Abstract
Inadequate apoptosis may contribute to the synovial hyperplasia associated with rheumatoid arthritis (RA). The Fas-associated death domain protein (FADD)-like interleukin (IL)-1β-converting enzyme (FLICE)-inhibitory protein (FLIP), which is an apoptotic inhibitor, has been implicated in the resistance to Fas-mediated apoptosis of synoviocytes. This study investigated whether hydroxychloroquine (HCQ), an anti-rheumatic drug, induces the apoptosis of rheumatoid synoviocytes, and modulates the expression of FLIP. Fibroblast-like synoviocytes (FLS) were prepared from the synovial tissues of RA patients, and were cultured with various concentrations of HCQ in the presence or absence of the IgM anti-Fas monoclonal antibodies (mAb) (CH11). Treatment with HCQ, ranging from 1 to 100 µM, induced the apoptosis of FLS in a dose- and time-dependent manner. The increase in synoviocytes apoptosis by HCQ was associated with caspase-3 activation. A combined treatment of HCQ and anti-Fas mAb increased FLS apoptosis and caspase-3 activity synergistically, compared with either anti-Fas mAb or HCQ alone. The Fas expression level in the FLS was not increased by the HCQ treatment, while the FLIP mRNA and protein levels were decreased rapidly by the HCQ treatment. Moreover, time kinetics analysis revealed that the decreased expression of FLIP by HCQ preceded the apoptotic event that was triggered by HCQ plus anti-Fas mAb. Taken together, HCQ increases the apoptosis of rheumatoid synoviocytes by activating caspase-3, and also sensitizes rheumatoid synoviocytes to Fas-mediated apoptosis. Our data suggest that HCQ may exert its anti-rheumatic effect in rheumatoid joints through these mechanisms.
Keywords: apoptosis, Fas, FLIP, hydroxychloroquine, synoviocyte
Introduction
Rheumatoid arthritis (RA) is characterized by a tumour-like expansion of the synovium, inflammatory infiltrates and progressive destruction of the cartilage and bone [1]. In the rheumatoid synovium, both the synovial fibroblasts and mononuclear cells express the functional Fas antigen, and these cells can undergo apoptosis by anti-Fas monoclonal antibody (mAb) [2,3]. In animal models of RA, the active induction of apoptosis in the rheumatoid synovium by anti-Fas mAb or Fas ligand (FasL) gene transfer improves the arthritis due to the elimination of both the proliferating synoviocytes and infiltrating lymphocytes in the inflamed synovium [4,5]. During Fas-mediated apoptosis of RA synoviocytes, the Fas-associated death domain protein (FADD) is recruited selectively to the Fas death domain [6], which suggests that the sensitivity to Fas-mediated apoptosis in synoviocytes may be regulated by the recruitment of FADD to the Fas death domain, resulting in the formation of a death-inducing signalling complex (DISC).
The FADD-like interleukin (IL)-1β-converting enzyme (FLICE) inhibitory protein, FLIP (also known as FLAME-1, a caspase-8 inhibitory molecule), has been identified as a regulator of caspase-8 activation at the DISC [7]. It contains a caspase-like domain that shares significant homology with caspase 8 [8]. FLIP interacts with the adaptor protein FADD and the protease FLICE, and inhibits potently the apoptosis induced by the human death receptors [7,8]. FLIP is expressed during the early stage of T cell activation, but disappears when the T cells become susceptible to Fas ligand-induced apoptosis [7]. The expression of FLIP in macrophages confers resistance to Fas-mediated apoptosis [9], which may contribute to the development of inflammatory disease. In RA patients FLIP is expressed strongly in the synovium, particularly at the sites of cartilage invasion and bone destruction [10], suggesting that it contributes to joint destruction.
Hydroxychloroquine (HCQ) has been used widely to treat RA for more than a century. Similar to most anti-rheumatic drugs, HCQ has a wide range of actions. It interferes with the cellular function in the compartments with an acidic microenvironment, such as the lysosomes [11]. This may have different effects on the cellular function, including the inhibition of intracellular processing and protein secretion, the interference of autoantibody production, decreased lymphocyte proliferation and decreased cytokine production, such as tumour necrosis factor (TNF)-α[12]. Recently, it has been observed that HCQ induces apoptosis in several cell types. It induces apoptosis in peripheral blood T lymphocyte through caspase cascades [13]. The apoptosis of leukaemic cells is also induced by HCQ, which is associated with the activation of caspase-3 and the modulation of bcl-2/bax ratio [14]. However, there is no information on the effect of HCQ on synoviocyte apoptosis in RA. In this study, we investigated whether HCQ induces the apoptosis of rheumatoid synoviocytes, and whether it modulates the Fas-mediated apoptosis and the expression of FLIP, the apoptosis inhibitor.
Materials and methods
Isolation and culture of RA synoviocytes
The fibroblast-like synoviocytes (FLS) were prepared from the synovial tissues of six RA patients, who had undergone total joint replacement surgery. The isolation of the FLS from the synovial tissues was performed according to a procedure described elsewhere [15]. Briefly, the tissues were minced into 2–3-mm pieces, and treated for 4 h with 4 mg/ml of type I collagenase (Worthington Biochemical, Freehold, NJ, USA) in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C in a 5% CO2 atmosphere. The dissociated cells were then resuspended in DMEM, supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, penicillin and streptomycin, and then plated in 75 cm2 flasks. After culturing overnight, the non-adherent cells were removed and the adherent cells were cultivated in DMEM plus 10% FCS. The cultures were kept at 37°C in a 5% CO2 atmosphere, and the medium was replaced every 3 days. At confluence, the cells were passed by diluting them 1 : 3 with fresh medium and recultured until used. The synoviocytes, from passages 3–8, were used for each experiment. The cells were morphologically homogeneous and had the appearance of FLS, with a typical bipolar configuration under inverse microscopy. The purity of the cells was examined by flow cytometry analysis (> 95% CD90, < 2% CD14, < 1% CD3 and < 1% CD19 positive).
The FLS were seeded in 24-well plates (Nunc, Roskilde, Denmark) at 3 × 104 cells per well or in a 100 mm culture dish at 5 × 105 cells in 1 ml DMEM/5% FCS, supplemented with 5% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM l-glutamine, and incubated at 37°C in the presence of various HCQ concentrations (kindly provided by Kyung-Poong Pharmaceuticals, Seoul, Korea) ranging from 1 to 100 µM. In some experiments, the FLS were cultured with HCQ in the presence of anti-Fas mAb IgM (CH11; Immnotech, Marseille, France) in order to determine the effect of HCQ on Fas-mediated apoptosis of FLS. Caspase activation was inhibited by preincubating the cells with caspase inhibitors, Z-VAD-FMK (R&D, Minneapolis, MN, USA) and Z-DEVD-FMK (R&D) 30 min before adding the HCQ or HCQ plus anti-Fas mAb. All the cultures were performed in either duplicate or triplicate.
Apoptosis assay
The FLS (3 × 104 cells) undergoing apoptosis were assessed by the level of cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA). The cellular DNA fragmentation ELISA kit (Roche Applied Science, Indianapolis, IN, USA) is based on the quantitative sandwich ELISA principle using two mouse mAbs directed against the DNA and BrdU. Briefly, an anti-DNA antibody was fixed in the wells of a microtitre plate. The BrdU-labelled DNA fragments contained in the sample were bound to the immobilized anti-DNA antibody. The immune-complexed BrdU-labelled DNA fragments were denatured and fixed on the surface of the plate by microwave irradiation. In the final step, the anti-BrdU peroxidase conjugate was reacted with the BrdU incorporated into the DNA. After removing the unbound peroxidase conjugates, the quantity of peroxidase bound in the immune complex was determined photometrically with tetramethylbenzidine (TMB) as a substrate.
Analysis of caspase activity
The enzymatic activity of caspase-3 was determined using the apotarget caspase-3/cpp32/colourimetric protease assay kit (Biosource, Camarillo, CA, USA), as suggested by the manufacturer. Briefly, 2 × 106 cells were resuspended in 50 µl of lysis buffer and reaction buffer, and a chromogenic cpp32 substrate DEVD-p-nitroanilide (DEVD-pNA) was added. Reactions were incubated at 37°C for 1 h and samples were measured at 405 nm. Fold increase in caspase-3 activity was determined by direct comparison with the level of untreated cells. In some experiments, caspase-8 activity in FLS was also determined by protease assay kit (Biosource).
Flow cytometry for the determination of Fas expression on FLS
After treatment of FLS (5 × 105 cells) with various concentrations of HCQ for 12 h, the cells were harvested, incubated for 20 min on ice in a blocking buffer [phosphate buffered saline (PBS) with 3% FCS] and 0·02% 1 M sodium azide), and subsequently stained for 30 min on ice with phycoerythrin (PE)-conjugated mouse anti-human CD95 antibody (Pharmingen, San Diego, CA, USA), which is specific against Fas. PE-conjugated mouse IgG1 (Pharmingen) was used as the isotype control antibody. The cells were washed and resuspended twice in a staining buffer (PBS containing 3% FCS and 0·02% 1 M sodium azide), and analysed on a fluorescence activated cell sorter (FACScan) cytometer (Becton Dickinson, Mountain View, CA, USA). At least 5000 events were acquired from each sample and subsequently analysed using Lysis II and cellQuest software (Becton Dickinson).
RNA isolation and semiquantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis for FLIPL
The FLS (5 × 105 cells) were incubated with various concentrations of HCQ (0–100 µM) and analysed for FLIPL (the long form of FLIP) mRNA expression by semiquantitative RT–PCR. Briefly, after culture for 4 h, the mRNA was extracted using RNAzol B according to the manufacturer’s instruction (Biotec Laboratories, Houston, TX, USA). The RNA was converted to cDNA using SuperscriptII RT (Gibco brl, Gaithersburg, MD, USA), 10 mM dNTP, 0·1 M DTT, RNase inhibitor (Rnasin, Toyobo, Osaka, Japan) and random hexamer oligonucleotide priming (GibcoBRL). The PCR amplification of the cDNA aliquots was performed by adding 2·5 mM deoxyribonucleoside triphosphate (dNTPs), 2·5 U Taq DNA polymerase (Boehringer, Mannheim, Germany) and 0·25 µM each of the sense and anti-sense primers. The reaction was performed in a PCR buffer (1·5 mM MgCl2, 50 mM KCl, 10 mM Tris HCl, pH 8·3) in a total volume of 25 µl. The following sense and anti-sense primers for FLIPL and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) were used (all written in 5′→3′ direction): FLIPL sense GTTAGGTAGCCAGTTGG, anti-sense CCTGCCT TGCTTCAGC; GAPDH sense CGATGCTGGGCGTGAG TAC, GAPDH anti-sense CGTTCAGTCCAGGGATGACC. The reactions were processed in a DNA thermal cycler (Hybaid, Teddington, UK). The cycling conditions were as follows: 1 min denaturation at 94°C for FLIPL, 30 s denaturation at 94°C for GAPDH; 1 min annealing at 56°C for FLIPL and at 55°C for GAPDH; and 1 min elongation at 72°C. The PCR rounds were repeated for 30 cycles for FLIPL and 25 cycles for GAPDH, which had been determined to fall within the exponential phase of amplification for each molecule. The PCR products were run on a 1·5% agarose gel and stained with ethidium bromide. The mRNA expression level is presented as a ratio of the cytokine product over the GAPDH product.
Western blotting analysis for FLIP protein
The FLS (5 × 105 cells) were treated with 100 µM of HCQ for a different culture time. The total cellular protein extracts were obtained by washing the cells twice in PBS and resuspending them in a lysis buffer (0·5% Triton X-100, 300 mM NaCl, 50 mM Tris HCl, pH 7·6, containing 1 mM phenylmethylsulphonyl fluoride, 2 µg/ml aprotinin and 10 µg/ml leupeptin). The cells were kept on ice for 30 min and then centrifuged at 10 000 g for 10 min. The amount of the cellular protein present in the clarified supernatant was evaluated using a Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of the cellular protein (20 µg) from each sample were then separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membranes (Amersham Pharmacia, Roosendaal, the Netherlands). The blots were hybridized with rat anti-human FLIP mAb (clone Dave 3, recognizing both FLIPL and FLIPS isoforms; Alexis Biochemicals, Lausanne, Switzerland) or mouse anti-human β-actin mAb (Sigma), followed by horseradish peroxidase-conjugated anti-rat IgG (Alexis Biochemicals) or anti-mouse IgG (Amersham Biosciences, Little Chalfont, UK). The proteins were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA) and exposed to X-ray films (Hyperfilm ECL, Amersham Biosciences) according to the manufacturer’s instructions.
Statistical analysis
The data are expressed as mean ± standard deviation (s.d.). Comparisons of the numerical data between the groups were performed by using a Mann–Whitney U-test. P-values less than 0·05 were considered statistically significant.
Results
Induction of apoptosis by HCQ in rheumatoid synoviocytes
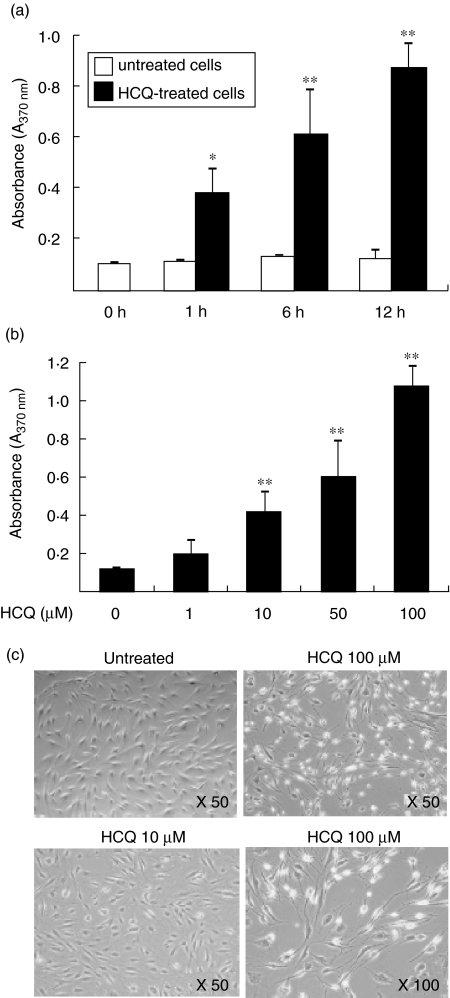
As shown in Fig. 1a, a rapid increase in apoptosis of FLS was observed as early as 1 h after treatment with 100 µM of HCQ, as determined by cellular DNA fragmentation ELISA. The percentage of apoptotic cells was time-dependently increased after the HCQ treatment, compared with the level in the untreated cells. The HCQ-induced increase in the synoviocyte apoptosis was dose-dependent, and the maximum increase was 8·4-fold over the spontaneous levels, as determined 12 h after the treatment with 100 µM of HCQ (Fig. 1b). In contrast, another immunosuppressive agent cyclosporin, ranging from 40 to 4000 nM, failed to increase the degree of apoptosis of RA synoviocytes (data not shown). The dose-dependent induction of apoptosis by HCQ was also evident on phase-contrast microscopy (Fig. 1c). The HCQ-treated FLS became spherical, shrunk and detached from the bottom of the culture plates, which is in contrast to the appearance of the untreated cells with a typical bipolar and attached configuration.
Fig. 1.
Hydroxychloroquine (HCQ) increases the apoptosis of synovial fibroblasts from rheumatoid arthritis (RA) patients. Fibroblast-like synoviocytes (FLS) were cultured with 1–100 µM of HCQ in duplicate. The cells undergoing apoptosis were assessed by cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA). (a) Time-dependent increase in the apoptosis of FLS treated with 100 µM of HCQ. (b) Dose-dependent increase in the apoptosis of FLS cultured with 1–100 µM of HCQ for 12 h. Data are expressed as the mean ± s.d. of six independent experiments. *P < 0·05; **P < 0·01 versus the untreated cells. (c) Phase-contrast microscopy of FLS apoptosis induced by a treatment with HCQ. RA synovial fibroblasts were cultured with 10 or 100 µM of HCQ for 12 h, and the cytolytic activity was observed under phase-contrast microscopy.
HCQ-induced synoviocytes apoptosis is caspase-3 dependent
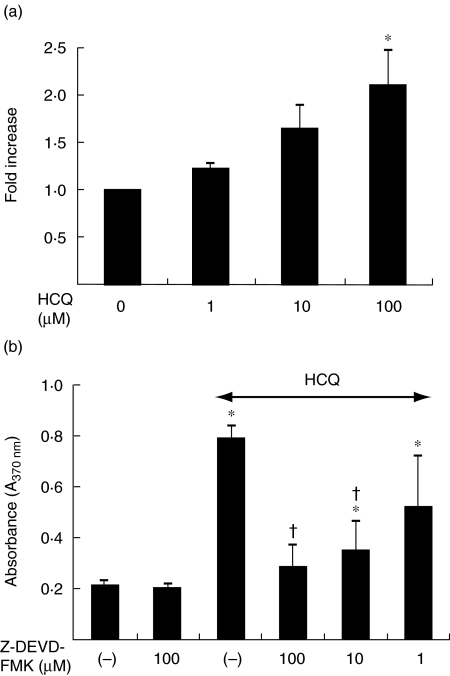
It has been reported that HCQ activates caspase-3 in some cell types [14,16], whereas it induces the apoptosis of HL-60 cells independently of caspase-3 [17]. Therefore, we next examined whether HCQ-induced apoptosis of FLS is dependent or independent on the casepase-3 activity. As shown in Fig. 2a, caspase-3 protease activity was increased dose-dependently following treatment with HCQ, which is in parallel with the data from DNA fragmentation ELISA. Moreover, pretreatment of the FLS with a caspase-3 inhibitor, Z-DEVD-FMK, prevented the apoptosis in response to 100 µM of HCQ (Fig. 2b), suggesting that HCQ increased apoptosis of FLS through the activation of capase-3. However, the activity of caspase-8, a critical enzyme for Fas-mediated apoptosis, was not changed by treating cells with HCQ (1–100 µM) for 12 h (data not shown).
Fig. 2.
Hydroxychloroquine (HCQ) induces the synoviocytes apoptosis through the activation of caspase-3. (a) Increase in caspase-3 activity by HCQ. The fibroblast-like synoviocytes (FLS) were treated with various concentrations of HCQ for 12 h, and then examined for the enzymatic activity of caspase-3 by the colourimetric assay. Data are the mean ± s.d. of four independent experiments, presented as fold increase relative to the optical density of untreated cells. *P < 0·05 versus untreated cells. (b) Effect of a pretreatment with a caspase-3 inhibitor, Z-DEVD-FMK on the FLS apoptosis induced by 100 µM of HCQ. Data represented the mean ± s.d. of four independent experiments. *P < 0·05 versus untreated cells. †P < 0·05 versus cells treated with HCQ 100 µM.
HCQ synergistically increases Fas-mediated apoptosis of synoviocyte
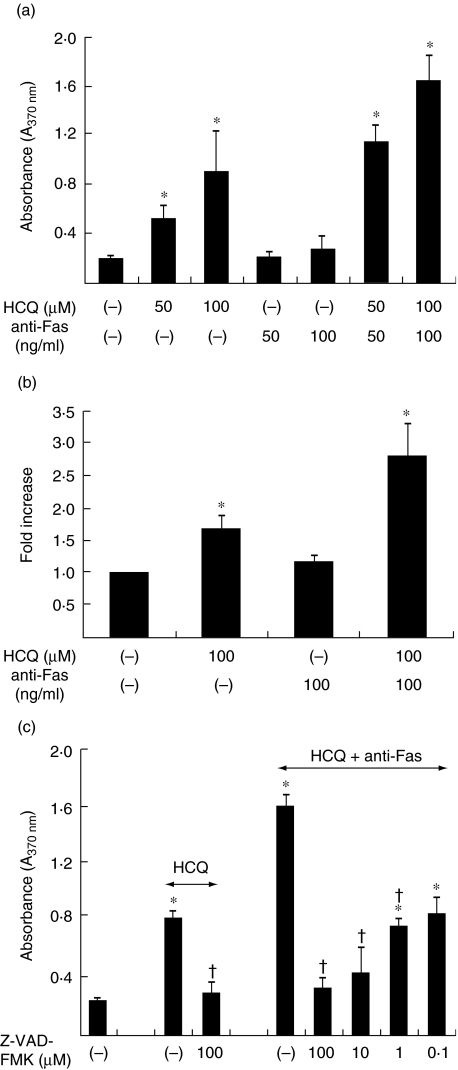
The Fas antigen (CD95) is a representative molecule that transmits the apoptotic signal to several types of cells. It has been documented that the FLS express the functional Fas antigen on their surface and undergo apoptosis that is mediated by IgM anti-Fas mAb in vitro[2]. Our next goal was to determine if HCQ could affect the sensitivity of Fas-mediated apoptosis in FLS. As shown in Fig. 3a, the combined treatment of HCQ and IgM anti-Fas mAb enhanced synergistically the degree of FLS apoptosis, compared with either anti-Fas mAb or HCQ alone. For example, when compared with the spontaneous level, the level of apoptosis of the FLS was increased 1·2-fold by 50 ng/ml of anti-Fas mAb, 2·7-fold by 50 µM of HCQ and 5·8-fold by anti-Fas mAb (50 ng/ml) plus HCQ (50 µM). The caspase-3 activity was also increased synergistically by anti-Fas mAb plus HCQ (Fig. 3b). In addition, pretreatment of the FLS with a caspase inhibitor, Z-VAD-FMK (pan-caspase inhibitor), prevented apoptosis in response to either 100 µM of HCQ alone or anti-Fas mAb (100 ng/ml) plus HCQ (100 µM) (Fig. 3c). The caspase-3 inhibitor, Z-DEVD-FMK, showed a similar pattern (data not shown). Taken together, these results indicate that HCQ may sensitize the FLS to Fas-mediated apoptosis via a caspase-3-dependent pathway.
Fig. 3.
Hydroxychloroquine (HCQ) synergistically increases the Fas-mediated apoptosis of synoviocyte. (a) Apoptosis induction by the combined treatment with IgM anti-Fas monoclonal antibody (mAb) and HCQ. Synovial fibroblasts of the rheumatoid arthritis (RA) patients were incubated for 12 h with anti-Fas mAb (50 or 100 ng/ml) (CH11) in the absence or presence of HCQ (50 or 100 µM). The degree of apoptosis was assessed by cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA). Data are presented as the mean ± s.d. of four independent experiments. *P < 0·05 versus untreated cells. (b) Synergistic increase in caspase-3 activity by the treatment with HCQ plus anti-Fas mAb. The fibroblast-like synoviocytes (FLS) were treated with IgM anti-Fas mAb (100 ng/ml) in the absence or presence of HCQ (100 µM) for 12 h, and then examined for the enzymatic activity of caspase-3 by the colourimetric assay. Data are the mean ± s.d. of two independent experiments in triplicate, presented as fold increase relative to the optical density of untreated cells. *P < 0·05 versus untreated cells. (c) Effect of a pretreatment with a caspase inhibitor, Z-VAD-FMK on the FLS apoptosis induced by 100 µM of HCQ alone or HCQ (100 µM) plus anti-Fas mAb (100 ng/ml). Data are presented as the mean ± s.d. of four independent experiments. *P < 0·05 versus untreated cells, †P < 0·05 versus cells treated with HCQ or HCQ plus anti-Fas IgM.
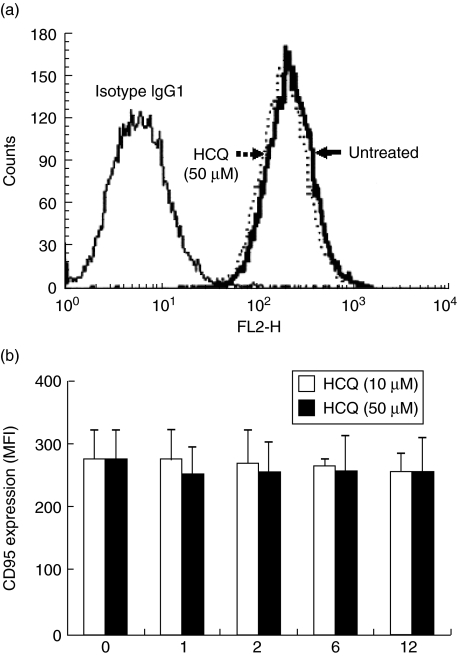
HCQ down-regulates FLIP expression in synoviocytes
Down-regulation of FLIP sensitizes the FLS to Fas-mediated apoptosis [18]. Based on the knowledge that HCQ increases synergistically Fas-mediated apoptosis of the synoviocytes, two possibilities for the mechanism of this synergism were examined. First, HCQ might affect Fas expression on the FLS. Secondly, HCQ could potentiate the Fas-mediated signalling pathway. The results showed that Fas expression, as determined by flow cytometry, was almost unaffected by any HCQ concentration tested (1–50 µM) (Fig. 4a,b). Indeed, the Fas expression level was decreased slightly by the treatment of FLS with 100 µM of HCQ for 12 h, down to approximately 75% of the basal expression level (data not shown). This suggests that the activation of the intracellular signalling pathway, rather than an alteration of the Fas expression level, may be the major mechanism responsible for the increased susceptibility to Fas ligation. Therefore, an experiment was conducted to determine the effect of HCQ on the expression of FLIP, which is an inhibitory signalling molecule of Fas-mediated apoptosis. As shown in Fig. 5a, FLIP mRNA was highly expressed in the unstimulated FLS, but was decreased dose-dependently by the HCQ treatment. The protein expression of both FLIPL and FLIPs was also decreased rapidly as early as 1 h after the treatment with 100 µg of HCQ, and remained down-regulated to 6 h (Fig. 5b). Moreover, time kinetics analysis revealed that the apoptosis triggered by HCQ plus anti-Fas mAb was followed by a decrease in the FLIP protein expression level by HCQ (Fig. 5c), and both were correlated inversely with each other. These results suggest that HCQ may increase the Fas-mediated apoptosis of FLS by modulating FLIP expression.
Fig. 4.
Flow cytometry analysis of Fas expression on synovial fibroblasts. The cells were treated with 10 and 50 µM of hydroxychloroquine (HCQ), and stained with phycoerythrin (PE)-conjugated mouse anti-human Fas (CD95). PE-conjugated mouse IgG1 was used as an isotype control antibody. (a) A representative result from three independent experiments using different cells is shown. The dotted line indicates Fas expression on fibroblast-like synoviocytes (FLS) treated with 50 µM of HCQ for 12 h, and a thick solid line on the left shows the constitutive Fas expression on the untreated cells. The thin solid line on the right indicates the cells stained with the isotype control IgG1. (b) Mean fluorescence intensity (MFI) of Fas expression on the cells treated with 10 or 50 µM of HCQ for a different culture period. Data are presented as the mean ± s.d. of three independent experiments.
Fig. 5.
Down-regulation of FADD-like interleukin (IL)-1β-converting enzyme (FLICE) inhibitory protein, (FLIP) in the synovial fibroblasts by hydroxychloroquine (HCQ). (a) Dose-dependent reduction of FLIP mRNA expression by HCQ. Fibroblast-like synoviocytes (FLS) were incubated without (lane 1) or with 1, 10 and 100 µM of HCQ for 4 h (lane 2–4), and then analysed for FLIPL (the long form of FLIP) mRNA expression by semiquantitative reverse transcription–polymerase chain reaction (RT–PCR). Amplification of the housekeeping gene, glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), was used as the control. The mRNA levels are expressed as the fold increase relative to the mRNA from the cells treated with 100 µM of HCQ (lane 4), and were corrected for the levels of the GAPDH mRNA signal. The data represent the results from one of three similar experiments. (b) Time-dependent decrease in FLIP protein expression by HCQ. The FLS were treated without (lane 1) or with HCQ 100 µM for 1–6 h (lane 2–4), and then examined for FLIP protein expression by Western blotting analysis using anti-human FLIP monoclonal antibodies (mAb), which recognizes both FLIPL (55 kDa) and FLIPS (27/28 kDa) isoforms. The data are a representative result of three independent experiments. (c) Time kinetics analysis of FLIPL protein expression (solid circle) in the HCQ (100 µM)-treated FLS and the extent of apoptosis (solid triangle) in the cells treated with HCQ (100 µM) plus ant-Fas IgM (CH11, 100 ng/ml), which were determined by Western blotting analysis and DNA fragmentation enzyme-linked immunosorbent assay (ELISA), respectively. Data are presented as the mean of two independent experiments.
Discussion
The synovial fibroblasts contribute to the chronic inflammatory responses of RA as a major part of the invasive pannus [19]. They express strongly a variety of activation markers including surface molecules (e.g. MHC-II, VCAM-1), and thereby can present antigens efficiently to the T cells. The fibroblast cell lines isolated from RA patients have the potential to produce matrix-degrading enzymes and several cytokines, such as IL-1, IL-6 and IL-8 [20–22]. Moreover, synovial fibroblasts proliferate abnormally, and invade the local environments and exhibit the characteristics of tumour cells such as somatic mutations in H-ras and p53 [23,24]. Even though synoviocytes express the functional Fas antigen, most cells escape the process of apoptosis in vivo, which may contribute to the activation and hyperplasia of FLS [25,26]. Therefore, the development of agents that enhance the apoptosis of synoviocytes, such as anti-Fas mAb [27], would represent a new therapeutic strategy for human RA. In the present study we have demonstrated that an anti-rheumatic drug, HCQ, could induce the apoptosis of FLS. The apoptotic effect of HCQ was observed very early (at 1 h after treatment), and increased dose- and time-dependently. The HCQ concentration used in our culture system was relevant physiologically in that HCQ 100 µM, the maximum dose tested, was similar to the concentration achieved in the plasma of patients receiving anti-rheumatic therapy with 400 mg HCQ daily [28]. Together, these results suggest that the enhancement of synoviocyte apoptosis is one of the major mechanisms explaining the therapeutic benefit of HCQ in RA patients.
FLIP contains two death effector domains and an inactive caspase domain, binds to FADD and caspase-8, and thereby inhibits the death receptor-mediated apoptosis [7,8]. In adjuvant-induced arthritis, FLIP was strongly expressed at the erosion sites and was localized to the pannus [29]. In RA patients, FLIP expression was also found in both the lining and sublining layers, and was identified at the sites of cartilage invasion and bone destruction [10]. The synovial fibroblasts of RA patients had a 50% higher FLIP expression level than those of osteoarthritis patients [10]. Moreover, synovial macrophages in the RA synovium express the FLIP and are refractory to Fas-mediated apoptosis [30]. Overall, it can be postulated that in rheumatoid synovial cells FLIP may regulate the Fas-mediated apoptosis by interfering with the interaction between FADD and caspase-8 [7,8,31]. In this respect, the inhibition of FLIP may augment synovial apoptosis [18], thereby ameliorating the RA inflammation. In the present study, we demonstrated first that HCQ synergistically increased the level of Fas-mediated apoptosis of synoviocytes. Moreover, HCQ (0·1–50 µM) inhibited the mRNA and protein expression levels of FLIP, but not Fas. This suggests that HCQ may sensitize the Fas-mediated apoptosis of rheumatoid synoviocytes by down-regulating FLIP expression.
HCQ activates caspase-3 in some cell types [14,16], which is a critical step for DNA fragmentation and apoptotic cell death. In this study, HCQ was able to increase the caspase-3 activity in FLS. Moreover, the caspase inhibitors, Z-VAD-FMK (pan caspase inhibitor) and Z-DEVD-FMK (caspase-3 inhibitor), block synoviocyte apoptosis almost completely by either HCQ or HCQ plus anti-Fas IgM. Therefore, it is conceivable that the increased apoptosis of FLS by HCQ may have been caused by the cumulative action of it on at least two different pathways, e.g. the direct activation of caspase-3 and the potentiation of intracellular Fas signalling by suppressing FLIP expression, which may trigger caspase activity indirectly. However, because there are many more molecules participating in the apoptotic death of FLS, other molecules such as bcl-2 and bax, whose expression level are significantly modified by HCQ in other types of cells [14,16], are probably also involved in this process.
It is unclear how HCQ regulates FLIP mRNA expression. It has been proposed that HCQ interferes with the post-transcriptional events [11,12]. HCQ is a weak base that is known to affect the acid vesicles leading to a dysfunction of the enzymes essential for the post-translational modifications of proteins. Therefore, HCQ might alter the function of some proteins critical for maintaining the stability of FLIP mRNA, just as it modified the gp120 protein of human immunodeficiency virus-1 (HIV-1) [32,33]. Otherwise, it is possible that FLIP mRNA transcription may be disrupted by HCQ without interfering with the mRNA stability. The relevant evidence was obtained from the earlier findings that chloroquine inhibited TNF-α mRNA expression in a macrophage cell line and peripheral blood mononuclear cells [34,35], and that the activation of some transcriptional factors, such as AP-1, are dependent on a chloroquine-sensitive step [36]. Further studies to evaluate these possibilities are currently under way.
In summary, HCQ induced the apoptosis of the FLS in dose- and time-dependent fashion. A combined treatment of HCQ and anti-Fas mAb increased the apoptosis of FLS synergistically, compared with either anti-Fas mAb or HCQ alone. Moreover, treatment with HCQ plus anti-Fas mAb resulted in a rapid decrease in FLIP expression in the FLS, which was followed by the apoptotic event. These results suggest that HCQ sensitizes rheumatoid synoviocytes to Fas-mediated apoptosis by down-regulating FLIP expression, and may exert its therapeutic effect against RA via this mechanism.
Acknowledgments
This work was supported by grants from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (0405-DB01-0104–0006) and St Vincent’s Hospital in Suwon.
References
- 1.Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999;103:3–4. doi: 10.1172/JCI5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima T, Aono H, Hasunuma T, et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485–91. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- 3.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631–8. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujisawa K, Asahara H, Okamoto K, et al. Therapeutic effect of the anti-Fas antibody on arthritis in HTLV-1 tax transgenic mice. J Clin Invest. 1996;98:271–8. doi: 10.1172/JCI118789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto K, Asahara H, Kobayashi T, et al. Induction of apoptosis in the rheumatoid synovium by Fas ligand gene transfer. Gene Ther. 1998;5:331–8. doi: 10.1038/sj.gt.3300597. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K, Kobayashi T, Kobata T, et al. Fas-associated death domain protein is a Fas-mediated apoptosis modulator in synoviocytes. Rheumatology. 2000;39:471–80. doi: 10.1093/rheumatology/39.5.471. [DOI] [PubMed] [Google Scholar]
- 7.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 8.Tschopp J, Irmler M, Thome M. Inhibition of fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–8. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 9.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–88. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schedel J, Gay RE, Kuenzler P, et al. FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum. 2002;46:1512–8. doi: 10.1002/art.10309. [DOI] [PubMed] [Google Scholar]
- 11.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–9. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rynes RI. Antimalarial drugs in the treatment of rheumatological diseases. Br J Rheumatol. 1997;36:799–805. doi: 10.1093/rheumatology/36.7.799. [DOI] [PubMed] [Google Scholar]
- 13.Meng XW, Feller JM, Ziegler JB, Pittman SM, Ireland CM. Induction of apoptosis in peripheral blood lymphocytes following treatment in vitro with hydroxychloroquine. Arthritis Rheum. 1997;40:927–35. doi: 10.1002/art.1780400522. [DOI] [PubMed] [Google Scholar]
- 14.Lagneaux L, Delforge A, Dejeneffe M, Massy M, Bernier M, Bron D. Hydroxychloroquine-induced apoptosis of chronic lymphocytic leukemia involves activation of caspase-3 and modulation of Bcl-2/bax/ratio. Leuk Lymph. 2002;43:1087–95. doi: 10.1080/10428190290021506. [DOI] [PubMed] [Google Scholar]
- 15.Min SY, Hwang SY, Jung YO, et al. Increase of cyclooxygenase-2 expression by interleukin 15 in rheumatoid synoviocytes. J Rheumatol. 2004;31:875–83. [PubMed] [Google Scholar]
- 16.Lagneaux L, Delforge A, Carlier S, Massy M, Bernier M, Bron D. Early induction of apoptosis in B-chronic lymphocytic leukaemia cells by hydroxychloroquine: activation of caspase-3 and no protection by survival factors. Br J Haematol. 2001;112:344–52. doi: 10.1046/j.1365-2141.2001.02553.x. [DOI] [PubMed] [Google Scholar]
- 17.Meng XW, Fraser MJ, Feller JM, Ziegler JB. Caspase-3-dependent and caspase-3-independent pathways leading to chromatin DNA fragmentation in HL-60 cells. Apoptosis. 2000;5:61–7. doi: 10.1023/a:1009689710184. [DOI] [PubMed] [Google Scholar]
- 18.Palao G, Santiago B, Galindo M, Paya M, Ramirez JC, Pablos JL. Down-regulation of FLIP sensitizes rheumatoid synovial fibroblasts to Fas-mediated apoptosis. Arthritis Rheum. 2004;50:2803–10. doi: 10.1002/art.20453. [DOI] [PubMed] [Google Scholar]
- 19.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–90. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 20.Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–74. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bédard PA, Golds EE. Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol. 1993;154:433–41. doi: 10.1002/jcp.1041540227. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 23.Roivainen A, Jalava J, Pirila L, Yli-Jama T, Tiusanen H, Toivanen P. H-ras oncogene point mutations in arthritic synovium. Arthritis Rheum. 1997;40:1636–43. doi: 10.1002/art.1780400913. [DOI] [PubMed] [Google Scholar]
- 24.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mountz JD, Wu J, Cheng J, Zhou T. Autoimmune disease. A problem of defective apoptosis. Arthritis Rheum. 1994;37:1415–20. doi: 10.1002/art.1780371002. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto S, Muller-Ladner U, Gay RE, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996;23:1345–52. [PubMed] [Google Scholar]
- 27.Matsuno H, Yudoh K, Nakazawa F, et al. Antirheumatic effects of humanized anti-Fas monoclonal antibody in human rheumatoid arthritis/SCID mouse chimera. J Rheumatol. 2002;29:1609–14. [PubMed] [Google Scholar]
- 28.French JK, Hurst NP, O’Donnell ML, Betts WH. Uptake of chloroquine and hydroxychloroquine by human blood leucocytes in vitro: relation to cellular concentrations during antirheumatic therapy. Ann Rheum Dis. 1987;46:42–5. doi: 10.1136/ard.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlman H, Liu H, Georganas C, et al. Differential expression pattern of the antiapoptotic proteins, Bcl-2 and FLIP, in experimental arthritis. Arthritis Rheum. 2001;44:2899–908. doi: 10.1002/1529-0131(200112)44:12<2899::aid-art478>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Perlman H, Pagliari LJ, Liu H, Koch AE, Haines GK, III, Pope RM. Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1β-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum. 2001;44:21–30. doi: 10.1002/1529-0131(200101)44:1<21::AID-ANR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Okamoto K, Kobata T, et al. Differential regulation of Fas-mediated apoptosis of rheumatoid synoviocytes by tumor necrosis factor-α and basic fibroblast growth factor is associated with the expression of apoptosis-related molecules. Arthritis Rheum. 2000;43:1106–14. doi: 10.1002/1529-0131(200005)43:5<1106::AID-ANR21>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Sperber K, Kalb TH, Stecher VJ, Banerjee R, Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retroviruses. 1993;9:91–8. doi: 10.1089/aid.1993.9.91. [DOI] [PubMed] [Google Scholar]
- 33.Savarino A, Gennero L, Sperber K, Boelaert JR. The anti-HIV-1 activity of chloroquine. J Clin Virol. 2001;20:131–5. doi: 10.1016/s1386-6532(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X, Ertel W, Ayala A, Morrison MH, Perrin MM, Chaudry IH. Chloroquine inhibits macrophage tumour necrosis factor-α mRNA transcription. Immunology. 1993;80:122–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Weber SM, Levitz SM. Chloroquine interferes with lipopolysaccharide-induced TNF-α gene expression by a nonlysosomotropic mechanism. J Immunol. 2000;165:1534–40. doi: 10.4049/jimmunol.165.3.1534. [DOI] [PubMed] [Google Scholar]
- 36.Yi AK, Krieg AM. Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J Immunol. 1998;161:4493–7. [PubMed] [Google Scholar]