Abstract
Surface proteins of schistosomes are exposed to host tissues and thus present as potential candidate molecules for the development of new intervention strategies. Herein, we have identified a new tegumental protein of Schistosoma mansoni, termed Sm29. In silico analysis revealed a signal peptide, three glycosylation sites and a transmembrane region on Sm29 amino acid sequence. Sm29 transcription in mammalian developmental stages cDNA libraries of S. mansoni was verified by PCR using specific primers for Sm29 nucleotide sequence and it revealed the presence of transcripts in schistosomula and adult worm stages of the parasite. Sm29 (40–169) fragment was produced in Escherichia coli and purified by affinity chromatography to be used in the immunological assays. Confocal microscopy confirmed bioinformatic studies, revealing that Sm29 is a membrane-bound protein localized on the tegument of S. mansoni adult worm. ELISA was performed using rSm29 protein to investigate the antibody isotype profile to Sm29 in sera of patients living in endemic areas for schistosomiasis. IgG1 and IgG3 subclass antibodies to rSm29 were predominant in sera of individuals naturally resistant to infection and resistant to re-infection whereas low levels of IgM, IgA or IgE were measured. Since, IgG1 and IgG3 are involved in parasite killing and in protective immunity the findings reported here suggest the use of Sm29 as a potential candidate vaccine against schistosomiasis.
Keywords: Sm29, immunolocalization, Schistosoma mansoni, vaccine, antibody response
Introduction
Schistosomiasis is an important parasitic disease that affects more th an 200 million people worldwide causing more than 250 000 deaths per year [1]. The pathology characteristic of this disease is a granulomatous reaction around parasite eggs within the liver and other organ [2]. Currently, schistosomiasis control strategy is mainly based on the treatment of infected individuals by chemotherapy with safe and effective drug [3]. In spite of decades of chemotherapy, the number of infected people remains almost the same [4]. Large extension of endemic areas and constant reinfection of individuals together with poor sanitary conditions in tropical countries requires other controlling strategies besides drug treatment [5]. Therefore, vaccination as a way to control schistosomiasis would contribute enormously to disease eradication. In the case of schistosomiasis, a sterilizing vaccine, although desirable, is not essential. Since schistosomes do not multiply within the final host, a vaccine that induces even a partial reduction in worm burdens could considerably reduce pathology, limit parasite transmission and be less expensive than repetitive drug treatment [6].
The best long-term strategy to control schistosomiasis is thought to be the immunization with an antischistosomiasis vaccine [7]. Many world health agencies agree that the development of an antischistosomiasis vaccine should be seeked. Several studies are in progress in this field, testing different antigens of the parasite and different vaccination strategy [6]. Vaccine candidate antigens are often secreted by or anchored on the surface of pathogens. Proteins that are secreted or anchored on the surface of schistosomes are exposed to host tissues and thus present as potential candidate molecules for the development of new vaccines. It has been shown that isolated tegumental membranes are capable of stimulating protective immunity in mice [8]. Additionally, the principal membrane-associated antigens contained in the adult S. mansoni tegument do not cross-react with egg antigens of the parasite, which are involved in immunopathology [9]. Therefore, characterization of proteins within the tegument is relevant at a more basic level to improvement of the functional understanding of this structure and at a more applied level to identification of molecules that may act as targets for protective immune responses or may be useful in diagnosis [10].
In this study, we identified in a S. mansoni adult-worm cDNA library a gene encoding a new tegument protein of the parasite termed Sm29. In silico analysis of Sm29 amino acid sequence identified an N-terminal signal peptide, three glycosylation sites and a hydrophobic transmembrane helix. Herein, we produced the recombinant protein Sm29 in Escherichia coli and raised polyclonal antibodies anti-Sm29 in mice. Further, we confirmed the bioinformatics analysis localizing Sm29 on the tegument of adult worms by confocal microscopy. Lastly, we tested rSm29 as antigen with sera from individuals living in endemic areas for schistosomiasis in Brazil. IgG1 and IgG3 anti-Sm29 were the predominant isotypes identified in sera of individuals naturally resistant to infection and resistant to reinfection. Experiments are underway in mice testing this new molecule as a vaccine candidate against experimental infection.
Materials and methods
Study population
Peripheral blood was obtained from individuals with different genetic background living in three different endemic areas for schistosomiasis (‘Melquiades’, ‘Côrrego do Onça’ and ‘Caatinga do Moura’). These individuals were classified in five groups, regarding their infection status and the selection of subjects was performed based only on the criteria for inclusion and exclusion of each group independent of previous knowledge of immune responses for each individual. Non-infected (NI) individuals are healthy people without any parasite infection or contaminated water contact. Some individuals, despite contact with contaminated water, showed repeated stool-negative examination and no clinical signs of disease for at least three consecutive years and were classified as naturally resistant to S. mansoni infection (NR). The water contact exposure was objectively evaluated by observers and studied population had at least one contact daily. Another group showed stool-negative examination after treatment and was classified as resistant to S. mansoni reinfection (RR). Individuals classified as susceptible to S. mansoni reinfection (SR) showed stool-positive examination following treatment (praziquantel, 40 mg/kg). The sera from RR and SR groups were obtained six months after praziquantel treatment and these individuals were examined for S. mansoni infections using the Kato–Katz technique before treatment and one, six and 12 months after treatment to check for reinfection rates. Individuals grouped as infected (INF) showed stool-positive examination and no treatment history. These infected patients had infection levels that varied from 48 to 224 epg (egg counts per gram of faeces). These patients or their legal guardians gave informed consent after explanation of the protocol that had been previously approved by the Ethical Committee of the Federal University of Minas Gerais. Details regarding sex and age of the individuals included in this study are described in Table 1.
Table 1.
Study population.
| Group | Infection status | Description | Age (mean ± SD) | Sex (M/F) |
|---|---|---|---|---|
| Infected (I) n = 7 | Infected with S. mansoni | Individuals living in an endemic area for schistomiasis with stool positive examinations | 17.1 ± 14.9 | 5/2 |
| Suseptible to reinfection (SR) n = 9 | Infected with S. mansoni | Individuals living in a endemic area for schistosomiasis that present stool positive examination after praziquantel treatment | 22.8 ± 12.1 | 3/6 |
| Resistant to reinfection (RR) n = 9 | Not infected | Individuals living in a endemic area for schistosomiasis that although water contact, present stool negative examination after praziquantel treatment | 18.8 ± 9.3 | 5/4 |
| Naturally resistant (NR) n = 9 | Not infected | Individuals living in a endemic area for schistosomiasis that although water contact, present stool negative examination | 45.8 ± 8.1 | 5/4 |
| Not infected (NI) n = 8 | Not infected | Individuals living out of endemic area for schistosomiasis that never present this disease in their lives | 28.3 ± 4.5 | 3/5 |
Sm29 DNA sequencing analysis
DNA sequencing analysis of the clone AW1127/E11 containing the full-length Sm29 gene from a adult worm cDNA library [11] gifted by Dr Gloria Franco (Federal University of Minas Gerais-UFMG) was performed using the ‘DYEnamic™ ET dye terminator cycle sequencing (MegaBACE™)’ kit and the MegaBACE 1000 capillary sequencer (GE Healthcare, São Paulo, Brazil). Subsequent homology searches were performed using BLAST programs [12].
In silico Sm29 amino acid sequence analysis
Primary structure analysis of the deduced amino acid sequence was performed using ExPASy (Expert Protein Analysis System) computer program [13]. The signal peptide was identified using the SignalP 3·0 [14]. Transmembrane protein topology prediction and GPI anchor site was analysed by TMHMM (using the hidden Markov model) and DGPI bioinformatic tools (http://129.194.185.165/dgpi/index_en.html), respectively, and glycosylation sites were verified by YinOYang (http://www.cbs.dtu.dk/services/YinOYang/). Finally, SYFPEITHI algorithm [15] was used in the prediction of human MHC II binding epitopes. To compare multiple sequences we used CLUSTAL and T-COFFEE Program [16]. To identify sequences similar to Sm29 we used the BLAST network server at the National Center for Biotechnology Information (NCBI).
Sm29 transcript analysis in parasite life-stage cDNA libraries
In order to identify Sm29 mRNA transcripts in the cDNA libraries from specific human developmental stages of S. mansoni, PCR was performed using specific primers flanking the complete Sm29 DNA sequence (sense: 5′- ATGAAAAGTGGCTGGGAGT-3′ and antisense: 5′-GAATC AGTAAATACATTCTATGA-3′). Adult worm, lung-stage, egg and cercariae cDNA libraries were gifted by the Minas Gerais Genome Network. PCR parameters using 1 µl of each library as template were as follows: denaturation for 30 s at 94 °C, annealing for 30 s at 57 °C and extension for 1 min at 72 °C (35 cycles). Agarose gel (1%) electrophoresis and ethidium bromide staining were performed to visualize the Sm29 cDNA sequence amplified from each library.
Expression and purification of recombinant Sm29 in Escherichia coli
Recombinant Sm29 (40–169) amino acid fragment without the signal peptide and the transmembrane domain was expressed as a His (histidine)-tag fusion protein in E. coli. The cDNA coding for Sm29 was subcloned into pET21a expression vector (Novagen, Madison, WI, USA) coding for six N-terminally located histidine residues. For this purpose, PCR was performed with Sm29 specific primers and the following restriction sites (underlined): EcoRI for the sense (5′-CCGGAATTCATGAGCGTATCAATATCAGAAGA-3′) and XhoI for the antisense (5′-CTATGTAACGGAATGACAAA ACTCGAGGCC-3′). The parameters for PCR reaction were as follows: denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C and extension for 1 min at 72 °C (35 cycles). Subcloning and confirmation of DNA sequences followed standard procedure [17]. One litre of E. coli (BL21 DE3) culture containing the recombinant plasmid was grown at 37°C to an A600 (nm) of 0·5, and the expression of rSm29 was induced by 1 mM isopropylthiogalactoside (IPTG). After incubation for additional 5 h, cells were harvested by centrifugation and resuspended in 50 ml 10 mM Na2HPO4, 10 mM NaH2PO4, 0·5 M NaCl and 10 mM imidazole. Cells were lysed by sonication 3 times at 30% of amplitude and centrifuged at 5400 g, 4 °C for 20 min. Recombinant Sm29 was recovered as inclusion bodies and resuspended in 50 ml 10 mM Na2HPO4, 10 mM NaH2PO4, 0·5 M NaCl, 10 mM imidazole and 8 M urea. The recombinant protein was then purified by metal affinity chromatography (GE Healthcare, São Paulo, Brazil) under denaturing conditions according to the manufacturer’s protocol. Further, using a desalting column (GE Healthcare), the denaturing buffer was exchanged by renaturing PBS buffer before the use of this antigen in in vitro experiments.
SDS-PAGE and immunobloting
SDS-PAGE of purified rSm29 was performed according to Laemmli [18]. The gel was electroblotted onto nitrocellulose membrane according to Towbin et al. [19]. Membrane was blocked with TBST (0·5 M NaCl−0·02 M Tris (pH 7·5), 0·05% Tween 20) containing 5% dry milk for 16 h at room temperature. Subsequently, the membrane was incubated in a 1 : 3000 dilution of mouse alkaline phosphatase (AP) conjugated anti6xHIS antibodies (Invitrogen) in TBST plus 5% dry milk for 3 h at room temperature. After three washes using TBST, the membrane was treated with AP reaction developing buffer containing nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-1-phosphate (BCIP). After reaction had developed, the membrane was washed using distilled water and dried in filter paper.
Production of anti-Sm29 antibodies in mice
For production of specific anti-Sm29 antibodies, C57BL/6 mice were subcutaneously injected in the nape of the neck with 25 µg of rSm29 on days 0, 15 and 30 with Freund’s adjuvant as previously described [20]. Mouse anti-Sm29 serum was collected from the animals 45 days after the first immunization. ELISA was performed to confirm the titre of specific IgG anti-Sm29 in the serum of immunized animals. For this, 96 well flat-bottom microtitre plates (Nunc) were coated overnight at 4 °C with 100 µl of rSm29–6xHIS at a concentration of 5 µg/ml in 0·1 M carbonate bicarbonate buffer (pH 9·6) per well. The plates were then blocked with 10% bovine fetal serum in PBS for 2 h at room temperature. Subsequently, the plates were washed three times with PBS plus 0·05% Tween-20 (PBST). Serum samples diluted 1 : 50 (IgG) in PBST (100 µl/well) were added in duplicate and the plates incubated for 1 h at room temperature. Peroxidase-labelled antimouse IgG was added at dilution of 1 : 10 000 (100 µl/well). After 1 h at 37 °C, the plate was washed and orthophenyl-diaminobenzidine plus 0·05% hydrogen peroxide in phosphate citrate buffer (pH 5) was added (100 µl/well). This mixture was than incubated for 30 min at room temperature, and the reaction was stopped by addition of 5% H2SO4 (50 µl/well). Absorbance was read at 492 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). Animals immunized with PBS were used as negative control.
Immunolocalization in male adult worm of S. mansoni
Paraffin sections of male adult worm were deparaffinized with xylol series, blocked with PGN (0·25% gelatin, 0·1% sodium azide and 0·1% saponin in PBS) for 1 h, washed four times with PBS and incubated for 1 h with anti-Sm29 serum diluted 1 : 20 in PGN. Serum from naive C57BL/6 mice was used as negative control. Samples were washed four times with PBS, incubated for 1 h with FITC-labelled antibodies antimouse IgG (Sigma, St. Louis, MO, USA) diluted 1 : 50 in PGN containing 10 µM DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, Molecular Probes, Eugene, Oregon, USA) to visualize nuclei, and 0·1 µg/ml phalloidin-rhodamine (Sigma) to stain actin microfilaments. Following a 1 h incubation, samples were washed in three times in PBS, mounted in 0·1 M Tris-HCl (pH 8·8) buffered glycerol containing 0·1%p-phenylenediamine as antibleaching agent. S. mansoni worms were then imaged in a Bio-Rad 1024UV confocal system attached to a Zeiss Axiovert 100 microscope using a 1·2 NA 40× water immersion PlanApochromatic objective with differential interference contrast. Images were acquired with LaserSharp 1024 version 3·2T (Bio-Rad) then processed with NIH-Image-J (http://rsb.info.nih.gov/ij/).
Measurement of human antibody isotypes anti-Sm29
Sera of schistosomiasis patients living in endemic areas in Brazil were used in an enzyme-linked inmunosorbent assay (ELISA) as previously described by our group [21] to measure the levels of immunoglobulin isoytpes to rSm29 and SWAP (soluble adult worm antigen preparation) antigens. For this assay, 96 well flat-bottom microtitre plates (Nunc) were coated overnight at 4 °C with 100 µl of rSm29–6xHIS or SWAP at a concentration of 5 µg/ml in 0·1 M carbonate bicarbonate buffer (pH 9·6) per well. The plates were then blocked with 10% bovine fetal serum in PBS (pH 7·4) for 2 h at room temperature. Subsequently, the plates were washed three times with PBS plus 0·05% Tween-20 (PBST). Serum samples diluted 1 : 50 (IgG), 1 : 25 (IgM) and 1 : 40 (IgA) in PBST (100 µl/well) were added in duplicate, and the plates incubated for 1 h at room temperature. Peroxidase-labelled anti-IgG, anti-IgM and anti-IgA (Sigma) were added at dilutions of 1 : 10 000, 1 : 7500 and 1 : 1000 (100 µl/well), respectively. After 1 h at 37 °C, the plates were washed and orthophenyl-diaminobenzidine plus 0·05% hydrogen peroxide in phosphate citrate buffer (pH 5) was added (100 µl/well). This mixture was than incubated for 30 min at room temperature, and the reaction was stopped by addition of 5% H2SO4 (50 µl/well). Absorbance was read at 492 nm using a microplate reader (Bio-Rad, Hercules. CA, USA). To measure IgG subclasses and IgE levels, the previous protocol was modified. For IgE detection, serum was treated with RF–Absorbent (Behring Diagnostics, Marburg, Germany) for 1 h at room temperature to deplete IgG avoiding IgE under-estimation. The serum dilution was changed based on the isotype or subclass to be detected. Serum samples diluted 1 : 30 (IgG1), 1 : 5 (IgG2 or IgG4), 1 : 80 (IgG3) and 1 : 4 (IgE) were added to the plates and incubated for 2 h at 37°C as previously described [21]. After washing, peroxidase-labelled mouse antihuman antibody was added in each well at concentrations of 1 : 1000 (IgG1 or IgG3), 1 : 500 (IgG2, IgG4 or IgE) and the plates were incubated for 16 h at 4°C. The subsequent steps were identical to those described for the other isotypes.
Statistical analysis
Kruskal–Wallis test was used to evaluate the significance of the results of all groups compared to NI. Mann–Whitney test was used to evaluate the significance of antibody measurements obtained from NR versus I groups and between RR versus SR groups.
Results
Bioinformatic analysis of Sm29
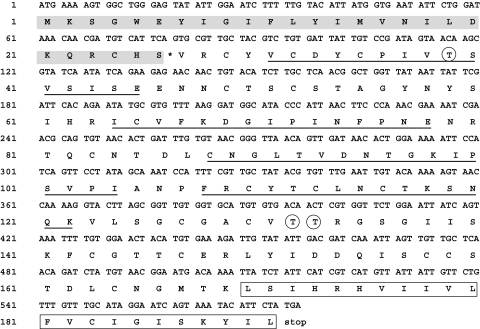
The Sm29 amino acid sequence contains 576 nucleotides and the amino acid sequence deduced is 192 residues long with a predicted molecular mass of approximately 18 kD without the signal peptide. Sm29 amino acid sequence showed 100% identity to a previous Sm29 adult worm sequence deposited in the GenBank (AF029222). Additionally, amino acid comparison analysis revealed that Sm29 amino acid sequence showed 52% of identity with an unknown S. japonicum protein (SJCHGC05668). The primary amino acid sequence of the Sm29 is composed by a signal peptide of 26 amino acids, with the signal cleavage site between Ser26 and Val27 (Fig. 1). Three glycosylation sites are present in the mature sequence on the Thr39, Thr132 and Thr133. Transmembrane protein topology prediction analysis showed that the region within 169–191 amino acids, exhibited high hydrophobicity and therefore, represents primary transmembrane helix (Fig. 1). No GPI anchor domain was found within Sm29 amino acid sequence. The in silico analysis performed here suggest that Sm29 is a membrane-bound protein. Additionally, prediction of human MHC class II epitopes revealed that Sm29 possess five promiscuous peptides which have high binding score for the HLA DRB1*0101, HLA-DRB1*0301, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*1101 and HLA-DRB1*1501 alleles (data not shown). The predicted peptides are shown in Fig. 1.
Fig. 1.
In silico analysis of Sm29 amino acid sequence. Sm29 is composed by a signal peptide of 26 amino acids (grey bar), with the signal cleavage site between Ser26 and Val27 (*). Three glycosylation sites are present in the mature sequence on the Thr39, Thr132 and Thr133 (circles). The transmembrane helix is found from the Leu169 to the Leu191 (box). Five promiscuous HLA binding peptides predicted in Sm29 are underlined. (Peptides CNGLTVDNTGKIPSV and GLTVDNTGKIPSVPI are overlapped).
Sm29 transcripts are detected in adult worm and lung-stage cDNA libraries
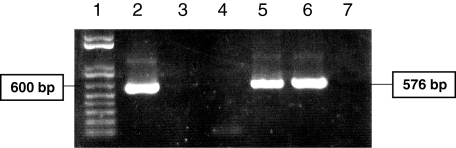
Sm29 transcripts with 576 bp were detected in the adult worm and lung-stage cDNA libraries of S. mansoni but not in egg or cercariae (Fig. 2). Since, the lungs seem to be the major site of parasite elimination during the irradiated cercariae model of vaccination, the use of Sm29 as a vaccine candidate could be an interesting approach in the control of schistosomiasis [22].
Fig. 2.
PCR analysis of cDNA libraries using specific primers for the complete sequence of Sm29. 1, ladder (bp); 2, positive control; 3, negative control; 4, egg cDNA library; 5, lung-stage cDNA library; 6, adult worm cDNA library; 7, cercariae cDNA library.
Production of recombinant Sm29
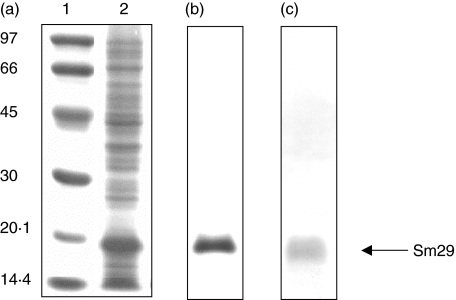
To confirm that Sm29 was expressed in E. coli as a 6xHis-tag fusion, SDS-PAGE and Western blot analysis were performed revealing a 16·7 kD protein, as expected for the rSm29 (40–169) fragment (Fig. 3a,c, respectively). This recombinant protein was obtained as inclusion bodies and purified under denaturing conditions in 8 M urea by affinity chromatography (Fig. 3b). The refolding in renaturing PBS pH 7·4 buffer results in a great lost of protein in a precipitated form, but yielded enough soluble recombinant protein to be used in in vitro experiments.
Fig. 3.
SDS-PAGE and Western blot analysis of the recombinant Sm29–6xHIS fusion protein. (a) Coomassie blue stained SDS-12% PAGE profile of induced E.coli expressing the pET21a-Sm29 construct; 1, ladder (kD); 2, E. coli lysate expressing Sm29. (b) Comassie blue stained SDS-12% PAGE profile of the purified Sm29–6xHIS fusion protein. (c) Western blot analysis of the purified protein using anti6xHIS antibody. Arrow indicates the rSm29.
Sm29 is a membrane-bound protein located in the tegument of S. mansoni adult worm
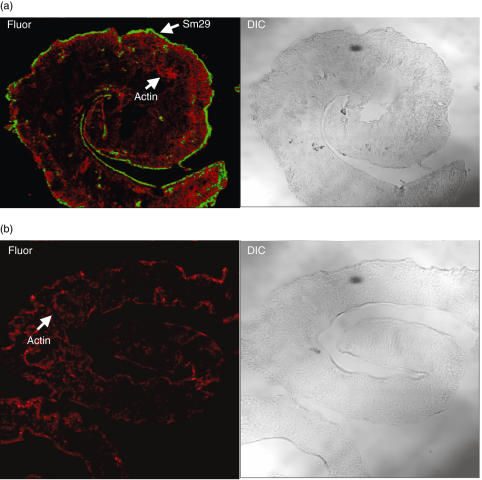
A mouse serum raised against rSm29 revealed through confocal fluorescence microscopy that Sm29 is located at the tegument of the adult worm stage of S. mansoni(Fig. 4a). This membrane-bound antigen appears to be highly expressed on the tegument of this helminth and is absent in the internal tissues of the parasite body. No Sm29 antibody staining was observed in sections incubated with preimmune mouse serum (data not shown).
Fig. 4.
Immunolocalization of Sm29 on S. mansoni tegument. Fluorescence confocal microscopy images (Fluor) and corresponding differential interface contrast (DIC) images of male adult worm of S. mansoni are shown. Polyclonal anti-Sm29 and secondary antibody coupled to FITC were used for fluorescence detection of Sm29 on male adult worm sections. Serum from naive mice was used as negative control. Flavoidin and Rodamin were used for the actin localization. The Sm29 localization is represented in (a) and the negative control is shown in (b).
Immunoglobulin isotype profile of schistosomiasis patients to rSm29
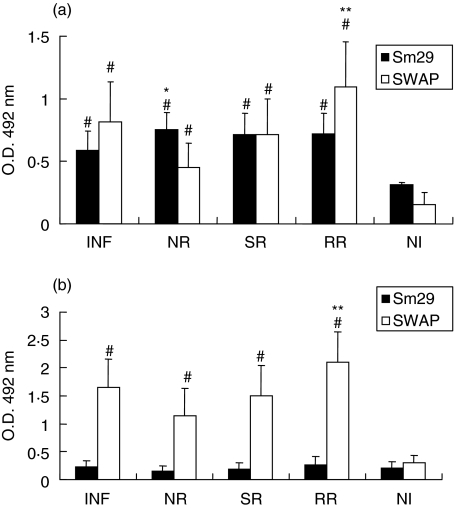
To investigate the presence of specific antirSm29 antibodies in sera of individuals according to their status of resistance and susceptibility to S. mansoni infection and reinfection, ELISA was performed. Figure 5 shows anti-SWAP or antirSm29 total IgG levels in sera of schistosomiasis patients and noninfected individuals. All the schistosomiasis groups studied had significant levels of anti-SWAP or antirSm29 IgG compared to the noninfected group. In order to determine if rSm29 could be recognized by resistant versus susceptible individuals, we compared the isotype antibody profile of the NR group to INF individuals, and RR patients compared to the SR group. The NR group produced statistically significant levels of IgG anti-Sm29 compared to INF patients (Fig. 5a). Additionally, the RR group had increased levels of IgG anti-SWAP when compared to individuals susceptible to reinfection (SR). Regarding IgA, statistically significant levels of antibodies to SWAP were observed in all groups when compared to NI (Fig. 5b). Further, the RR group produced more IgA anti-SWAP compared to SR individuals. However, as for IgA anti-Sm29, no significant levels of this isotype were detected compared to NI individuals. For IgM, low levels were detected anti-Sm29 and anti-SWAP in all studied groups (data not shown). Low IgM anti-SWAP antibody levels were also observed in others studies using adult patients infected with S. haematobium[23]. Regarding IgE, we were not able to detect significant amounts of this antibody isotype in all different groups studied in response to either SWAP or rSm29 (data not shown). These results demonstrate that antirSm29 or anti-SWAP IgG is the dominant isotype measured in patients’ sera.
Fig. 5.
Isotype profile of schistosomiasis patients’ sera to rSm29 or SWAP. Analysis of (a) IgG and (b) IgA antibody responses in sera of infected patients (INF), naturally resistant individuals (NR), susceptible to reinfection individuals (SR), resistant to reinfection individuals (RR) and noninfected individuals (NI). Results are expressed as means of individual measurement. Error bars indicate SD of the means. #Statistically significant compared to the noninfected group (P < 0·05). *Statistically significant compared to the infected group. **Statistically significant compared to the susceptible to reinfection group.
IgG1 and IgG3 antibody responses to rSm29
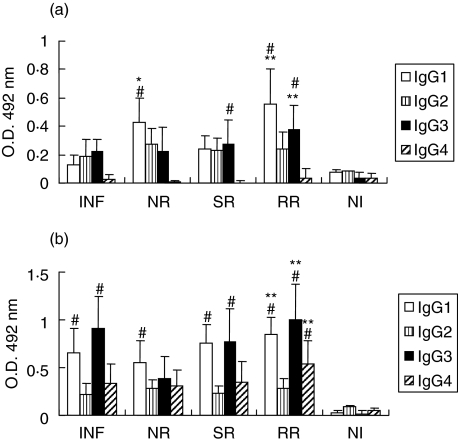
The IgG subclass profile of schistosomiasis patients was characterized predominantly by IgG1 and IgG3 antibody responses to rSm29 and SWAP. Regarding anti-Sm29 antibody isotypes, we detected IgG1 in NR individuals compared to INF patients and IgG1 and IgG3 in RR group compared to SR (Fig. 6a). As for SWAP, we observed statistically significant levels of IgG1, IgG3 and IgG4 in RR group compared to SR patients (Fig. 6b). IgG1 and IgG3 are immunoglobulin molecules that have functional activity of opsonization, cell-dependent cytotoxicity, and the ability to activate the classical complement pathway therefore involved in resistance to S. mansoni infection.
Fig. 6.
Analysis of IgG subclass responses to rSm29 (A) or SWAP (B). IgG subclass levels in sera of infected patients (INF), natural resistant individuals (NR), susceptible to reinfection individuals (SR), resistant to reinfection individuals (RR) and noninfected individuals (NI) were determined. Results are expressed as means of individual measurements. Error bars indicate SD of the means. #Statistically significant compared to the noninfected group (NI). *Statistically significant compared to the infected group (I). **Statistically significant compared to the susceptible to reinfection group (SR).
Discussion
Schistosomiasis ranks as one of the most serious parasitic disease worldwide [24]. The schistosomes have a complex life cycle and display sexual dimorphism as adults, making difficult the studies of its biological features and the host–parasite interaction. The identification of new tegument proteins is the focus of contemporary research to enhance our understanding of host–parasite interactions as well as provide new potential vaccine antigens against schistosomiasis [25,26].
In the present study, we identified and described in silico analysis, immunolocalization and humoral immune responses of schistosomiasis patients to a new tegumental protein present in S. mansoni adult worm and lung-stage of the parasite. The cDNA coding for the Sm29 antigen was sequenced and analysed by bioinformatic techniques. Based on in silico data, we conclude that Sm29 is a membrane-bound protein and this group of glycoproteins has interesting characteristics as a vaccine candidate as described by Verjovski-Almeida et al. [27]. Sm29 amino acid sequence possesses a signal peptide, and a transmembrane region. Additionally, Sm29 sequence has cysteine rich domains that confer stability for this molecule by the dissulphide bridges formed between the cysteins. To confirm bioinformatic analysis, confocal fluorescence microscopy revealed that Sm29 is located at the tegument of the adult worm stage of S. mansoni. This membrane-bound antigen appears to be highly expressed on the tegument of this helminth and is absent in the internal tissues of the parasite body.
The immune interactions that are necessary to eradicate invasive helminth parasites are extremely complex and likely require both humoral and cell-mediated components of the immune system [28]. Previous analysis of immune responses in experimental and human schistosomiasis has shown that antibodies are able to kill schistosomula in the presence of phagocytic cell populations such as macrophages, platelets, and eosinophils. Another study using the B cell knockout mouse model demonstrated the pivotal role of antibody molecules in inducing resistance to schistosomiasis [29]. Thus, the different functional properties of antibody isotypes make them interesting to study as they may give predictive information regarding disease progression and efficacy of vaccination. The Sm29 recombinant antigen produced here was used in the investigation of antibody isotype profile specific to Sm29 in sera of resistant versus susceptible individuals to infection and re-infection living in endemic areas in Brazil. The IgG1 and IgG3 antibodies specific to rSm29, isotypes related to opsonization, cell-dependent cytotoxicity and activation of classical complement pathway were present in significant levels in the resistant to infection and re-infection (RR) individuals. Knowing that Sm29 has been located by confocal microscopy in the surface of adult worms, this molecule could be available as a target to antibody-dependent cell cytotoxicity (ADCC) effector mechanisms mediated by IgG1 and IgG3. In addition to opsonization, cell-dependent cytotoxicity, and the ability to activate the classical complement pathway, IgG1 and IgG3 are also involved in protective immunity in vivo based on passive transfer experiments performed in mice [30]. High levels of IgG1 and IgG3 are related to resistant status in the Sm23, Sm28-TPI, PN18-cyclophilin, Sm37-SG3PDH and Sm14-FABP studies as reported by Al-Sherbiny et al. [31].
IgM, IgG2 and IgG4 elicited in response to antigens released during S. mansoni infection react with parasite surface glycoproteins and block the effect of protective antibodies mediating ADCC reactions against the schistosomula. IgM and IgG2 antibodies are usually induced by parasite carbohydrate epitopes therefore the absence of significant levels of these isotypes in response to rSm29 could be due to lack of sugars on the recombinant protein or that Sm29 does not induce the production of these antibodies isotypes. IgG2 and IgG4 bind in vitro to relevant schistosomula surface epitopes thus preventing the functional effector mechanisms involved in parasite killing, so these subclasses have been considered blocking antibodies [32]. There is also evidence linking augmented IgG4 response with susceptibility, in contrast to IgE response that is related to resistance against reinfection [33]. Therefore, the absence of antirSm29 IgG2 and IgG4 might be an important humoral attribute that contributes to host protection against schistosomiasis when Sm29 will be tested as a vaccine candidate.
Regarding IgE and IgA, investigators have correlated the increased levels of these isotypes with resistance to reinfection engendered by schistosome antigens [34,35]. High levels of anti-adult worm antigens IgE have been detected in patients after treatment with both oxaminiquine and praziquantel especially to schistosome tegument proteins. However, low levels of this antibody isotype have been determined in individuals before treatment [36]. According to this hypothesis, Hota-Mitchell et al. [37] detected low IgE titres in S. mansoni-infected human sera when recombinant calpain was used as antigen, what parallels with our results where low levels of IgE and IgA antibodies to rSm29 were observed in all patients’ sera before treatment. These results suggest that the mechanisms related to natural resistance are different from resistance to reinfection following treatment, based on IgE responses.
This is the first report identifying a new tegument protein of S. mansoni termed Sm29. We have concluded that specific IgG1 and IgG3 to rSm29 were the antibody types prevalent in sera of individuals resistant to infection and re-infection. This is an important finding for vaccine development since these subclasses are associated with parasite killing. The characterization of human populations exposed to schistosomes over time and identification of subjects who appear epidemiologically resistant versus susceptible to re-infection are precious sources of information on the studies of protective human response on this disease Additionally, five promiscuous peptides were defined by bioinformatic analysis to bind to six different HLA-DRB1 molecules what confirms Sm29 antigenicity. Recently, we have predicted immunodominant epitopes in vaccine candidate antigens using algorithms and these peptides have been shown to be immunoreactive to human T cells [38–40]. Finally, experiments are underway using rSm29 to test this molecule as a vaccine candidate in the control of murine schistosomiasis.
Acknowledgments
This work was supported by FAPEMIG, CNPq, PADCT/CNPq and FAPESP.
References
- 1.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–39. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 2.Boros DL. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–69. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harder A. Chemotherapeutic approaches to schistosomes. current knowledge and outlook. Parasitol Res. 2002;88:395–7. doi: 10.1007/s00436-001-0588-x. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist NR. Schistosomiasis vaccine development: approaches and prospects. Mem Inst Oswaldo Cruz. 1995;90:221–7. doi: 10.1590/s0074-02761995000200017. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist NR. Schistosomiasis vaccine development. Prog Prospects Mem Inst Oswaldo Cruz. 1998;93:95–101. doi: 10.1590/s0074-02761998000700013. [DOI] [PubMed] [Google Scholar]
- 6.Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–3. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist NR. Schistosomiasis: from risk assessment to control. Trends Parasitol. 2002b;18:309–14. doi: 10.1016/s1471-4922(02)02301-2. [DOI] [PubMed] [Google Scholar]
- 8.Smithers SR, Hackett F, Braga V, Simpson AJ. Immunoblotting identifies additional antigens recognised by mice protectively vaccinated with adult Schistosoma mansoni tegumental membranes. Parasitol Res. 1990;76:454–6. doi: 10.1007/BF00933557. [DOI] [PubMed] [Google Scholar]
- 9.Simpson AJ. Schistosome surface antigens: developmental expression and immunological function. Parasitol Today. 1990;6:40–5. doi: 10.1016/0169-4758(90)90067-e. [DOI] [PubMed] [Google Scholar]
- 10.Abath FG, Xavier EM, Allen R, Gomes YM, Lucena-Silva N, Baliza M, Simpson AJ. Characterization of Sm13, a tegumental antigen of Schistosoma mansoni. Parasitol Res. 2000;86:745–52. doi: 10.1007/pl00008562. [DOI] [PubMed] [Google Scholar]
- 11.Franco GR, Rabelo EM, Azevedo V, et al. Evaluation of cDNA libraries from different developmental stages of Schistosoma mansoni for production of expressed sequence tags (ESTs) DNA Res. 1997;4:231–40. doi: 10.1093/dnares/4.3.231. [DOI] [PubMed] [Google Scholar]
- 12.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucl Acids Res. 2003;31:3784–8. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Engineering. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–9. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 16.Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–17. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook K, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Eletrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonseca CT, Brito CFA, Alves JB, Oliveira SC. IL-12 enhances protective immunity in mice engendered by immunization with recombinant 14 kDa Schistosoma mansoni fatty acid-binding protein through IFN-γ and TNF-α dependent pathway. Vaccine. 2004;22:503–10. doi: 10.1016/j.vaccine.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Brito CF, Fonseca CT, Goes AM, Azevedo V, Simpson AJ, Oliveira SC. Human IgG1 and IgG3 recognition of Schistosoma mansoni 14 kDa fatty acid binding recombinant protein. Parasite Immunol. 2002;22:41–8. [PubMed] [Google Scholar]
- 22.Coulson PS, Wilson RA. Recruitment of lymphocytes to the lung through vaccination enhances the immunity of mice exposed to irradiated schistosomes. Infect Immun. 1997;65:42–8. doi: 10.1128/iai.65.1.42-48.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutapi F, Ndhlovu PD, Hagan P, Woolhouse ME. A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol. 1997;19:255–63. doi: 10.1046/j.1365-3024.1997.d01-206.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross AG, Li Y, Williams GM, Jiang Z, McManus DP. Dam worms. Biologist (London) 2001;48:121–4. [PubMed] [Google Scholar]
- 25.Smyth D, McManus DP, Smout MJ, Laha T, Zhang W, Loukas A. Isolation of cDNAs encoding secreted and transmembrane proteins from Schistosoma mansoni by a signal sequence trap method. Infect Immun. 2003;71:2548–54. doi: 10.1128/IAI.71.5.2548-2554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curwen RS, Ashton PD, Johnston DA, Wilson RA. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol Biochem Parasitol. 2004;138:57–66. doi: 10.1016/j.molbiopara.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Verjovski-Almeida S, DeMarco R, Martins EA, et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–57. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Hoffmann KF. Defining a schistosomiasis vaccination strategy – is it really Th1 versus Th2? Parasitol Today. 2000;16:497–501. doi: 10.1016/s0169-4758(00)01788-9. [DOI] [PubMed] [Google Scholar]
- 29.Jankovic D, Wynn TA, Kullberg MC. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-γ-dependent effector mechanisms. J Immunol. 1999;162:345–51. [PubMed] [Google Scholar]
- 30.Delgado V, McLaren DJ. Evidence for enhancement of IgG1 subclass expression in mice polyvaccinated with radiation-attenuated cercariae of Schistosoma mansoni and the role of this isotype in serum-transferred immunity. Parasite Immunol. 1990;12:15–32. doi: 10.1111/j.1365-3024.1990.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 31.Al-Sherbiny M, Osman A, Barakat R, El Morshedy H, Bergquist R, Olds R. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 2003;88:117–30. doi: 10.1016/s0001-706x(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 32.Dunne DW, Bickle QD, Butterworth AE, Richardson BA. The blocking of human antibody-dependent, eosinophil-mediated killing of Schistosoma mansoni schistosomula by monoclonal antibodies which cross-react with a polysaccharide-containing egg antigen. Parasitology. 1987;94:269–80. doi: 10.1017/s0031182000053944. [DOI] [PubMed] [Google Scholar]
- 33.Hagan P, Blumenthal UJ, Dunn D. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–5. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 34.Auriault C, Gras-Masse H, Pierce RJ. Antibody response of Schistosoma mansoni infected humans subjects to the recombinant P28 glutathione-S-transferase and to synthetic peptides. J Clin Microbiol. 1990;28:1918–24. doi: 10.1128/jcm.28.9.1918-1924.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grzych JM, Grezel D, Xu CB. IgA Antibodies to a protective antigen in human Schistosomiasis mansoni. J Immunol. 1993;150:527–35. [PubMed] [Google Scholar]
- 36.Webster M, Fallon PG, Fulford AJ, Butterworth AE, Ouma JH, Kimani G, Dunne DW. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 1997;19:333–5. doi: 10.1046/j.1365-3024.1997.d01-211.x. [DOI] [PubMed] [Google Scholar]
- 37.Hota-Mitchell S, Siddiqui AA, Dekaban GA. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–40. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 38.Fonseca CT, Cunha-Neto E, Goldberg AC, et al. Identification of paramyosin T cell epitopes associated with human resistance to Schistosoma mansoni reinfection. Clin Exp Immunol. 2005;142:539–47. doi: 10.1111/j.1365-2249.2005.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca CT, Cunha-Neto E, Kalil J, Jesus AR, Correa-Oliveira R, Carvalho EM, Oliveira SC. Identification of immunodominant epitopes of Schistosoma mansoni vaccine candidate antigens using human T cells. Mem Inst Oswaldo Cruz. 2004;99:63–6. doi: 10.1590/s0074-02762004000900011. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca CT, Cunha-Neto E, Goldberg AC, Kalil J, de Jesus AR, Carvalho EM, Correa-Oliveira R, Oliveira SC. Human T cell epitope mapping of the Schistosoma mansoni 14-kDa fatty acid-binding protein using cells from patients living in areas endemic for schistosomiasis. Microbes Infect. 2005;7:204–12. doi: 10.1016/j.micinf.2004.10.012. [DOI] [PubMed] [Google Scholar]