Abstract
Common variable immunodeficiency (CVID) is the most frequent symptomatic primary immunodeficiency disease, characterized by low levels of circulating immunoglobulins and recurrent bacterial infections, particularly of the respiratory tract. T cell dysfunction is often present, and lymphoproliferative and autoimmune disorders as well as haematological cytopenias are frequently observed. In this study, we report a polyclonal expansion of large granular lymphocytes (LGL) in a substantial proportion of CVID patients, associated with splenomegaly, increased numbers of CD8+ T cells, inverted CD4 : CD8 T cell ratios and neutropenia. CVID patients who had both increased numbers of LGL and granulocytopenia had elevated levels of soluble Fas ligand (sFasL). Our observations indicate that CVID may be added to the list of inflammatory diseases associated with increased numbers of LGL. Furthermore, our findings suggest common pathogenic mechanisms of granulocytopenia in CVID and lymphoproliferative disease of granular lymphocytes.
Keywords: CVID, Fas, immunodeficiency, LGL, lymphoproliferative disease
Introduction
Common variable immunodeficiency (CVID) is the most frequent symptomatic primary immunodeficiency, defined by low levels of circulating IgG and other immunoglobulin subsets, leading to recurrent bacterial infections, particularly of the respiratory tract [1]. Although the B cell defect is the immunological hallmark of CVID, about half the cases show signs of T cell abnormalities [2], which may be of importance both for the defective antibody production and for the clinical manifestations in this disorder. Additionally, lymphoproliferative disorders and various malignancies are more frequent in CVID, and haematological cytopenias (i.e. thrombocytopenia, haemolytic anaemia and neutropenia), which may show a fluctuating course, are often observed [2]. However, the mechanisms leading to these cytopenias are not known.
Large granular lymphocytes (LGL) normally comprise 10% to 15% of peripheral blood mononuclear cells (PBMC) (0·12–0·32 109 LGL/l) [3], probably representing in vivo-activated CD3+ (T-LGL) or CD3–[natural killer (NK)-LGL] lymphocytes. Malignant expansions of LGL (LGL-leukaemia) have traditionally been defined by monoclonality [3]. However, the definition of malignancy is problematic, as a monoclonal expansion of T-LGL has been observed even in healthy individuals [4]. In this report, we refer to all conditions associated with an expansion of LGL as lymphoproliferative disease of granular lymphocytes (LDGL). The pathogenesis of LDGL is not known, but the observed association with rheumatoid arthritis (RA) [3] or chronic viral infections [5,6] suggests that chronic inflammation may be involved in this expansion of LGL.
Although LDGL is associated most commonly with hypergammaglobulinaemia, hypogammaglobulinaemia has been observed in a few cases [7]. Moreover, neutropenia is frequently observed in LDGL and CVID, and may determine treatment and prognosis in both diseases. In this study, we report increased numbers of LGL in peripheral blood of a substantial proportion of CVID patients, showing a relationship with the occurrence of neutropenia in these patients.
Materials and methods
Patients
Twenty-nine patients with CVID according to the diagnostic criteria of the International Union of Immunodeficiency Societies (IUIS) expert group for primary immunodeficiencies [1] were included consecutively in the study (Table 1). The patients did not have any clinically apparent infection when recruited (i.e. no body temperature > 38°C, mucopurulent expectorate or cough or other clinical sign of acute infection, elevated C-reactive protein (CRP or other laboratory sign of infection). Splenomegaly was defined as > 14 cm splenar axis defined by ultrasonography or computer tomography. Blood samples were obtained immediately before intravenous or subcutaneous immunoglobulin administration. Healthy blood donors (n = 17, age range 24–60 years) were included as controls, and informed consent was obtained from all study subjects before inclusion. The study was conducted according to the ethical guidelines at our hospital, which comply with the Helsinki declaration, and was approved by the hospital’s authorized representative.
Table 1.
Patient characteristics.
| Lymphocytes4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Age1 | Gender | LGL2 | Episodes of neutropenia3 | CD19 | CD56 | CD3+ CD56 | CD4 | CD8 | Splenomegaly5 |
| 1 | 26 | F | 0·05 | No | 1·06 | 0·51 | 0·21 | 51 | 4 | Yes |
| 2 | 70 | M | 0·88pc | Yes | 0·23 | 0·72 | 0·11 | 18 | 4 | Yes |
| 3 | 48 | M | 1·67 | Yes | 0·90 | 2·31 | 0·91 | 29 | 4 | No |
| 4 | 38 | M | 0·13 | No | 0·63 | 0·20 | 0·12 | 25 | 5 | No |
| 5 | 27 | M | 1·79pc | Yes | 1·95 | 2·40 | 0·44 | 12 | 3 | Yes |
| 6 | 33 | M | 0·18 | No | 0·29 | 0·20 | 0·19 | 40 | 16 | No |
| 7 | 39 | F | 0·08 | No | 0·42 | 0·67 | 0·26 | 18 | 8 | No |
| 8 | 63 | M | 3·45pc | No | 1·58 | 3·98 | 0·02 | 13 | 6 | Yes |
| 9 | 57 | F | 0·56 | n.a. | 0·55 | 1·07 | 0·16 | 16 | 9 | Yes |
| 10 | 21 | M | 0·25 | No | 3·64 | 1·63 | 1·64 | 30 | 6 | Yes |
| 11 | 45 | F | 2·17pc | No | 1·91 | 2·24 | 0·83 | 18 | 7 | No |
| 12 | 42 | F | 0·84pc | Yes | 0·71 | 0·24 | 0·22 | 17 | 3 | Yes |
| 13 | 50 | F | 0·20 | Yes | 0·34 | 0·31 | 0·01 | 17 | 9 | No |
| 14 | 66 | M | 0·11 | No | 0·66 | 0·49 | 0·11 | 32 | 1 | No |
| 15 | 33 | F | 1·81pc | Yes | 0·43 | 0·48 | 0·01 | 24 | 9 | Yes |
| 16 | 66 | F | 0·23 | No | 0·49 | 2·08 | 0·04 | 11 | 3 | No |
| 17 | 49 | F | 0·01 | Yes | 0·16 | 0·26 | 0·04 | 7 | 4 | Yes |
| 18 | 18 | F | 0·23 | No | 0·90 | 1·14 | 0·39 | 32 | 7 | No |
| 19 | 47 | M | 1·20pc | Yes | 1·91 | 1·24 | 0·16 | 6 | 3 | Yes |
| 20 | 54 | M | 0·11 | No | 0·53 | 0·39 | 0·02 | 42 | 19 | No |
| 21 | 51 | M | 0·80 | No | 0·57 | 2·30 | 0·15 | 32 | 19 | Yes |
| 22 | 64 | F | 0·31 | No | 0·39 | 0·52 | 0·19 | 48 | 4 | No |
| 23 | 26 | F | 0·12 | No | 0·37 | 0·75 | 0·32 | 20 | 1 | No |
| 24 | 39 | F | 0·61 | n.a. | 0·73 | 1·22 | 0·03 | 10 | 4 | Yes |
| 25 | 63 | M | 22·58pc | Yes | 0·63 | 3·28 | 0·07 | 13 | 12 | Yes |
| 26 | 54 | M | 0·24 | n.a. | 0·92 | 1·55 | 0·48 | 24 | 9 | Yes |
| 27 | 31 | F | 0·18 | Yes | 0·18 | 0·25 | 0·00 | 10 | 4 | Yes |
| 28 | 57 | F | 0·08 | No | 0·73 | 0·30 | 0·03 | 28 | 8 | Yes |
| 29 | 28 | F | 0·15 | No | 1·66 | 0·47 | 0·91 | 8 | 2 | No |
Age in years.
Number of large granular lymphocytes (LGL) as detected morphologically in blood smear analysis (109/l), bold figures indicate numbers exceeding normal ranges (0·12–0·32 109 LGL/l, see reference [3]).
Absolute count of neutrophil granulocytes below 0·15 109/l at any time during the last 5 years.
Number of cells in peripheral blood (109/l) (please note: these numbers were estimated using TruCount™ (see Methods section); thus, some of the largest LGL may have escaped the lymphocyte gate and not been counted in the flow cytometre).
Determined by ultrasound or computer tomography, splenectomized patients included. n.a.; not available, pc; polyclonal.
Characterization of large granular lymphocytes
Blood smears were prepared from ethylenediamine tetraacetic acid (EDTA) blood and stained with May–Grünwald–Giemsa staining solution. The proportion of LGL was assessed by light microscopy. A minimum of 500 leucocytes was assessed per blood smear. The absolute number of LGL was then calculated from the total leucocyte count procured from the same blood sample using an automated cell counter (CellDyn 4000; Abbot Diagnostics, Santa Clara, CA, USA). LGL are identified by their particular morphology: large cells with abundant, weakly basophilic cytoplasm with a few azurophilic granules, and a round or oval slightly eccentric nucleus with clumped chromatin and usually no visible nucleolus. Some, but not all LGL may express the surface marker CD57. Flow cytometric characterization of LGL is problematic, as there is no specific surface marker that may identify LGL reliably, and the gating of LGL by forward- and side-scatter properties may be unreliable, as LGL may escape the lymphocyte gate due to their increased size. For these reasons, we chose to define LGL morphologically using light microscopy in this study. For normal values of LGL we used internationally recognized estimates [3], which are in agreement with our own observations in healthy controls (see Fig. 1a).
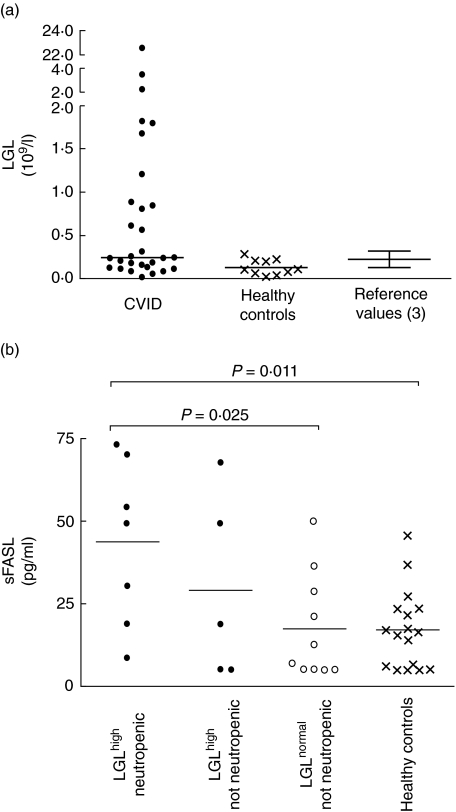
Fig. 1.
(a) Increased numbers of large granular lymphocytes (LGL) in common variable immunodeficiency (CVID) patients comparing healthy controls and reference values. The y-axis indicates absolute numbers of LGL in peripheral blood of 29 CVID patients and 10 healthy controls. Normal range (0·12–0·32 109 LGL/l) (see [3]) is illustrated as mean ± s.d. (b) Increased levels of sFasL in CVID patients with elevated LGL counts and neutropenia. The y-axis indicates serum levels of soluble Fas ligand (sFasL) in pg/ml, bar indicates median value. Filled symbols (left) indicate CVID patients with elevated LGL counts and neutropenia (n = 7) or no evidence of neutropenia (n = 4), Open symbols indicate CVID patients with normal LGL and neutrophil counts (n = 10), crosses indicate FasL levels in healthy controls (n = 17). P-values calculated using Mann–Whitney test.
Immunocytology
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood by Isopaque-Ficoll (Lymphoprep, Nycomed, Oslo, Norway) gradient centrifugation and fixed in 4% formaldehyde for 10 min. For immunocytological analysis, slides were incubated at room temperature for 30 min with commercially available mouse monoclonal antibodies against CD20, CD3, CD4, CD8, CD57 and CD11b (DakoCytomation, Glostrup, Denmark). After washing twice with phosphate-buffered saline for 5 min, the slides were incubated for 30 min with an unlabelled rabbit anti-mouse antibody (code no. Z 0259, DakoCytomation), washed again and incubated with the alkaline phosphatase anti-alkaline phosphate (APAAP) complex (code no. D 0651, DakoCytomation) for 30 min, followed by washing and incubation with Dako Fuchsin+™ Substrate Chromogene System (code no. K 0625, DakoCytomation) for 10 min. Slides were washed in running tap water for at least 5 min, counterstained with haematoxylin (Sigma, St Louis, MO, USA), mounted in aqueous mounting medium (i.e. Glycergel mounting medium, DakoCytomation) with cover slip and examined in a light microscope. Bound antibodies were seen as a red-coloured product localized to the cell membrane and/or to the cytoplasm.
Polymerase chain reaction (PCR) for rearrangement of the T cell receptor (TCR)-γ gene
DNA was extracted from cytological material using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). PCR was performed using seven forward primers complementary to common sequences in Vγ I-IV family genes and designated in our laboratory, and two reverse primers complementary to sequences in Jγ1, Jγ2, Jp1 and Jp2 gene segments [8]. The reverse primers were labelled with fluorochrom to enable detection by capillary electrophoresis (ABI3100). The forward primers were pooled (named Vmix) providing a concentration of each of the V primers of 5 µM in each tube. The PCR reaction mixture consisted of 1× PCR reaction buffer, 4·0 mM MgCl2, 200 µM 2′-deoxyribonucleoside 5′-triphosphate (dNTP), 0·625 U platinum Taq polymerase (Invitrogen, Carlsbad, CA, USA), Vmix 0·8 µM, Jp11 1·2 µM, Jγ11 0·05 µM and 2 µl of cell extract, to a total volume of 12·5 µl with sterile distilled water. Thirty-five PCR cycles were performed, each cycle consisting of a denaturing step at 94°C for 30 s, an annealing step at 60°C for 30 s and an elongation step at 72°C for 30 s. Following the 35 cycles there was a 45-min period at 60°C. The PCR products were detected by capillary electrophoresis on ABI3100 instrument. One µl PCR product was eluted in 10 µl of a mix of HiDi formamide (Applied Biosystems, Foster City, CA, USA) and size standard GS-500 ROX (Applied Biosystems) (3 µl ROX to 100 µl formamide). The PCR products were size-separated in polymer POP4 (Applied Biosystems). The result was a distribution of multiple peaks, representing many different PCR products in the case of polyclonal lymphoproliferation, and one or two single peaks consisting of one type of PCR product in the case of a monoclonal lymphoproliferation [expected size Vmix + Jγ11-Fam: ca. 190–250 bp and Vmix + Jp11-Hex ca. 215–225 base pairs (bp)].
Flow cytometry
Absolute numbers of CD3+, CD4+, CD8+ and CD19+ lymphocytes in peripheral blood were assessed using TruCount™ tubes and antibodies according to the manufacturer’s instructions (BD Biosciences, San Diego, CA, USA). For other flow cytometry analyses, 100 µl whole blood with EDTA were incubated with 5 µl of antibody, washed and fixed using 1% paraformaldehyde. Flow cytometry was performed using fluorescein isothiocyanate (FITC)-conjugated anti-CD8, anti-CD57 (BD Biosciences), anti-CCR7 (R&D Systems, Oxon, UK), anti-CD3, anti-human leucocyte antigen (HLA)-DR, anti-CD45RA and anti-CD45RO (DakoCytomation), phycoerythrin (PE)-conjugated anti-CD4, anti-CD56 (BD Biosciences) and anti-CD8 (DakoCytomation), and peridin chlorophyll protein (PerCP)-conjugated anti-CD3 (BD Biosciences), using a fluorescence activated cell sorter (FACSCalibur) instrument with CellQuestPro software (BD Biosciences). Isotype-matched control antibodies were used where indicated.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were obtained as described previously and stored at −80°C until analysis [9]. Serum concentrations of Fas ligand (FasL) were analysed using Duo-Set® ELISA (R&D Systems). All samples were thawed only once.
Statistical methods
Data are presented as medians and interquartile ranges unless indicated otherwise. Nominal data were compared using the χ2 test. The two-tailed Mann–Whitney U-test was used when comparing two groups of individuals. Correlations were calculated using Spearman’s signed rank test. Tests were considered significant when P < 0·05 (two-sided).
Results
LGL in CVID
To determine the occurrence of LGL in CVID patients we first examined blood smears of 29 patients with CVID, and found that 12 patients (41%) had absolute numbers of LGL > normal values (i.e. > 0·32 109 LGL/l) [3] (Fig. 1a, Table 1). These patients are referred hereafter to as CVIDLGL–high. To examine whether the expansion of LGL was a transient phenomenon, we reassessed the numbers of LGL in all available CVIDLGL–high patients (n = 10) 1–5 years after the initial assessment. Notably, the numbers of LGL were remarkably stable, also with increased absolute numbers of LGL in all 10 CVIDLGL–high patients at the second evaluation. Moreover, in four randomly chosen CVID patients with normal LGL counts in the initial assessment, all also showed LGL counts < 0·32 109 LGL/l at the second evaluation (data not shown).
Analyses of LGL phenotype and T cell clonality
Using immunocytology, we found that the LGL were CD3+, predominantly CD4– CD8+ (not shown). Notably, all patients were tested for TCRVβ-rearrangement, but we found no indication of a mono- or oligoclonal T cell expansion in any of the 12 CVIDLGL–high patients. Thus, our findings reveal a persistent polyclonal expansion of T-LGL with a predominance of CD8+ T cells in a considerable proportion of patients with CVID.
LGL in relation to clinical characteristics
Splenomegaly has been associated with chronic inflammation in CVID [9,10] and, notably, while 10/12 CVIDLGL–high patients had splenomegaly (of whom five were splenectomized), this was found in only 6/17 patients with LGL within normal ranges (P= 0·035). The presence of bronchiectasis in CVID may be related to the degree of recurring bacterial infections in the lower respiratory tract, but we found no association between the numbers of LGL and the presence of bronchiectasis in the CVID patients included (P= 0·55). Also, there was no association between number of LGL and certain other clinical characteristics registered, such as age, gender, mode of immunoglobulin substitution (intravenous or subcutaneous), age at onset, duration of symptoms or anamnestic or serological evidence of Epstein–Barr virus infection.
LGL in relation to immunological characteristics
In flow cytometric analyses of T cell subsets, we found that the CVIDLGL–high patients had lower CD4/CD8 T cell ratios compared with the other CVID patients (Table 2), which reflected higher CD8+ T cell counts, while the CD4+ T cell counts were similar in both groups (Table 2).
Table 2.
Immunological characteristics of common variable immunodeficiency (CVID) patients with high large granular lymphocyte (LGL) counts.
| CVID patients | High LGL counts1 (n = 12) | Normal LGL counts1 (n = 19) | Significance2 |
|---|---|---|---|
| CD4/CD8 T cell ratio | 0·48 (0·33–0·73) | 0·96 (0·73–1·58) | P= 0·001 |
| CD4+ T cells3 | 0·7 (0·55–1·83) | 0·5 (0·35–0·91) | P= 0·17 |
| CD8+ T cells3 | 1·7 (0·81–2·37) | 0·5 (0·28–0·94) | P= 0·004 |
| CD3+CD45RO+4 | 49 (42–63) | 49 (40–64) | P= 0·9 |
| CD4+CD45RO+5 | 31 (21–33) | 44 (29–52) | P= 0·017 |
| CD3+HLA-DR+4 | 53 (25–67) | 28 (17–48) | P= 0·09 |
| CD8+HLA-DR+6 | 23 (11–35) | 13 (3–20) | P= 0·044 |
| CCR7+4 | 32 (15–54) | 43 (36–69) | P= 0·08 |
Number of LGL > 0·32 109/l.
P-value determined using Mann–Whitney test.
Absolute number; 109/l.
Percentage of all T cells.
Percentage of all CD4+ T cells.
Percentage of all CD8+ T cells. HLA: human leucocyte antigen.
Furthermore, as reported previously in CVID [2], we found that the CVID patients as a whole had increased proportions of CD45RO+ T cells compared to healthy controls [63 (53–7) versus 50 (48–58)% CD45RO+ T cells; P = 0·048]. However, the CVIDLGL–high patients had significantly lower proportions of CD4+ T cells expressing CD45RO than the other CVID patients (see Table 2). Similar to previous observations in patients with LDGL [11], we also found increased proportions of T cells expressing the activation marker HLA-DR and a trend towards lower proportions of T cells expressing the CC-chemokine receptor 7 (CCR7) in the CVIDLGL–high patients compared to the remaining CVID patients (Table 2). Finally, increased expression of CD57 on circulating T cells has been associated previously with LDGL [7] and has also been observed in CVID. In agreement with this, we found increased proportions of T cells expressing CD57 in the CVID patients compared to healthy controls [29 (15–36) versus 4 (3–15)% CD57+ T cells; P = 0·037], but we found no statistical association between the expression of CD57 on peripheral T cells and numbers of LGL in CVID patients in the present study. Moreover, we did not find any relation between numbers of LGL and absolute numbers of B cells or NK cells as determined by surface expression of CD19 and CD56 (not shown).
LGL in relation to neutropenic episodes in CVID
The expansion of LGL is often associated with neutropenia [7], and therefore we next investigated the relationship between the numbers of LGL and the occurrence of neutropenia in CVID. Neutropenia in CVID often shows a fluctuating course. Of the 29 patients included in this study, 26 had been followed at our hospital for more than 5 years, mainly at 3-month intervals, with regular blood cell counts performed. In 10 of these (36%), we found at least one episode of neutropenia (defined as < 0·15 109 granulocytes/l [12]) without known cause during the last 5 years. Notably, these patients had significantly higher numbers of LGL than patients with no recorded neutropenia (n = 16) [1·0 (0·1–1·8) versus 0·2 (0·1–0·3) 109 LGL/l, P = 0·047], indicating a possible association between elevated numbers of LGL and occurrence of neutropenia in CVID. When comparing patients who were both CVIDLGL–high and had a history of neutropenia (n = 7) to the remaining CVID patients (n = 19), we found that six of these patients had a history of splenomegaly (85%versus 35%, respectively, P = 0·005). Notably, of these six patients, five had been splenectomized before the occurrence of neutropenia.
Soluble FasL related to LGL and neutropenia
Soluble forms of FasL (sFasL) have been suggested to play a role in the neutropenia associated with increased LGL [13]. To examine whether there was a similar association in CVID we next analysed the levels of sFasL in sera from CVID patients and healthy controls. We found a great variation in the sFasL levels in the CVID patients, with no significant difference compared to healthy controls (not shown). However, in the CVIDLGL–high patients in whom we had observed neutropenia (n = 7), we found nearly fourfold higher levels of sFasL than in the CVID patients who had normal LGL counts and no history of neutropenic episodes (n = 10) (P= 0·025, see Fig. 1b).
Discussion
CVID is a heterogeneous group of immunodeficiency diseases of mostly unknown aetiology [1], where T cell dysfunction seems to play a pathogenic role in a subgroup of patients. In this study, we report a polyclonal expansion of LGL in a considerable proportion of CVID patients. This was associated with splenomegaly, increased numbers of CD8+ T cells, inverted CD4/CD8 T cell ratios and increased proportions of HLA-DR+ and CCR7+ T cells, and with a history of neutropenia.
Expansions of LGL have previously been found in RA, chronic viral infections and other inflammatory disorders [3], but an expansion of LGL in CVID has not been reported previously. Furthermore, although the numbers of LGL were increased, we could not find evidence of any mono- or oligoclonal expansion in any patient. Polyclonal expansions of LGL are seen in acute infections [7], but no patient included had any apparent infection at the time of inclusion. Furthermore, the patients maintained similar numbers of LGL at repeated assessments after 1–5 years. In contrast to the association with splenomegaly, which has been shown to be a sign of persistent inflammation in CVID [9], there was no association between the occurrence of bronchiectasis and increased numbers of LGL. Thus, although persistent inflammation may be involved, intercurrent infections are an unlikely explanation for the increased numbers of LGL in our patients.
The de novo development of elevated numbers of LGL in CVID over time has not been determined in this study. Although we saw no rise in LGL numbers in four randomly selected CVID patients with normal LGL numbers re-examined after 1–5 years, the observation that 41% of CVID patients have elevated numbers of LGL suggests that the long-term development of LGL in CVID may be a target of future studies.
In RA and some chronic viral infections it has been proposed that chronic T cell stimulation with specific antigens may lead to a clonal expansion of LGL [7]. Chronic in vivo activation of T cells has also been suggested previously in CVID [14,15], and we have recently demonstrated increased proportions of T cells with an effector-memory phenotype in CVID [16], probably representing a further sign of chronic T cell activation in these patients. In agreement with this, increased proportions of CD45RO+ T cells, which may represent memory T cells, have been reported previously in CVID [2]. However, in this study we found lower proportions of CD45RO+ cells among CD4+ T cells in patients with high LGL counts compared to patients with LGL counts within normal ranges. In agreement with this, LGL expansions have been associated previously with increased proportions of CD45RA+ T cells [7]. Interestingly, recent reports suggest that T cells in certain chronic viral infections may re-express CD45RA in the terminal differentiation steps [17,18]. It is therefore possible that the increased expression of CD45RA associated with LGL may reflect terminally differentiated T cells, thus reflecting persistent T cell activation. It has been suggested that this persistent T cell activation in some CVID patients may represent ‘footprints’ of a chronic viral infection that may trigger immunodeficiency in genetically susceptible individuals [15,16,19]. However, it is unclear why CVID patients maintain a polyclonal expansion of LGL and other mechanisms may be operative, i.e. aberrant function of apoptotic pathways such as Fas/FasL [20,21].
In LDGL, neutropenia is frequent. In this study, we report a significant association also between expanded LGL and neutropenia in CVID, suggesting that a similar pathogenic mechanism for neutropenia may be operating in CVID as in LDGL. This mechanism is unknown, but sFasL has been shown to be involved in the development of neutropenia in LDGL, possibly through direct apoptotic effects mediated through binding of sFasL on Fas expressed on the neutrophil granulocytes [13]. It has been suggested that the sFasL is shed by LGL [13], but it has also been shown that activated monocytic cells may secrete sFasL [22]. Whether the elevation of sFasL in some CVID patients shown in this study is due to the elevated LGL or due to increased monocyte activation, as demonstrated previously in CVID [23], remains to be elucidated. In CVID, increased surface expression of Fas on T cells and reduced Fas-sensitivity have been observed in some patients [21], but this has not been related previously to elevated sFasL or neutropenia. Our observation of elevated serum levels of sFasL in the neutropenia-prone CVIDLGL–high patients compared to the other CVID patients may also suggest an involvement of sFasL in the development of neutropenia in CVID, and this issue should be addressed in future studies. Interestingly, despite persistent high numbers of LGL, favourable responses to methotrexate [24] or cyclosporin [25] have been reported concerning granulocytopenia in LGL-leukaemia, and these responses have been linked to a reduction in the serum levels of sFasL [13,26]. Whether similar positive responses would be achieved in CVID-related neutropenia has not been studied systematically. However, one of our patients with severe neutropenia (patient no. 25, Table 1) responded favourably to treatment with cyclosporin.
In conclusion, our study demonstrates increased numbers of polyclonal LGL in a considerable proportion of CVID patients, and may suggest common pathogenic mechanisms of neutropenia in both CVID and LDGL. We believe that these observations may increase our understanding of both conditions, and possibly contribute to the development of new therapeutic approaches in CVID.
Acknowledgments
This work was supported by a grant from the Norwegian Research Council.
References
- 1.Rosen FS, Eibl MM, Roifman CM, et al. Primary immunodeficiency diseases. Clin Exp Immunol. 1999;118(Suppl. 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. Report of an IUIS Scientific Committee. International Union of Immunological Societies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 3.Loughran TP., Jr Clonal diseases of large granular lymphocytes. Blood. 1993;82:1–14. [PubMed] [Google Scholar]
- 4.Fitzgerald JE, Ricalton NS, Meyer AC, et al. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995;154:3538–47. [PubMed] [Google Scholar]
- 5.Pulik M, Lionnet F, Genet P, Petitdidier C, Jary L, Fourcade C. CD3+ CD8+ CD56– clonal large granular lymphocyte leukemia and HIV infection. Br J Haematol. 1997;98:444–5. doi: 10.1046/j.1365-2141.1997.1913009.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong KF, Yip SF, So CC, Lau GT, Yeung YM. Cytomegalovirus infection associated with clonal proliferation of T-cell large granular lymphocytes: causal or casual? Cancer Genet Cytogenet. 2003;142:77–9. doi: 10.1016/s0165-4608(02)00739-2. [DOI] [PubMed] [Google Scholar]
- 7.Lamy T, Loughran TP., Jr Clinical features of large granular lymphocyte leukemia. Semin Hematol. 2003;40:185–95. doi: 10.1016/s0037-1963(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1:173–9. [PubMed] [Google Scholar]
- 9.Aukrust P, Lien E, Kristoffersen AK, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficienc − possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 10.Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–41. [PubMed] [Google Scholar]
- 11.Mitsui T, Maekawa I, Yamane A, et al. Characteristic expansion of CD45RA CD27 CD28 CCR7 lymphocytes with stable natural killer (NK) receptor expression in NK- and T-cell type lymphoproliferative disease of granular lymphocytes. Br J Haematol. 2004;126:55–62. doi: 10.1111/j.1365-2141.2004.05005.x. [DOI] [PubMed] [Google Scholar]
- 12.Boxer L, Dale DC. Neutropenia: causes and consequences. Semin Hematol. 2002;39:75–81. doi: 10.1053/shem.2002.31911. [DOI] [PubMed] [Google Scholar]
- 13.Liu JH, Wei S, Lamy T, et al. Chronic neutropenia mediated by fas ligand. Blood. 2000;95:3219–22. [PubMed] [Google Scholar]
- 14.Wright JJ, Wagner DK, Blaese RM, Hagengruber C, Waldmann TA, Fleisher TA. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990;76:2046–51. [PubMed] [Google Scholar]
- 15.Baumert E, Wolff-Vorbeck G, Schlesier M, Peter HH. Immunophenotypical alterations in a subset of patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1992;90:25–30. doi: 10.1111/j.1365-2249.1992.tb05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm AM, Sivertsen EA, Tunheim SH, et al. Gene expression analysis of peripheral T cells in a subgroup of common variable immunodeficiency shows predominance of CCR7(-) effector-memory T cells. Clin Exp Immunol. 2004;138:278–89. doi: 10.1111/j.1365-2249.2004.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 18.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 19.Kainulainen L, Nikoskelainen J, Vuorinen T, Tevola K, Liippo K, Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med. 1999;159:1199–204. doi: 10.1164/ajrccm.159.4.9807067. [DOI] [PubMed] [Google Scholar]
- 20.Camagna A, Cedrone L, Care A, et al. Polyclonal expansion of CD3(+)/CD4(+)/CD56(+) large granular lymphocytes and autoimmunity associated with dysregulation of Fas/FasL apoptotic pathway. Br J Haematol. 2001;112:204–7. doi: 10.1046/j.1365-2141.2001.02483.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Renzo M, Serrano D, Zhou Z, George I, Becker K, Cunningham-Rundles C. Enhanced T cell apoptosis in common variable immunodeficiency: negative role of the fas/fas ligand system and of the Bcl-2 family proteins and possible role of TNF-RS. Clin Exp Immunol. 2001;125:117–22. doi: 10.1046/j.1365-2249.2001.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiener PA, Davis PM, Rankin BM, et al. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–8. [PubMed] [Google Scholar]
- 23.Aukrust P, Froland SS, Muller F. Raised serum neopterin levels in patients with primary hypogammaglobulinaemia; correlation to other immunological parameters and to clinical and histological features. Clin Exp Immunol. 1992;89:211–6. doi: 10.1111/j.1365-2249.1992.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loughran TP, Jr, Kidd PG, Starkebaum G. Treatment of large granular lymphocyte leukemia with oral low-dose methotrexate. Blood. 1994;84:2164–70. [PubMed] [Google Scholar]
- 25.Sood R, Stewart CC, Aplan PD, et al. Neutropenia associated with T-cell large granular lymphocyte leukemia: long-term response to cyclosporine therapy despite persistence of abnormal cells. Blood. 1998;91:3372–8. [PubMed] [Google Scholar]
- 26.Saitoh T, Karasawa M, Sakuraya M, et al. Improvement of extrathymic T cell type of large granular lymphocyte (LGL) leukemia by cyclosporin A: the serum level of Fas ligand is a marker of LGL leukemia activity. Eur J Haematol. 2000;65:272–5. doi: 10.1034/j.1600-0609.2000.065004272.x. [DOI] [PubMed] [Google Scholar]