Abstract
Sera from patients with primary Sjögren Syndrome (pSS) or Systemic Lupus Erythematosus (SLE) often contain autoantibodies directed against La/SSB. The sequence 349–368aa represents the major B-cell epitope of La/SSB, also it contains, at position 366, a serine aminoacid residue which constitutes the main phosphorylation site of the protein. In this study we investigated the differential recognition of the 349–368aa epitope and its phosphorylated form by antibodies found in sera from patients with systemic autoimmune diseases. Peptides corresponding to the sequence of the unphosphorylated (pep349–368aa) and the phosphorylated form (pep349–368aaPh) of the La/SSB epitope 349–368aa, as well as to a truncated form spanning the sequence 349–364aa and lacking the phosphorylation site (pep349–364aa), were synthesized. Sera from 53 patients with pSS and SLE with anti-La/SSB specificity, 30 patients with pSS and SLE without anti-La/SSB antibodies, 25 patients with rheumatoid arthritis and 32 healthy individuals were investigated by ELISA experiments. Autoantibodies to pep349–368aaPh were detected in sera of anti-La/SSB positive patients with a higher prevalence compared to the pep349–368aa (66%versus 45%). Pep349–368aaPh inhibited the antibody binding almost completely (92%), while pep349–368aa inhibited the binding only partially (45%). Anti-La/SSB antibodies presented a higher relative avidity for the phosphorylated than the unphosphorylated peptide. Immunoadsorbent experiments using the truncated peptide pep349–364aa indicated that the flowthrough showed a selective specificity for pep349–368aaPh, while the eluted antibodies reacted with both peptide analogues of the La/SSB epitope. These data suggest that sera from pSS and SLE patients with anti-La/SSB reactivity possess autoantibodies that bind more frequently and with a higher avidity to the phosphorylated major B-cell epitope of the molecule.
Keywords: B-cell epitopes, autoantibodies, La/SSB, Sjogren’s syndrome, posttranslational modifications
Introduction
Patients with primary Sjogren’s syndrome (pSS) and Systemic Lupus Erythematosus (SLE) often have autoantibodies directed against the Ro/LaRNP ribonucleoprotein complex. This consists of small cytoplasmic RNA molecules (hYRNA) in noncovalent binding with different proteins, including La/SSB, Ro52 and Ro60 kD [1,2]. Human La/SSB is a phosphoprotein consisting of 408 aminoacids, with a molecular weight ranging from 48 to 51 kD. It contains three RNA recognition motifs (RRMs), aminoacid regions that are involved in binding to RNA molecules. A nuclear localization signal (NLS), which is located at the carboxy-terminal domain (CTD) of the molecule, allows the protein to move through the nuclear membrane. The major physiological role of La/SSB in the cell is to bind newly synthesized RNA molecules, transcribed by RNA polymerase III, such as tRNA and hYRNA, protecting them from the action of 3′-exonucleases and promoting their maturation [3,4].
La/SSB is phosphorylated primarily at the serine 366 aminoacid residue (Ser366) [5]. The phosphorylation of this residue is catalysed by the Casein Kinase II (CKII) enzyme. The phosphorylation of La/SSB has been shown to affect the ability of the protein to bind the nascent RNA transcripts. More specifically, studies have shown that phosphorylation of the Ser366 decreases the ability of La/SSB to bind and subsequently protect the 5′-end of the RNA molecules [3].
Previous experiments from our laboratory defined the linear B-cell epitopes of La/SSB protein and showed that the aminoacid region 349–368aa represented the major linear B-cell epitope of the protein. It was found, firstly, that this epitope is recognized by autoantibodies in the sera of pSS patients with the highest specificity and sensitivity, secondly, that it is the end result of epitope spreading in rabbits immunized with the other La/SSB epitopes and finally, that autoantibodies against the 349–364aa are highly correlated with the HLA-DQA1*0501, the genotype associated with pSS [6–8]. This epitope also contains the aminoacid Ser366 that is primarily phosphorylated by the CKII enzyme.
In this study, we investigated whether the addition of a phosphate group to the Ser366 residue of the major B-cell epitope 349–368aa of La/SSB protein might influence its antigenicity. Compared to the unphosphorylated peptide analogue, it was found that the phosphorylated peptide was recognized more frequently and with a higher relative avidity by autoantibodies derived from sera of patients with pSS and SLE.
Materials and methods
Human sera
Sera were obtained from 53 patients with either SLE (n = 13) [9] or pSS (n = 40) [10], with anti-La/SSB autoantibodies detected by counter-immunoelectrophoresis (CIE) and/or immunoblot. Twenty-five sera from patients with rheumatoid arthritis (RA) [11], without anti-La/SSB antibodies, were used as disease controls, 30 sera from pSS and SLE patients, without anti-La/SSB and Ro/SSA antibodies, were used as autoantibody controls and 32 sera from healthy individuals were used as negative controls. All sera had been taken for diagnostic purposes with the full consent of the patients that part of the serum will be used for research purposes. Ethical approval for the study was obtained from the Scientific Committee of Laiko Hospital.
Peptide synthesis
Two synthetic peptides corresponding to the sequence of the 349–368aa epitope of human La/SSB were synthesized, utilizing the solid phase peptide synthesis (Biosynthesis Inc., Lewisville, TX, USA). The first peptide corresponded to the sequence of La/SSB NH2–349GSGKGKVQFQGKKTKFA SDD368-CONH (pep349–368aa) and the second to its phosphorylated form (pep349–368aaPh), which has a phosphate group added to the Ser366. In addition, a peptide corresponding to the truncated form of the 349–368aa epitope NH2–349GSGKGKVQFQGKKTKF364-CONH (pep349–364aa) was synthesized. This peptide is the shortest form of the epitope 349–368aa of La/SSB recognized by antibodies found in the sera of patients with pSS, and lacks the phosphorylation site Ser366 [12]. An irrelevant peptide IASRYDQL, corresponding to the sequence 250–257aa of Leismania glycoprotein gp63, was also constructed to be used as a control peptide (Ctrl-pep). All peptides were purified by High Performance Liquid Chromatography (HPLC). Peptide purity and the correct orientation of the phosphorylation were evaluated by Mass Spectra (MS) analysis.
Purification of human anti-349–364aa antibodies
Total IgG antibodies from the sera of three patients, containing autoantibodies to both the unphosphorylated and the phosphorylated forms of the La/SSB epitope, were purified by affinity chromatography using a protein-A Sepharose 4B column. IgG fractions were concentrated and dialysed against phosphate buffer saline (PBS). A specific immunoaffinity column of cyanogen bromide (CNBr) activated Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden) was generated by standard methods, using 20 mg of the synthetic pep349–364aa. Anti-349–364aa IgG antibodies were purified from the three total IgG fractions. Pep349–364aa coupled beads were preincubated overnight at +4 °C, with approximately 100 mg of total IgG. Subsequently the beads were packed in a column washed with PBS and the flowthrough (IgG not bound to the beads) collected. Bound anti-349–364aa IgG antibodies were eluted with 0·1 M HCl-Gly, pH = 2·7. The flowthrough from the first purification was applied to the column once more to ensure that the majority of the anti-349–364aa antibodies were isolated. The two flowthroughs, as well as the eluents from the two consecutive experiments were mixed together, dialysed overnight against PBS (pH = 7·4), and protein concentration was measured using the Bradford assay.
Anti-peptide ELISA assays
All sera, as well as the flowthroughs and the eluents from the affinity column, were tested by specific anti-peptide ELISA experiments developed for the detection of antibodies against the peptide analogues of the 349–368aa La/SSB epitope. Briefly, 96-well polysterene plates with a hydrophilic surface (Multisorp™, NUNC, Denmark) were coated with either pep349–368aa or pep349–368aaPh, diluted in carbonate-bicarbonate buffer, pH = 8·6, at a concentration of 5 µg/ml. Non-specific binding on the plates was blocked with a 2% w/v solution of Bovine Serum Albumin (BSA) in PBS (blocking buffer). All sera were added to the plates at a dilution of 1 : 140 in blocking buffer. The flowthroughs and the eluents were added at a concentration corresponding to the 1 : 140 dilution of the initial IgG concentration in the human serum. After a 2-h incubation time, the plates were washed three times with PBS; subsequently alkaline phosphatase-conjugated, affinity purified, anti-human IgG (Jackson Immunoresearch, West Grove, PA, USA) diluted 1 : 1400 in blocking buffer, was added. p-Nitrophenyl phosphatase disodium substrate solution (pNPP, Sigma, St Louis, MO, USA) was subsequently added and the absorbance was measured at 410 nm by an ELISA reader (Molecular Devices, Sunnyvale, CA, USA). The optimum concentration of the reagents used was selected after preliminary experiments. The results from all ELISA experiments were transformed into binding units, to enable comparisons between different ELISA experiments, according to the formula:
The cut-off point value for all the assays was set as the mean OD values obtained from the sera of 32 normal individuals plus three standard deviations (SDs).
Inhibition assays
In order to examine the specificity of the reaction of IgG with the tested peptides, inhibition ELISA experiments were performed. A number of sera (n = 3) reacting with both peptides were selected. Each serum (1 : 140 dilution) was preincubated for 2 h at room temperature with different concentrations, ranging from 1 to 50 µg/ml, of the peptides pep349–368aa, pep349–368aaPh, pep349–364aa and the Ctrl-pep and subsequently examined by ELISA on plates coated with the pep349–368aaPh peptide as previously described. Similar inhibition assays were also performed with the flowthroughs and the eluents derived from the affinity chromatography column.
Estimation of the relative avidity
To estimate the relative avidity of the antibodies against pep349–368aa and pep349–368aaPh, antibody avidity designed ELISA experiments were performed. Sera, reacting with the pep349–368aaPh, were selected and incubated on plates coated with pep349–368aa and pep349–368aaPh. The plate was then washed three times for five minutes with either PBS or PBS containing 8 M urea. Urea is a chaotropic agent used to dissociate the low avidity antibodies from their target antigen [13]. Subsequently, the plate was washed three times with PBS and the ELISA assay was performed as described above. The relative avidity index (AI) was calculated as the percentile ratio between the absorbance readings obtained when the plates were washed with 8 M urea to the absorbance readings obtained when the plates were washed with PBS.
Statistical analysis
Continuous variables were compared using Students t-test. Mean values in binding units ± SD are reported. Categorical outcomes (anti-pep349–368aa positives versus anti-pep349–368aaPh positives) were compared using the χ2 test. Level of significance was set to 0·05.
Results
Recognition of pep 349–368aa and pep349–368aaPh peptides from sera of patients
Twenty-four (45%) of 53 anti-La/SSB positive sera were found positive for antibodies against the pep349–368aa peptide of the La/SSB. The prevalence of the autoantibodies against pep349–368aaPh was 66% (35 sera of 53) (Fig. 1). All sera positive against the unphosphorylated form of the epitope were also positive for the phosphorylated one. None of the normal control sera, the autoantibody control sera (except two sera that reacted with the pep349–368aaPh), or the disease control sera was found to be positive against either peptides. The phosphorylated and the unphosphorylated peptide recognition (pep349–368aa versus pep349–368aaPh), expressed in binding units, are statistically significantly different in patients’ sera with anti-Ro/SSA and anti-La/SSB reactivity (mean values ± SD: 166 ± 124 versus 129·2 ± 120, P = 0·001), as well as in the percentage of positive outcome (66%versus 45%, P = 0·03).
Fig. 1.
Recognition of the peptide analogues of the major linear B-cell La/SSB epitope 349–368aa, by sera from patients with pSS and SLE with anti-Ro/SSA and anti-La/SSB immune reactivity (Ro/La), sera from patients with pSS and SLE without anti-La/SSB reactivity (autoantibody controls, AC), sera from healthy individuals (NORMALS), and sera from patients with RA without anti-La/SSB reactivity (disease controls, DC); (a) the unphosphorylated peptide pep349–368aa and (b) the phosphorylated analogue pep349–368aaPh. The dotted lines in the figures represent the cut-off values of the experiments, and were calculated from the mean binding unit (BU) value of the sera from healthy individuals plus three SDs (mean + 3×SD). All of the values above the cut-off limit are considered as positive values. The percentages of the positive sera against the two synthetic peptides differ significantly (P < 0·05).
Inhibition experiments using whole sera
Three selected sera were incubated with various concentrations (ranging from 1 to 50 µg/ml) of soluble peptide inhibitors and tested by ELISA on plates coated with pep349–368aaPh. It was found that the phosphorylated peptide inhibited the binding of the antibodies to the solid phase almost completely (approximately 92%), while the unphosphorylated peptide inhibited the binding only partially (up to 42–45%) (Fig. 2). In contrast, pep349–364aa and ctrl-pep did not result in a significant inhibition of the binding of the antibodies onto the ELISA plate.
Fig. 2.
Inhibition of the binding of anti-La/SSB antibodies on the ELISA plates coated with pep349–368aaPh. Three selected patients’ sera (P1, P2, P3) were incubated with various concentrations (ranging from 1 to 50 µg/ml) of the peptides: (a) pep349–368aaPh, (b) pep349–368aa, (c) pep349–364aa and (d) ctrl-pep and subsequently tested on the coated plates.
Immunoadsorbent experiments with Sepharose 4B/349–364aa
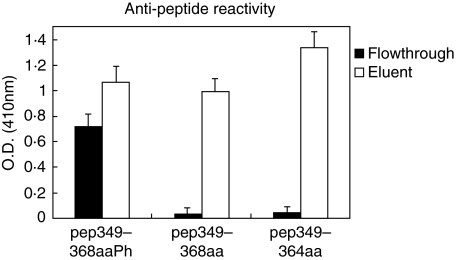
It was previously shown that the truncated peptide 349–364aa, which does not possess the Ser366 phosphorylation site, is recognized by the majority of anti-La/SSB antibodies present in patients’ sera [12]. In order therefore to examine whether the antibodies, reacting with the phosphorylated group of Ser366, constitute a distinct subpopulation different from the anti-349–364aa antibodies, affinity experiments were carried out, using a Sepharose 4B/349–364aa immunoadsorbent. Three selected sera were passed through this immunoadsorbent and the flowthroughs and eluents were tested by ELISA on plates coated with pep349–368aaPh, pep349–368aa, pep349–364aa and Ctrl-pep. It was found that the eluent presented reactivity against all peptides, except for the control peptide (Ctrl-pep) as expected. The flowthroughs of all sera showed a selective specificity against the phosphorylated peptide, while it did not react with the unphosphorylated pep349–368aa and the truncated pep349–364aa peptides (Fig. 3). All isolated antibodies did not react with the Ctrl-pep (data not shown).
Fig. 3.
Anti-peptide immune reactivity, detected by ELISA of the flowthrough and the eluent antibody fractions obtained using a Sepharose 4B/349–364aa immunoadsorbent. The specific anti-pep349–364aa antibodies (eluent) reacted with all three synthetic peptides (□). In contrast, the flowthrough (▪) reacted almost exclusively with the phosphorylated analogue pep349–368aaPh.
Inhibition experiments, performed with the flowthrough, but also with the eluent fraction showed that antibody recognition of the peptides was rather specific. Thus, the binding of the antibodies present in the flowthrough fraction, on plates coated with pep349–368aaPh was almost completely inhibited (approximately 97%) by the same peptide at a concentration of 10 µg/ml (Fig. 4). In contrast pep349–368aa inhibited only partially the antibody binding (23% at 10 µg/ml and 40% at 50 µg/ml concentration). The truncated form of La/SSB epitope 349–368aa, as well as the Ctrl-pep did not produce any significant inhibition of the antibody binding.
Fig. 4.
Inhibition experiments of the antibodies present in the flowthrough and the eluted fractions, obtained using a Sepharose 4B/349–364aa immunoadsorbent. Antibodies were tested on plates coated with the phosphorylated peptide pep349–368aaPh; (a) eluent; (b) flowthrough.
The binding of the eluted antibodies, on plates coated with pep349–368aaPh was inhibited by all peptides (pep349–364aa, pep349–368aa and pep349–368aaPh), at an inhibition range from 65% to 95%. This group of antibodies reacted with the peptide analogues of the La/SSB epitope, since all these peptides contain the aminoacid sequence 349–364aa.
Avidity experiments
The relative avidity of the antibodies found in sera from patients with anti-La/SSB immune response was evaluated by ELISA by measuring the antibody binding on plates coated with pep349–368aa and pep349–368aaPh before and after treatment with 8 M urea. Preliminary experiments using serial concentrations of urea in the dissociation buffer, ranging from 2 to 8 M, disclosed that a urea concentration of 8 M, produced the optimal discrimination results. In four of five sera examined, the relative avidity of the antibody binding to pep349–368aaPh was higher, compared to antibody binding to pep349–368aa (Fig. 5).
Fig. 5.
Estimation of the relative avidity index (AI) of the antibody binding to the two synthetic peptides pep349–368aa (▪) and pep349–368aaPh (□) using urea (8 M) disruption of the antigen-antibody bonds. Four of five tested sera bound the phosphorylated form of the La/SSB epitope (pep349–368aaPh) with higher avidity than the unphosphorylated peptide (pep349–368aa).
Discussion
Previous studies have identified that the B-cell epitopes of the La/SSB protein span the sequences 145–164aa, 289–308aa, 301–320aa and 349–368aa. The epitope 349–368aa represents the major B-cell epitope of the protein, since it is recognized with high sensitivity and specificity by the majority of anti-La/SSB positive sera [12,8].
La/SSB is a phosphoprotein phosphorylated primarily at the Ser366 aminoacid residue, which is located within the major epitope of La/SSB. This aminoacid residue, together with the three following residues, comprise a conserved aminoacid motif 366SDDE369 that represents the specific aminoacid pattern, required to be recognized by CKII. Other putative phosphorylation sites include two threonine residues, Thr302 and Thr362, and one serine, Ser325, all located in the carboxy-terminous of the autoantigen [14].
In this study, using the unphosphorylated and phosphorylated forms of the 349–368aa epitope, we showed that the latter peptide is recognized more frequently and with higher avidity by anti-La/SSB antibodies present in sera of patients with pSS or SLE compared to the unphosphorylated one. A phosphate group has a negative charge, while the 349–368aa La/SSB epitope is highly positively charged (pI = 10·4). The incorporation of a negatively charged group into a positively charged protein region would be expected to alter the local tertiary structure via ion–ion interactions [15–17], affecting eventually anti-La/SSB antibody binding. The inhibition experiments showed that pep349–368aaPh inhibited almost completely the binding of antibodies onto the ELISA plate, coated with pep349–368aaPh, whereas the unphosphorylated analogue inhibited this reaction only partially. When the truncated form (pep349–364aa) of the epitope, lacking the Ser366 phosphorylation site, and the irrelevant peptide were used as inhibitors no significant inhibition was observed. These data suggest that anti-La/SSB positive sera contain autoantibodies binding specifically the region 365–368aa of the epitope, which contains the phosphorylated Ser366 residue. Previous studies have demonstrated the existence of autoantibodies against the truncated sequence 349–364aa of La/SSB protein [12]. Therefore, we purified antibodies against the 349–364aa region by affinity chromatography. The absorbed and eluted antibodies and the flowthrough (the fraction of IgG that did not recognize and attach to pep349–364aa) were tested for reactivity against all the synthesized peptide analogues. It was found that the flowthrough reacted only against the pep349–368aaPh peptide. In contrast the eluent IgG from the column reacted against all three peptide analogues of the 349–368aa epitope of La/SSB, since it is directed against a common aminoacid region (349–364aa) present in all the synthetic peptides. The inhibition experiments confirmed the specificity of the immune reactivity of each antibody cluster, obtained from the affinity chromatography. These experiments disclosed two distinct antibody subpopulations reactive against different regions of the same epitope, one targeting specifically the region that is phosphorylated by the CKII (365–368aa) and the other targeting the remainder of the epitope sequence (349–364aa). Interestingly, the inhibition experiments revealed that the antibodies against the 365–368aa region of the epitope bound more strongly to the phosphorylated epitope (pep349–368aaPh) that the unphosphorylated one (pep349–368aa). This was confirmed from the urea antigen-antibody dissociation experiments, where the autoantibodies from the autoimmune sera, reactive against the La/SSB epitope, bound the phosphorylated form with higher avidity than the unphosphorylated form of the epitope, since they resisted the urea disruption of their binding to the peptide target.
Several studies have implicated the post-translational modifications (PTMs), as possible triggering factors for the breakdown of the immune tolerance against self proteins [18–20]. According to this hypothesis, new potentially antigenic epitopes (neoepitopes) are created and presented to the immune system [21]. Furthermore, preexisting epitopes hidden from the immune system (cryptotopes) may become available to the immunological cells through the disruption of the tertiary structure, by post-translational modifications of the target protein.
Common modifications include proteolytic cleavage of proteins from virus encoded proteases [22], or by caspases during apoptosis, producing novel autoantigen forms [23–25]. In addition, proteins are phosphorylated and dephosphorylated during apoptosis [26,27]. Since many of the modified autoantigens, are sequestered in apoptotic blebs of the cell [28], it is believed that they constitute putative sources of neoepitopes or cryptotopes [20].
Previous experiments had demonstrated that adenovirus infection increases both the extend of phosphorylation and the antigenicity of La/SSB protein, as well as it induces the translocation of the protein to the cell membrane [29,30]. It is still unknown if these changes involve the epitope 349–368aa of the protein. Adenovirus infected HeLa cells have also been used as antigen source for the detection of anti-La/SSB antibodies in the sera from patients with systemic autoimmune diseases. Slobbe et al.[31] showed that the use of lysates from these infected cells can improve the sensitivity of the anti-La/SSB antibody detection, compared with other commonly used immunological assays, such as CIE. The factors that are involved in this enhanced recognition of La/SSB antigen are still unknown.
Previous studies in corneal epithelial cells of pSS have shown that La/SSB is redistributed and localized on the cellular membranes [32]. Moreover, in the pathological lesion of pSS, affected epithelial cells have been found to present increased levels of La/SSB mRNA [33] and the B-cells contain intracytoplasmic immunoglobulins with anti-Ro/SSA and/or anti-La/SSB reactivity [34]. Taken together it appears that the affected salivary glands in pSS constitue an active site for anti-La/SSB response. The activated phenotype of the epithelial cells of exocrine glands in pSS [35] has suggested that they are infected by a virus. We recently presented evidence that coxsackievirus may persistently infect the salivary glands of pSS patients. We hypothesize that the coxsackieviruses may play a permissive role for the perpetuation and possibly the induction of the autoimmune disease in pSS [36].
Until now little is known about the possible effect of the phosphorylation status of La/SSB on its antigenicity. Phosphorylation of Ser366 has previously been reported to have multiple effects on the function of the protein. Firstly, it diminishes the ability of the protein to protect the nascent transcripts of the RNApolIII and alters its interactions with several viral RNAs [37,38]. The second effect is a change in the distribution of the protein within the cell. However, Broekhuis et al. [14] have reported that phosphorylation of La/SSB does not affect the distribution of the protein in the nucleus and cytoplasm. However, it has been found that nonphosphorylated human La/SSB is concentrated in nucleolar sites, where it interacts with a protein called nucleolin, while the phosphorylated form of the protein resides in the nucleoplasm [39].
Taken together, conditions, such as virus infection of epithelial cells, can increase the phosphorylation rate of the La/SSB protein, enchancing its recognition by autoantibodies. Moreover, a new epitope adjacent to the Ser366 residue of the autoantigen is formed upon phosphorylation of the protein (neoepitope at 349–368aa), with the potential to interact specifically with a subpopulation of anti-La/SSB antibodies. Our observation, of the increased antibody recognition of the phosphorylated major B-cell epitope of the La/SSB protein, may lead to the development of new improved diagnostic ELISA assays for the detection of anti-La/SSB antibodies. The recombinant protein that is used lacks post-translational modifications such as phosphorylation. In this case, it is likely that these assays do not detect antibodies that are directed against the chemically modified epitopes, such as the anti-pep349–368aaPh antibodies, the existence of which we demonstrated in this study. Thus, the use of the phosphorylated protein or the phosphorylated epitope as the coating antigen in the diagnostic ELISA assays will have an advantage in the detection of more anti-La/SSB antibodies, compared to the unmodified antigen.
Acknowledgments
This work has been supported by a grant (PENED-01ED164) from the Hellenic Secretariat for Research and Technology.
References
- 1.Harley JB, Alexander EL, Bias WB, Fox OF, Provost TT, Reichlin M, Yamagata H, Arnett FC. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjogren’s syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 2.Fabini G, Rutjes SA, Zimmermann C, Pruijn GJ, Steiner G. Analysis of the molecular composition of Ro ribonucleoprotein complexes. Identification of novel Y RNA-binding proteins. Eur J Biochem. 2000;267:2778–89. doi: 10.1046/j.1432-1327.2000.01298.x. [DOI] [PubMed] [Google Scholar]
- 3.Fan H, Goodier JL, Chamberlain JR, Engelke DR, Maraia RJ. 5′ processing of tRNA precursors can be modulated by the human La antigen phosphoprotein. Mol Cell Biol. 1998;18:3201–11. doi: 10.1128/mcb.18.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufel J, Allmang C, Chanfreau G, Petfalski E, Lafontaine DL, Tollervey D. Precursors to the U3 small nucleolar RNA lack small nucleolar RNP proteins but are stabilized by La binding. Mol Cell Biol. 2000;20:5415–24. doi: 10.1128/mcb.20.15.5415-5424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan H, Sakulich AL, Goodier JL, Zhang X, Qin J, Maraia RJ. Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell. 1997;88:707–15. doi: 10.1016/s0092-8674(00)81913-3. [DOI] [PubMed] [Google Scholar]
- 6.Tzioufas AG, Wassmuth R, Dafni UG, et al. Clinical, immunological, and immunogenetic aspects of autoantibody production against Ro/SSA, La/SSB and their linear epitopes in primary Sjogren’s syndrome (pSS): a European multicentre study. Ann Rheum Dis. 2002;61:398–404. doi: 10.1136/ard.61.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiannaki E, Vlachoyiannopoulos PG, Manoussakis MN, Sakarellos C, Sakarellos-Daitsiotis M, Moutsopoulos HM, Tzioufas AG. Study of antibody and T cell responses in rabbits immunized with synthetic human B cell epitope analogues of La (SSB) autoantigen. Clin Exp Immunol. 2000;121:551–6. doi: 10.1046/j.1365-2249.2000.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yiannaki EE, Tzioufas AG, Bachmann M, Hantoumi J, Tsikaris V, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin Exp Immunol. 1998;112:152–8. doi: 10.1046/j.1365-2249.1998.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Tzioufas AG, Yiannaki E, Sakarellos-Daitsiotis M, Routsias JG, Sakarellos C, Moutsopoulos HM. Fine specificity of autoantibodies to La/SSB. epitope mapping, and characterization. Clin Exp Immunol. 1997;108:191–8. doi: 10.1046/j.1365-2249.1997.d01-1003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn NK, Besselaar TG, Schoub BD, O’Connell KF. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J Med Virol. 1991;33:6–9. doi: 10.1002/jmv.1890330103. [DOI] [PubMed] [Google Scholar]
- 14.Broekhuis CH, Neubauer G, van der Heijden A, Mann M, Proud CG, van Venrooij WJ, Pruijn GJ. Detailed analysis of the phosphorylation of the human La (SS-B) autoantigen. (De) phosphorylation does not affect its subcellular distribution. Biochemistry. 2000;39:3023–33. doi: 10.1021/bi992308c. [DOI] [PubMed] [Google Scholar]
- 15.Gelsthorpe M, Pulumati M, McCallum C, Dang-Vu K, Tsubota SI. The putative cell cycle gene, enhancer of rudimentary, encodes a highly conserved protein found in plants and animals. Gene. 1997;186:189–95. doi: 10.1016/s0378-1119(96)00701-9. [DOI] [PubMed] [Google Scholar]
- 16.Milani M, Leoni L, Rampioni G, Zennaro E, Ascenzi P, Bolognesi M. An active-like structure in the unphosphorylated StyR response regulator suggests a phosphorylation-dependent allosteric activation mechanism. Structure (Camb) 2005;13:1289–97. doi: 10.1016/j.str.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Wan C, Tempel W, Liu ZJ, Wang BC, Rose RB. Structure of the conserved transcriptional repressor enhancer of rudimentary homolog. Biochemistry. 2005;44:5017–23. doi: 10.1021/bi047785w. [DOI] [PubMed] [Google Scholar]
- 18.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–9. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 19.Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjogren’s syndrome: implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004;15:156–64. doi: 10.1177/154411130401500304. [DOI] [PubMed] [Google Scholar]
- 20.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–54. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neugebauer KM, Merrill JT, Wener MH, Lahita RG, Roth MB. SR proteins are autoantigens in patients with systemic lupus erythematosus. Importance of phosphoepitopes. Arthritis Rheum. 2000;43:1768–78. doi: 10.1002/1529-0131(200008)43:8<1768::AID-ANR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Tsubota K, Ono M, et al. Possible involvement of EBV-mediated alpha-fodrin cleavage for organ-specific autoantigen in Sjogren’s syndrome. J Immunol. 2001;166:5801–9. doi: 10.4049/jimmunol.166.9.5801. [DOI] [PubMed] [Google Scholar]
- 23.Bockenstedt LK, Gee RJ, Mamula MJ. Self-peptides in the initiation of lupus autoimmunity. J Immunol. 1995;154:3516–24. [PubMed] [Google Scholar]
- 24.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 26.Rutjes SA, Utz PJ, van der Heijden A, Broekhuis C, van Venrooij WJ, Pruijn GJ. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 1999;6:976–86. doi: 10.1038/sj.cdd.4400571. [DOI] [PubMed] [Google Scholar]
- 27.Utz PJ, Hottelet M, van Venrooij WJ, Anderson P. Association of phosphorylated serine/arginine (SR) splicing factors with the U1-small ribonucleoprotein (snRNP) autoantigen complex accompanies apoptotic cell death. J Exp Med. 1998;187:547–60. doi: 10.1084/jem.187.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizer LI, Deng JS, Stenberg RM, Tan EM. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983;3:1235–45. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin Exp Immunol. 1989;78:454–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Slobbe R, Van Esch B, Kveder T, Van Venrooij WJ. The use of adenovirus-infected HeLa cells for the detection of low titer autoantibodies. J Immunol Meth. 1991;138:237–44. doi: 10.1016/0022-1759(91)90172-c. [DOI] [PubMed] [Google Scholar]
- 32.Yannopoulos DI, Roncin S, Lamour A, Pennec YL, Moutsopoulos HM, Youinou P. Conjunctival epithelial cells from patients with Sjogren’s syndrome inappropriately express major histocompatibility complex molecules, La (SSB) antigen, and heat-shock proteins. J Clin Immunol. 1992;12:259–65. doi: 10.1007/BF00918149. [DOI] [PubMed] [Google Scholar]
- 33.Tzioufas AG, Hantoumi I, Polihronis M, Xanthou G, Moutsopoulos HM. Autoantibodies to La/SSB in patients with primary Sjogren’s syndrome (pSS) are associated with upregulation of La/SSB mRNA in minor salivary gland biopsies (MSGs) J Autoimmun. 1999;13:429–34. doi: 10.1006/jaut.1999.0333. [DOI] [PubMed] [Google Scholar]
- 34.Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren’s syndrome. Arthritis Rheum. 1998;41:2238–48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Tapinos NI, Polihronis M, Tzioufas AG, Moutsopoulos HM. Sjogren’s syndrome. Autoimmune epithelitis. Adv Exp Med Biol. 1999;455:127–34. [PubMed] [Google Scholar]
- 36.Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsackievirus infection in primary Sjogren’s syndrome. Arthritis Rheum. 2004;50:2897–902. doi: 10.1002/art.20463. [DOI] [PubMed] [Google Scholar]
- 37.Ehlers I, Horke S, Reumann K, Rang A, Grosse F, Will H, Heise T. Functional characterization of the interaction between human La and hepatitis B virus RNA. J Biol Chem. 2004;279:43437–47. doi: 10.1074/jbc.M402227200. [DOI] [PubMed] [Google Scholar]
- 38.Heise T, Guidotti LG, Chisari FV. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J Virol. 1999;73:5767–76. doi: 10.1128/jvi.73.7.5767-5776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Intine RV, Dundr M, Vassilev A, Schwartz E, Zhao Y, Zhao Y, Depamphilis ML, Maraia RJ. Nonphosphorylated human La antigen interacts with nucleolin at nucleolar sites involved in rRNA biogenesis. Mol Cell Biol. 2004;24:10894–904. doi: 10.1128/MCB.24.24.10894-10904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]