30.Croft D, McIntyre P, Wibulswas A, Kramer I. Sustained elevated levels of VCAM-1 in cultured fibroblast-like synoviocytes can be achieved by TNF-alpha in combination with either IL-4 or IL-13 through increased mRNA stability. Am J Pathol. 1999;154:1149–58. doi: 10.1016/s0002-9440(10)65367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang PL, Shirasu S, Shinohar M, Azuma Y, Daito M, Yasuda H, Ohura K. IL-10 inhibits Porphyromonas gingivalis LPS-stimulated human gingival fibroblasts production of IL-6. Biochem Biophys Res Commun. 1999;263:372–7. doi: 10.1006/bbrc.1999.1381. [DOI] [PubMed] [Google Scholar]
- 32.Chatelain R, Wollenberg A, Martin C, Panhans-Gross A, Bieber T, Degitz K, Heckmann M. IL-10 inhibits ICAM-1 expression on human Langerhans cells but not on keratinocytes, dermal endothelial cells or fibroblasts. Arch Dermatol Res. 1998;290:477–82. doi: 10.1007/s004030050339. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Chou C, Sun Y, Huang W. Tumor necrosis factor alpha-induced activation of downstream NF-kappaB site of the promoter mediates epithelial ICAM-1 expression and monocyte adhesion. Involvement of PKCalpha, tyrosine kinase, and IKK2, but not MAPKs, pathway. Cell Signal. 2001;13:543–53. doi: 10.1016/s0898-6568(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 34.Ju JW, Kim SJ, Jun CD, Chun JS. p38 kinase and c-Jun N-terminal kinase oppositely regulates tumor necrosis factor alpha-induced vascular cell adhesion molecule-1 expression and cell adhesion in chondrosarcoma cells. IUBMB Life. 2002;54:293–9. doi: 10.1080/15216540215674. [DOI] [PubMed] [Google Scholar]
- 35.De Cesaris P, Starace D, Riccioli A, Padula F, Filippini A, Ziparo E. Tumor necrosis factor-alpha induces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J Biol Chem. 1998;273:7566–71. doi: 10.1074/jbc.273.13.7566. [DOI] [PubMed] [Google Scholar]
- 36.Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-kappaB in IL-1beta-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L227–37. doi: 10.1152/ajplung.00224.2004. [DOI] [PubMed] [Google Scholar]
- 37.Rezavandi K, Palmer RM, Odell EW, Scott DA, Wilson RF. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31:59–64. doi: 10.1046/j.0904-2512.2001.joptest.doc.x. [DOI] [PubMed] [Google Scholar]
- 38.Fraser HS, Palmer RM, Wilson RF, Coward PY, Scott DA. Elevated systemic concentrations of soluble ICAM-1 (sICAM) are not reflected in the gingival crevicular fluid of smokers with periodontitis. J Dent Res. 2001;80:1643–7. doi: 10.1177/00220345010800070901. [DOI] [PubMed] [Google Scholar]