Abstract
Respiratory syncytial virus (RSV) is a common cause of lower respiratory tract disease (LRTD) in infants. Eosinophils have been suggested to play a role in the disease pathogenesis of LRTD. Inflammation can induce functional and morphological alterations of peripheral blood granulocytes. In patients with RSV LRTD, we aimed to investigate the eosinophil activation status by analysing surface markers. In vitro stimulation of eosinophils with cytokines leads to up-regulation of CD11b and priming markers recognized by the recently developed priming markers A17 and A27, whereas interleukin (IL)-5Rα is being down-regulated. In 51 patients and 10 controls we examined the expression of these surface markers on eosinophils in moderate to severe RSV-induced LRTD patients at the time of admission and 6 weeks later during the convalescence phase. RSV-patients were characterized by a higher eosinophil CD11b expression compared to controls. Although basal A17 and A27 expression was not increased, we observed a significantly higher expression of these priming epitopes on N-formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated cells of RSV patients compared with cells of controls, indicative of prior in vivo priming. Furthermore, IL-5Rα expression was down-regulated on peripheral blood eosinophils of these patients. Follow-up blood samples showed normalization of all markers but CD11b, which was persistently increased. Utilizing cellular markers, we observed that peripheral blood eosinophils from infants with RSV LRTD are in a more activated state compared to eosinophils of controls, which normalizes only partially during convalescence.
Keywords: CD11b, granulocytes, lower respiratory tract disease, peripheral blood, priming, RSV
Introduction
The majority of infants in the western world have encountered at least one respiratory syncytial virus (RSV) infection before the age of 2 years. A total of 1–3% of infected infants require hospitalization for lower respiratory tract disease (LRTD). No effective therapy is currently available, and only supportive care such as supplemental oxygen and intravenous fluid can be provided to assist spontaneous recovery.
Research into the pathogenesis of this common respiratory infection was advanced by the dramatic results of the vaccination studies in the 1960s. Infants who were immunized with formalin-inactivated (FI) RSV vaccine became severely ill upon exposure to wild-type RSV in the subsequent winter epidemic [1]. In the following years, this FI-RSV vaccine has been used in a murine model to study RSV pathogenesis [2]. The presence of large amounts of eosinophils in the lungs of mice after segmental immunization and infection with RSV, as well as in the lungs and blood of infants who died after vaccination, was a characteristic feature [3,4]. Eosinophil presence is linked to the clinical symptoms of bronchial hyperresponsiveness during an acute infection, which sometimes persevere several weeks to months after viral clearance [5]. Although the levels of eosinophilic cationic protein (ECP) were highly increased in bronchoalveolar lavage (BAL) fluid of children with a severe primary RSV infection [6,7], no increase in eosinophil cell numbers was found. Also in asthma, discrepancies between eosinophil numbers in sputum or peripheral blood and pulmonary tissue have been described. Flood-Page and colleagues have shown that even after several months of anti-interleukin (IL)-5 therapy 50% of eosinophils were still present in the tissue, whereas in blood, bone marrow and sputum only very low levels of eosinophils could be found [8]. A small number of eosinophils present in airway tissue is thought to be enough to cause symptoms.
Involvement of eosinophils in RSV LRTD requires migration of cells from the peripheral blood to the lungs. For optimal extravasation of eosinophils through the pulmonary vasculature, these cells need cytokine priming in the peripheral blood [9,10]. As in most local inflammatory conditions, cytokine levels in the peripheral blood are below detection level and are often not useful in studying immune cell involvement. However, cellular markers associated with priming and activation are indicative for exposure to picomolar concentrations of cytokines and may be used as a systemic read-out of local inflammation [11,12].
Recently, novel priming-specific monoclonal antibodies were used to demonstrate increased priming of peripheral blood eosinophils in whole blood samples during allergen challenge in adults [13,14]. Activation of eosinophils can also be measured by analysing the expression of CD11b [11,15], which is known to be up-regulated on the surface of activated cells due to relocation from specific granules in granulocytes [16–18]. Furthermore, it has been shown that during allergen challenge, eosinophils in the lung down-regulate their IL-5Rα, a high-affinity receptor for IL-5 [19,20]. In vitro experiments have demonstrated a down-regulation of IL-5Rα when cultured in the presence of IL-5, IL-3 or granulocyte–macrophage colony-stimulating factor (GM-CSF) [21]. Down-regulation of IL-5Rα expression may therefore reflect stimulation of eosinophils by cytokines and could be used as a potential marker for eosinophil-mediated inflammation.
In the present study we aimed to investigate the involvement of eosinophils in RSV LRTD. We observed systemic activation of eosinophils cells in peripheral blood, using cellular surface markers as a read-out and related systemic activation to clinical disease severity.
Materials and methods
Patients
Infants under 2 years of age, admitted to the hospital with RSV-induced lower respiratory tract infection, were enrolled during two winter epidemics. The diagnosis of RSV LRTD was based on: (1) the presence of a positive immunofluorescence test for RSV on nasopharyngeal secretions; (2) first-ever episode of wheezing; and (3) a paediatrician made a clinical diagnosis of RSV-LRTD with fine crackles, wheeze and/or ronchi present on auscultation of their lungs.
In 51 patients and 10 healthy controls, eosinophil activation markers were measured. Children with pre-existing wheezing, chronic lung disease, congenital heart disease or immunodeficiency were excluded from the study. Standard care patients were enrolled from five different small regional hospitals and intensive care patients from the University Medical Centre in Utrecht (UMCU), whereas all blood tests were performed at the laboratory of pulmonary diseases at the UMCU. The study was approved by the Central Committee on Research Involving Human Subjects of the Ministry of Health and the Medical Ethical Committees in all participating centres. Both written and oral permission were obtained from parents or guardians from all patients. To evaluate the disease severity, duration of hospitalization, number of days on supplemental oxygen support and duration of mechanical ventilation were recorded.
Control patients were enrolled at the Urology Department of Paediatrics. Only patients under 2 years of age, undergoing small urological operations without a history of allergy, LRTD or wheezing, were included. None of these patients had a current or recent (urinary tract) infection or were known to have any kind of immunodeficiency.
Collection of materials and staining of eosinophil surface markers
Heparinized venous blood was drawn from the RSV patients within 36 h of admission. A second blood sample was taken during the convalescent phase 6 weeks later. Blood from control patients was drawn when venous access was obtained for the induction of intravenous anaesthesia. The samples were directly placed on ice, preventing ex-vivo phagocyte activation. Unstimulated blood samples were stained using fluorescein isothiocyanate (FITC)-labelled human monoclonal phage antibodies (MoPhab) A17 and A27, isolated from a synthetic bacteriophage antibody library as described previously [13]. These priming epitopes are expressed on granulocytes in vitro after exposure to picomolar concentrations of cytokines such as GM-CSF and IL-5. Staining of leucocytes was performed as described previously [14]. In brief, FITC-labelled MoPhab A17 and A27 were diluted in phosphate buffered saline (PBS; 1 : 10). Whole blood samples (50 µl each) were incubated on ice for 60 min with 100 µl of the (1 : 10) dilution of FITC-labelled MoPhab before lysing the erythrocytes. CD11b and IL-5Rα expression was measured by staining 50 µl of whole blood with anti-CD11b (1 : 100) (clone 2LPM19c; Dako, Glostrup, Denmark) and anti-IL-5Rα (1 : 100) (clone A14; BD PharMingen, San Diego, CA, USA), respectively, for 30 min on ice. Ex-vivo stimulated blood samples were preincubated at 37°C for 5 min and then stimulated for 10 min with N-formyl-methionyl-leucyl-phenylalanine (fMLP) (1 µM) (Sigma, St Louis, MO, USA). After stimulation, the samples were placed on ice and stained as described above.
After staining, erythrocytes of all samples were lysed by adding ice-cold isotonic NH4Cl [22] and cells were washed and resuspended in PBS/1% human serum albumin (HSA) (Sanquin, Amsterdam, the Netherlands) for analysis. All samples were analysed in a fluorescence activated cell sorter (FACS) vantage flow cytometer (Becton Dickinson, Mountain View, CA, USA). Eosinophils were identified according to their specific side- and forward-scatter signals [23]. The percentage of eosinophils was determined as the percentage of total leucocytes measured by FACS.
Statistical analysis
Surface marker expression levels in peripheral blood eosinophils had non-parametric distributions. They are expressed as median values and range. Mann–Whitney U-tests were used to analyse differences between patient groups and controls. Within-group analysis was performed using Wilcoxon’s rank sum test. Spearman’s correlation coefficient was used to analyse the relation between the different surface markers. Statistical tests were performed by using the statistical software package (SPSS Inc., version 12·0, Chicago, IL, USA). All tests of significance were two-sided. A P-value < 0·05 was considered statistically significant.
Results
The patient population comprised of 51 patients with RSV LRTD, 11 of whom required mechanical ventilation (RSV-Intensive Care; RSV-IC group), and 40 required standard care (RSV-SC group). Ten control patients were enrolled with a median age of 56·4 weeks (range: 35–106). Median age in the RSV-SC group was 9·6 weeks (range 2·1–67) and in the RSV-IC group 7·8 weeks (range 1·3–68). None of the patients received anti-viral agents or systemic corticosteroids. Table 1 shows baseline characteristics of the RSV cohort and controls. No correlation was present between age or post-conceptional age and basal or fMLP-stimulated levels of eosinophil expression of the A17 or A27 epitope, CD11b and/or IL-5Rα (data not shown).
Table 1.
Demographic characteristics of patient groups.
| Controls n = 10 | RSV standard care n = 40 | RSV intensive care n = 11 | |
|---|---|---|---|
| Sex (male/female) | 8/2 | 26/14 | 6/5 |
| Age (weeks) | 56·4 (35·0–106·4) | 9·6 (2·1–67) | 7·8 (1·3–67·7) |
| Post-conceptional age (weeks) | 96·3 (75·6–143·4) | 49·5 (40–107) | 44·9 (38·7–107·7) |
| Length of hospital stay (days) | – | 4 (0–12) | 13·5 (9–21) |
| Oxygen support (days) | – | 6·5 (3–14) | 12 (9–18) |
RSV: Respiratory syncytial virus.
Eosinophils of RSV patients are characterized by a primed phenotype: a higher baseline of CD11b expression and an increased sensitivity for ex vivo stimulation with fMLP
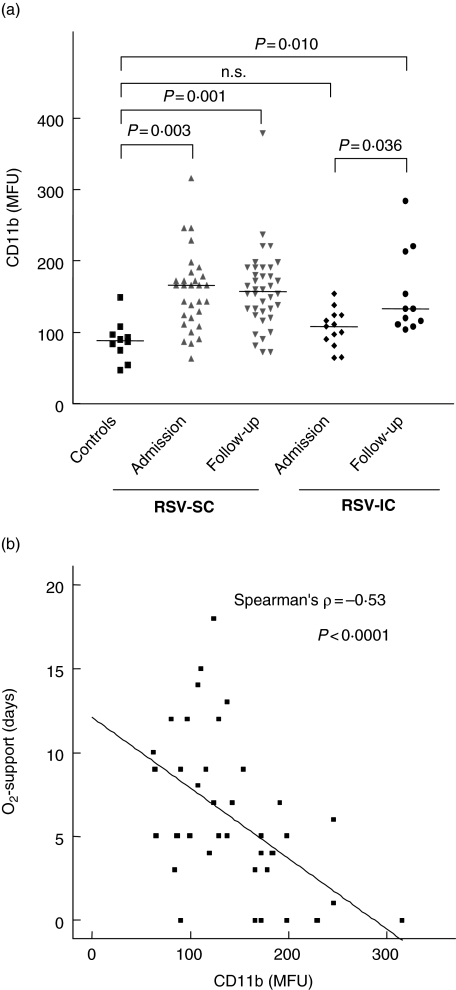
Significant differences were found in basal expression of Mac-1 (CD11b/CD18) of eosinophils between the different groups (Fig. 1a). Median CD11b expression in controls was 91·5 median fluorescence units (MFU) (range 75–149), in RSV-SC patients 166 MFU (range 63–316, P = 0·003). The CD11b expression in RSV-IC patients was comparable to controls, with a median expression of 104 MFU (range 64–154, P = 0·34). These data show clearly the pre-activation of the eosinophil compartment in RSV-SC patients.
Fig. 1.
CD11b expression on eosinophils respiratory syncytial virus (RSV) patients (standard care: RSV-SC and intensive care: RSV-IC) and controls is expressed in median fluorescence units (MFU) (a). For RSV-SC patients (grey triangles) and RSV-IC patients, both CD11b (MFU) expression of eosinophils at the time of admission and during follow-up are shown (a). The median of each group is represented by a horizontal line. (b) Inverse correlation between CD11b expression and the number of days of oxygen support the infants had received.
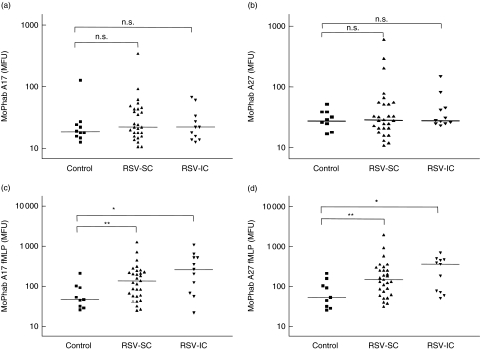
In analogy to Mac-1 the basal expression of priming epitopes A17 and A27 were measured on peripheral blood eosinophils. In marked contrast to CD11b, we did not observe a significant difference in the expression of A17 and A27 epitopes on eosinophils from RSV patients compared to healthy controls (Fig. 2). The median MoPhab A17 binding of controls was 17·5 MFU (range 12–124). In RSV-SC patients, median eosinophil MoPhab A17 binding was 21 MFU (range 10–340) and in RSV-IC patients, eosinophil priming was 21 MFU (range 12–65) (Fig. 2a). Expression of the A27 epitope followed a similar pattern. Basal A27 expression in controls was 28 MFU (range 9–52), in RSV-SC patients 28 MFU (range 11–604) and in RSV-IC patients 30 MFU (range 23–149) (Fig. 2b).
Fig. 2.
Monoclonal phage antibody (MoPhab) A17 (a)and A27 (b) (both markers of priming) binding to eosinophils of patients with respiratory syncytial virus (RSV) admitted to a standard care ward (RSV-SC), intensive care (RSV-IC) and of control subjects. Eosinophil priming is expressed as MoPhab in median fluorescence units (MFU). (c, d) MoPhab A17- and A27-binding, respectively, after ex-vivo stimulation with N-formyl-methionyl-leucyl-phenylalanine (fMLP). The median of each group is indicated by a horizontal line. *P-value < 0·05; a **P-value < 0·01.
This mixed primed phenotype under basal conditions was accompanied by an enhanced sensitivity to ex-vivo stimulation with fMLP stimulation. This resulted in a significant increase in: (1) CD11b expression in RSV-SC patients (P= 0·017) and RSV-IC patients (P= 0·008); (2) A17-expression in RSV-SC patients (P= 0·005) and RSV-IC patients (P= 0·015) (Fig. 2c); and (3) A27 expression in RSV-SC patients (P= 0·020) and RSV-IC patients (P= 0·019) (Fig. 2d) compared to normal controls.
The sensitivity for ex-vivo stimulation with fMLP normalized during convalescence at 6 weeks after RSV disease (results not shown). Comparison between groups did not show a significant difference. Also, A27 binding upon ex-vivo stimulation with fMLP was increased in RSV patients (P= 0·009), with the highest levels in RSV-IC patients (Fig. 2d).
Basal A17 and A27 binding correlate well with each other (R= 0·87, Spearman’s rho = 0·63, P < 0·0001). Basal and stimulated expression of MoPhab A17 or A27 of eosinophils did not correlate with basal or stimulated binding of CD11b.
CD11b expression on eosinophils of RSV-SC patients does not return to normal levels during convalescence, whereas CD11b on cells of RSV-IC patients increases
Blood was drawn from patients in the convalescence phase, 6 weeks after admission. In the convalescence phase, the increased sensitivity for stimulation with fMLP (in the context of A17 and A27 epitope expression) had decreased (P< 0·0001) to levels comparable to controls (see above). However, the expression of CD11b on eosinophils from RSV-SC patients was comparable to the levels during acute disease and was still higher than in controls (Fig. 1a, P = 0·001). In the RSV-IC patients an apparent increase in CD11b expression was observed compared to baseline (P= 0·036), to levels significantly higher than control values (P= 0·010) (Fig. 1a).
CD11b expression is negatively correlated to disease severity
During acute respiratory illness, a significant inverse correlation was found between CD11b expression and the number of days of oxygen support the child had received (Spearman’s rho = −0·53, P < 0·0001) (Fig. 1b). This correlation was still present when only the RSV-SC children were analysed.
Decreased eosinophil counts and down-regulation of IL-5Rα on eosinophils in peripheral blood of patients during acute lower respiratory tract infection
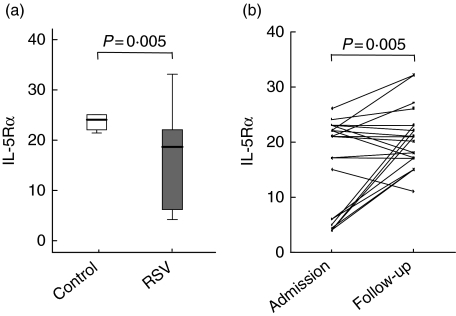
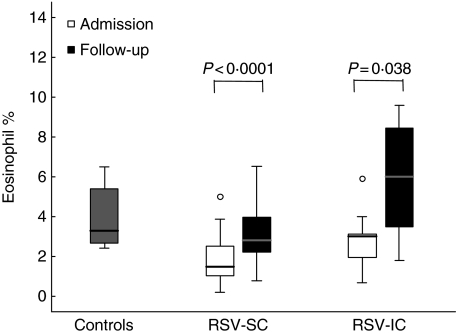
IL-5Rα expression on eosinophils in the peripheral blood of RSV patients (median 18, range 4–33) was decreased compared to control values (median 24 MFU, range 21·3–25) (P= 0·02) (Fig. 3a). At the 6 weeks’ convalescence time-point, IL-5Rα expression was again normalized comparable to control levels (Wilcoxon’s rank sum test, P = 0·005), indicating that IL-5Rα was down-regulated during acute disease (Fig. 3b). The same pattern was observed in eosinophil percentages. The percentage of eosinophils found in RSV patients (both IC and SC) (median 1·5% of total leucocytes) were lower than in controls (median 3·3%) (P< 0·0001). At 6 weeks, eosinophil numbers had increased significantly (P< 0·0001) (Fig. 4). Furthermore, there was no difference between RSV-SC and RSV-IC patients in the percentage of eosinophils at either time-point.
Fig. 3.
(a) Interleukin (IL)-5Rα-expression on peripheral blood eosinophils of infants with respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD) and controls (P= 0·005). (b) IL-5Rα expression, expressed in median fluorescence units (MFU) was measured by fluorescence activated cell sorter (FACS) as described in Materials and methods both during acute disease and at the follow-up time-point 6 weeks later. A Wilcoxon’s rank test showed that IL-5Rα expression significantly increased during convalescence.
Fig. 4.
The percentage of eosinophils (from total leucocytes analysed by FACS) is represented for both respiratory syncytial virus (RSV) standard care (RSV-SC) and intensive care (RSV-IC) groups at the initial time-point and during follow-up 6 weeks post-infection (Wilcoxon’s rank test).
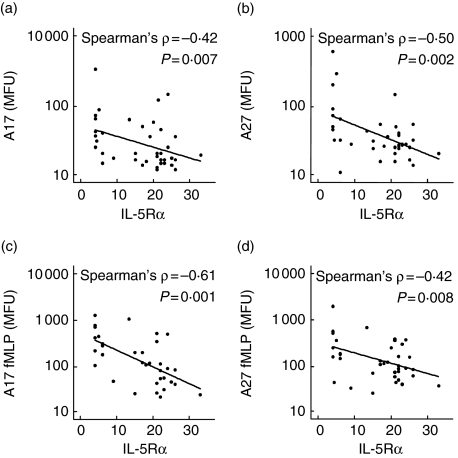
During acute disease IL-5Rα expression was correlated inversely to both the basal and stimulated level of priming epitope-A17/A27 expression (Fig. 5), indicating that acute disease may induce up-regulation of priming epitopes and a down-regulation of IL-5Rα expression on eosinophils in the peripheral blood.
Fig. 5.
Scatterplots of eosinophil interleukin (IL)-5Ra expression and monoclonal phage antibody (MoPhab) A17/A27 binding of respiratory syncytial virus (RSV) patients. Unstimulated (a and b) and N-formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated (c and d) priming of eosinophils (measured as expression of A17 and A27 epitopes) in median fluoresence units (MFU) was observed to correlate with IL-5Rα expression (MFU).
Discussion
This study was conducted because of the controversy regarding the involvement of eosinophils in RSV LRTD. We investigated eosinophil (pre)activation or priming in peripheral blood during RSV LRTD, utilizing cellular markers and found several arguments supporting activation of the eosinophil compartment. First, in comparison with healthy controls, we observed a higher basal CD11b expression on eosinophils from RSV hospitalized infants, and secondly an increased sensitivity to ex-vivo stimulation with fMLP, which is very typical for primed eosinophils [24]. A cellular response with increasing expression of eosinophil CD11b and A17/A27 priming epitopes suggests strongly a primed eosinophil phenotype in the peripheral blood of children with RSV LRTD and implies the involvement of eosinophils in the clinical course of this disease. Unfortunately, our study cannot differentiate between a pre-existing sensitivity of eosinophils for an immunological insult such as induced by RSV or that RSV or RSV products are actively inducing eosinophil activation. However, pre-existence of an ‘atopic type’ immune system does not seem to be a risk factor for increased sensitivity of the eosinophil compartment during RSV infection, because only few infants (n = 6) were positive in the Phadiatop Infant test (results not shown). This is in line with the finding that atopy does not seem to be a dominant risk factor for RSV-LRTD, as the majority of atopic children do not develop this disease after infection with RSV. None the less, our data show that the host response to RSV infection, leading to LRTD, is associated with the activation of peripheral eosinophils (also see below).
RSV patients were compared to a control group which also consisted of infants less than 2 years of age, but with a mean age of a few months older than the RSV patients. However, no relation could be observed between (post-conceptional) age and eosinophil marker expression, nor has it has been described elsewhere that eosinophil function or marker expression change during early infancy. The controls were screened for a negative history regarding respiratory conditions or (family) atopy.
CD11b is an accepted marker for activated phagocytes and is induced in vitro by both cytokines and chemokines [24–26]. Interestingly, we did not observe a significant basal up-regulation of priming markers A17 and A27, both of which are up-regulated in vitro by the same mediators (such as GM-CSF) as are involved in up-regulation of CD11b. Apparently, the signals operating in vivo are distinct from those which are used in vitro to activate eosinophils. This hypothesis is strengthened by our previous studies, where we have shown that a well-controlled inflammatory stimulus in vivo, in mild asthmatic patients in the form of an allergen challenge, caused a transient priming of peripheral blood eosinophils characterized by enhanced expression of A17 expression and a stable expression of CD11b. This priming response was detectable only within a few hours after challenge [14]. Hereafter, A17/A27 signals from peripheral blood eosinophils normalized to pre-challenge levels whereas the late asthmatic response was developing. These findings are consistent with the hypothesis that under different inflammatory conditions eosinophils are characterized by subtle differences in priming phenotype.
In RSV patients we measured priming markers within the first 36 h of admission, which could be a considerable time after occurrence of the first symptoms. Thus, although A17 and A27 have been shown to be up-regulated in more chronic inflammatory diseases such as mild asthma, in this acute respiratory tract disease sampling may have been too late to see this initial burst of A17/A27 epitope-expressing eosinophils in the peripheral blood in all patients.
The observation of an increased sensitivity to ex-vivo stimulation with fMLP demonstrates that residual cells in the peripheral blood are certainly pre-activated, albeit to a lesser or different extent than observed 6 h after allergen challenge [14]. Apparently, eosinophils of RSV patients exhibit an increased sensitivity to fMLP, which is well known to occur after cytokine exposure in vitro, and is also seen in allergic asthmatics in vivo.
Two additional arguments for modulation of the eosinophil compartment during RSV LRTD stem from the findings of a decreased IL-5Rα expression on eosinophils of RSV patients and the decrease in peripheral blood eosinophil numbers. Liu et al. have shown that stimulation of leucocytes with cytokines in vitro and in the tissue in vivo leads to down-regulation of the IL-5Rα on the surface of eosinophils [19]. Interestingly, we observed a similar down-regulation on the surface of eosinophils in the peripheral blood of patients with acute RSV LRTD. It is unlikely that the low IL-5Rα-expressing cells represent a population of eosinophils newly recruited from the bone marrow, as CD34+ bone marrow progenitors are known to up-regulate their IL-5Rα during differentiation to eosinophils [27]. Furthermore, blood levels of eosinotrophic cytokines are below detection levels (our unpublished data). Therefore, we propose that circulating eosinophils may also originate from redistributed cells from the lung, in which down-modulation has already taken place, resulting in a lower IL-5Rα expression.
The lower percentage of eosinophils in RSV LRTD fits the concept that eosinophils extravasate to the lung during a pulmonary inflammatory process. Alternatively, a lowered percentage of eosinophils in the peripheral blood could be merely a reflection of increased total leucocyte numbers. However, RSV bronchiolitis in infants is associated with a transient but prominent decline in circulating lymphocytes but only a small increase in neutrophil numbers [28]. The reduction in eosinophil percentage is due therefore to a true decrease in peripheral blood eosinophil numbers and is most probably a reflection of eosinophil migration to the pulmonary tissues in response to chemoattractants [IL-8, macrophage inflammatory protein (MIP)-1α, eotaxin] produced in the lung during RSV LRTD [29].
We have shown here that, during RSV LRTD, the peripheral blood eosinophil compartment is changed, represented by changes in expression of inflammation markers with different kinetics. The basal expression of A17/A27 epitopes on eosinophils of RSV patients was not significantly different from the controls, but the range of expression in the RSV patients was quite large. An interesting inverse correlation could be observed between the stimulated and unstimulated expression of A17/A27 epitopes and eosinophil IL-5Rα expression. Similar observations have been described in the literature on BAL eosinophils of asthmatics after allergen challenge [19]. It is tempting to speculate that cells expressing high levels of A27/A17 and low levels of IL-5Rα have interacted with the inflamed tissue in the lung and might originate from this site. Although eosinophils are considered to be tissue-dwelling cells that become activated after extravasation and die by apoptosis, the possibility of redistribution of eosinophils from the lung to other compartments is not unprecedented. In the lungs of patients with asthma, which is characterized by pulmonary tissue eosinophilia, apoptotic eosinophils are rarely found [30–32]. Several authors have shown that eosinophils instilled in the lungs of mice migrate to the thoracic lymph node, expressing a higher level of activation markers on their surface than peripheral blood eosinophils [33–35].
It is intriguing as to why CD11b is up-regulated on eosinophils both during acute disease and during convalescence, whereas all other parameters normalized. Although initially not increased in the RSV-IC patients, in convalescence CD11b levels were still significantly higher than in controls. In’t Veen et al. found a positive correlation between bronchial hyperresponsiveness of asthmatic patients and CD11b expression of peripheral blood eosinophils [36]. RSV LRTD is characterized by a period of increased bronchial responsiveness in the post-bronchiolitis period. The increased expression of CD11b present in both the RSV-SC and RSV-IC groups 6 weeks post-infection can therefore be explained by residual activation of eosinophils under these conditions.
The expression of CD11b was lower in the RSV-IC patients and correlated inversely with disease severity, determined by the number of days of oxygen support. Again, this may reflect migration of activated eosinophils to the lung, which is expected to be most prominent in ventilated patients, whereas the more subtle changes in fMLP-responsiveness are still measurable in these infants. Alternatively, the RSV-IC patients could be characterized by an aberrant activation process of immune cells resulting in lower expression of activation markers.
Taken together, we have shown for the first time, to our knowledge, that the systemic eosinophil compartment is modulated during RSV LRTD. Peripheral blood eosinophils from infants with RSV LRTD are more highly activated than eosinophils of control patients, and this normalizes only partially during convalescence. IL-5Rα expression is down-modulated on peripheral blood eosinophils and is another indication of an eosinophil response. We speculate that this observation could also reflect relocation to the peripheral blood of pulmonary eosinophils on which IL-5Rα has been down-regulated by cytokine exposure. Our observation, that circulating eosinophils are primed, suggests the functional involvement of eosinophils during RSV LRTD.
Acknowledgments
This study was supported by a research grant of the Netherlands Asthma Foundation (project number 3·2.01·49).
References
- 1.Openshaw PJ, Culley FJ, Olszewska W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine. 2001;20(Suppl. 1):S27–31. doi: 10.1016/s0264-410x(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 2.Openshaw PJ. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am J Respir Crit Care Med. 1995;152:S59–62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 3.Chin J, Magoffin RL, Shearer LA, et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 5.Bont L, Aalderen WM, Kimpen JL. Long-term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr Respir Rev. 2000;1:221–7. doi: 10.1053/prrv.2000.0052. [DOI] [PubMed] [Google Scholar]
- 6.Garofalo R, Kimpen JL, Welliver RC, Ogra PL. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 7.Dimova-Yaneva D, Russell D, Main M, et al. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:555–8. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- 8.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 9.Boomars KA, Schweizer RC, Zanen P, et al. Eosinophil chemotactic activity in bronchoalveolar lavage from idiopathic pulmonary fibrosis is dependent on cytokine priming of eosinophils. Eur Respir J. 1998;11:1009–14. doi: 10.1183/09031936.98.11051009. [DOI] [PubMed] [Google Scholar]
- 10.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 11.Bochner BS. Systemic activation of basophils and eosinophils: markers and consequences. J Allergy Clin Immunol. 2000;106:S292–S302. doi: 10.1067/mai.2000.110164. [DOI] [PubMed] [Google Scholar]
- 12.Seely AJ, Pascual JL, Christou NV. Science review: cell membrane expression (connectivity) regulates neutrophil delivery, function and clearance. Crit Care. 2003;7:291–307. doi: 10.1186/cc1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenderman L, Kanters D, Maesen BPL, et al. Monitoring of neutrophil priming in whole blood by antibodies from asynthetic phage library. J Leukoc Biol. 2000;68:58–64. [PubMed] [Google Scholar]
- 14.Luijk B, Lindemans CA, Kanters D, et al. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol. 2005;115:997–1003. doi: 10.1016/j.jaci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Berends C, Hoekstra MO, Dijkhuizen B, et al. Expression of CD35 (CR1) and CD11b (CR3) on circulating neutrophils and eosinophils from allergic asthmatic children. Clin Exp Allergy. 1993;23:926–33. doi: 10.1111/j.1365-2222.1993.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 16.Diez-Fraile A, Meyer E, Paape MJ, Burvenich C. Analysis of selective mobilization of 1-selectin and Mac-1 reservoirs in bovine neutrophils and eosinophils. Vet Res. 2003;34:57–70. doi: 10.1051/vetres:2002053. [DOI] [PubMed] [Google Scholar]
- 17.Buyon JP, Philips MR, Merrill JT, et al. Differential phosphorylation of the beta2 integrin CD11b/CD18 in the plasma and specific granule membranes of neutrophils. J Leukoc Biol. 1997;61:313–21. doi: 10.1002/jlb.61.3.313. [DOI] [PubMed] [Google Scholar]
- 18.Graves V, Gabig T, McCarthy L, et al. Simultaneous mobilization of Mac-1 (CD11b/CD18) and formyl peptide chemoattractant receptors in human neutrophils. Blood. 1992;80:776–87. [PubMed] [Google Scholar]
- 19.Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils. I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–8. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 20.Liu LY, Sedgwick JB, Bates ME, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils. II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–66. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura-Uchiyama C, Yamaguchi M, Nagase H, et al. Changing expression of IL-3 and IL-5 receptors in cultured human eosinophils. Biochem Biophys Res Commun. 2003;309:26–31. doi: 10.1016/s0006-291x(03)01526-2. [DOI] [PubMed] [Google Scholar]
- 22.Mengelers HJ, Maikoe T, Hooibrink B, et al. Down modulation of L-selectin expression on eosinophils recovered from bronchoalveolar lavage fluid after allergen provocation. Clin Exp Allergy. 1993;23:196–04. doi: 10.1111/j.1365-2222.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 23.Mengelers HJ, Maikoe T, Brinkman L, et al. Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994;149:345–51. doi: 10.1164/ajrccm.149.2.8306028. [DOI] [PubMed] [Google Scholar]
- 24.Detmers PA, Powell DE, Walz A, et al. Differential effects of neutrophil-activating peptide 1/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J Immunol. 1991;147:4211–7. [PubMed] [Google Scholar]
- 25.Warringa RA, Mengelers HJ, Raaijmakers JA, et al. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J Allergy Clin Immunol. 1993;91:1198–205. doi: 10.1016/0091-6749(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 26.Wittmann S, Rothe G, Schmitz G, Frohlich D. Cytokine upregulation of surface antigens correlates to the priming of the neutrophil oxidative burst response. Cytometry. 2004;57A:53–62. doi: 10.1002/cyto.a.10108. [DOI] [PubMed] [Google Scholar]
- 27.Hellman C, Hallden G, Hylander B, Lundahl J. Regulation of the interleukin-5 receptor alpha-subunit on peripheral blood eosinophils from healthy subjects. Clin Exp Immunol. 2003;131:75–81. doi: 10.1046/j.1365-2249.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell DR, Carrington D. Peripheral blood lymphopenia and neutrophilia in children with severe respiratory syncytial virus disease. Pediatr Pulmonol. 2002;34:128–30. doi: 10.1002/ppul.10140. [DOI] [PubMed] [Google Scholar]
- 29.McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191:1225–32. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 30.Erjefalt JS, Greiff L, Andersson M, et al. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med. 1999;160:304–12. doi: 10.1164/ajrccm.160.1.9809048. [DOI] [PubMed] [Google Scholar]
- 31.Aalbers R, de Monchy JG, Kauffman HF, et al. Dynamics of eosinophil infiltration in the bronchial mucosa before and after the late asthmatic reaction. Eur Respir J. 1993;6:840–7. [PubMed] [Google Scholar]
- 32.Djukanovic R, Wilson JW, Britten KM, et al. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142:863–71. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- 33.Duez C, Dakhama A, Tomkinson A, et al. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–5. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Shi HZ, Xiao CQ, Li CQ, et al. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–35. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 35.Shi HZ, Humbles A, Gerard C, et al. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–53. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.in’t Veen Grootendorst DC, Bel EH, et al. CD11b and 1-selectin expression on eosinophils and neutrophils in blood and induced sputum of patients with asthma compared with normal subjects. Clin Exp Allergy. 1998;28:606–15. doi: 10.1046/j.1365-2222.1998.00279.x. [DOI] [PubMed] [Google Scholar]