Abstract
Influenza virus causes a contagious and potentially serious infection of the upper respiratory tract. While neutralizing antibodies are protective against infection, the problem of antigenic drift remains, requiring the constant monitoring and development of new vaccines. The magnitude of this situation is underscored by the emergence of new potentially human pathogenic influenza strains, avian H5N1 being the most recent example. We present evidence that antibodies against T cell immunoglobulin mucin-1 (TIM-1), a recently identified immunomodulatory molecule, stimulate cellular immunity against influenza viruses and cross-strain immune reactivity. To determine potential immunostimulatory properties of anti-TIM-1, mice were vaccinated with inactivated influenza virus in the presence or absence of TIM-1-specific monoclonal antibodies. Development of cellular immunity against both the influenza strain used for immunization and serotypically distinct virus strains was monitored 3 weeks after vaccination by determining antigen-specific lymphocyte proliferation and cytokine production. Results show that TIM-1 antibodies enhance antigen-specific cellular proliferation (P < 0·05) and interferon (IFN)-γ production (P < 0·01). Using blocking anti-CD4 and CD8 antibodies, it was observed that antigen-specific cellular proliferation is CD4-dependent and that the majority of proliferating cells are CD4+. Finally, vaccination with inactivated influenza virus with TIM-1 antibody results in the significant (P < 0·001) induction of proliferation and IFN-γ production upon stimulation with one of three serologically distinct strains. TIM-1 antibodies demonstrate an adjuvant effect promoting antigen-specific cellular proliferation and IFN-γ production, which are important for the promotion of cell-mediated immunity. These results are the first to suggest that TIM-1 antibody may serve as a potent adjuvant in the development of new influenza virus vaccines.
Keywords: adjuvant, IFN-γ, influenza, heterosubtypic, TIM-1
Introduction
Influenza virus is the causative agent of contagious, and potentially serious, infections of the upper respiratory tract. Worldwide, some 250 000–500 000 people die each year from complications due to influenza virus [1]. Failure to identify correctly or supply adequately a particular vaccine for a given year, as observed for the 2003–04 and 2004–05 seasons, can have serious consequences [2,3]. The recent outbreaks of human infections with avian influenza virus suggests the possibility of a devastating pandemic similar to the 1918 ‘Spanish influenza pandemic’[4,5]. While there exists some debate about the virulence of the current H5N1 virus in humans, there is broad consensus that a highly virulent strain may occur in the near future. Despite the existence of vaccines that produce protective antibodies, influenza, in general, is still a worldwide problem. While neutralizing antibodies are the correlate of protection against influenza virus, rapid mutations of the haemagglutinin (HA) and neuraminidase (NA) coat proteins of the virus necessitate rigorous identification and virus-specific vaccine production. Febrile reactions associated with higher doses of influenza virus antigens precludes most strategies using a large number of serotypes in a single vaccine (reviewed in [6]). In addition to generating neutralizing antibody responses, another strategy is to generate protective cell-mediated immunity against influenza virus [7]. Vaccine adjuvants, primarily aluminium salts, have been used for more than 70 years, and their safety and efficacy for certain indications is well established [8]. However, one potential drawback to the use of aluminium salts is their failure to stimulate Th1 immunity and CD8+ T cell effector function [9]. As salts of aluminium hydroxide are the only clinically approved adjuvants, new adjuvants with the ability to enhance immunity, especially cell-mediated immunity, are urgently needed.
The TIM molecules are a recently discovered class of proteins with the ability to regulate the immune system. Cross-linking of TIM-1 on T cells by TIM-1 antibodies results in the co-stimulation of T cells [10]. Furthermore, enhanced proliferation and cytokine secretion was observed using T cells from DO11·10 mice cultured in the presence of the cognate antigen, ovalbumin and increased interferon (IFN)-γ as well as interleukin (IL)-4 in response to antigen was reported from mice treated with TIM-1 antibody [10]. We report here for the first time the use of TIM-1 antibody as an adjuvant in combination with a known infectious disease immunogen, influenza virus. Use of TIM-1 antibody results in an antigen dose-dependent enhancement of lymphocyte proliferation, IFN-γ production and cross-strain reactivity. We propose that approaches targeting TIM-1 may be used to develop novel prophylactic and/or therapeutic vaccination strategies.
Materials and methods
Viruses
Whole inactivated influenza viruses, A/Beijing/292/95 (Beijing, H1N1); A/Taiwan/1/86 (Taiwan, H1N1), A/Kiev/301/94-like A/Johannesburg/33/94 (Kiev, H3N2), A/Panama/2007/99 (Panama, H3N2) and A/Shandong/9/93 (Shandong, H3N2), were purchased from BioDesign Inc. (Houston, TX, USA) and Advanced Immunochemicals (Long Beach, CA, USA).
Mice and immunizations
Six- to 8-week-old female BALB/c mice (Jackson Laboratories Bar Harbor, ME, USA) were used for this study. Treatment of animals was in accordance with regulations outlined in the USDA Animal Welfare Act and specifications in the Guide for Care and Use of Laboratory Animals [11]. Mice were vaccinated with 10 µg of whole inactivated virus mixed with either 100 µg of TIM-1 antibody or isotype-control antibody in a volume of 200 µl in phosphate-buffered saline (PBS). All immunizations were conducted via the intraperitoneal route (i.p.).
Antibodies
Initially, preservative-free rat anti-mouse TIM-1 monoclonal antibody (clone 222414, rat IgG2b, low endotoxin) and a rat anti-KLH isotype-control antibody (clone 141945, IgG2b) were purchased from R&D Systems (Minneapolis, MN, USA). More recent studies were performed with in-house-generated rat anti-mouse TIM-1 monoclonal antibodies, Am1-005 and Am1-006, or with the commercial antibody RMT1-4 (e-Biosciences, San Diego, CA, USA), yielding essentially identical results. Antibodies and antigen reagents were evaluated for low endotoxin using a chromogenic limulus amebocyte lysate endotoxin assay (Cambrex Bioscience, Walkersville, MD, USA). To block the proliferation of CD4+ and CD8+ T cells, blocking antibodies GK1·5 (rat anti-mouse CD4 [12]) and 53–6·7 (rat anti-mouse CD8 [13]) were used at a final concentration of 10 µg/ml in the proliferation assays.
Proliferation assay
Twenty-one days after vaccination, spleens were harvested from immunized and control mice and splenocytes prepared for in vitro assays. Single-cell splenocyte suspensions were prepared by mechanical disruption. After red blood cell (RBC) lysis with ACK lysing solution (Invitrogen, Carlsbad, CA, USA), the cells were washed and resuspended in complete media [RPMI-1640, 10% fetal bovine serum (FBS), GlutaMAX™, 5 µmβ-ME] and adjusted to 5 × 106 viable cells/ml. Cells (100 µl per well) were incubated in quadruplicate with increasing amounts of whole influenza virus in a final volume of 200 µl in flat-bottomed, opaque white-wall plates for 96 h at 37°C and 5% CO2. In other experiments, incubating cultures for 72 h yielded similar results (data not shown). Sixteen hours prior to harvest, the cells were pulsed with 10 µM bromodeoxyuridine (BrdU) and processed according to the procedures for the Delfia Proliferation Assay (Perkin-Elmer, Wellesley, MA, USA). Anti-BrdU Europium-based fluorescence was detected using a Wallac-1420 Victor-2 time-resolved fluorimeter. Results are represented as relative fluorescence units (RFU) ± standard error of the mean (s.e.m.).
Cytokine assays
Supernatants were derived from the cultures described above. Briefly, supernatants were harvested after 96 h and assayed for the presence of IFN-γ (R&D Systems, DuoSet no. 04485) and IL-4 (BD Biosciences, San José, CA, USA; capture antibody, no. 11B11; detection antibody, no. BVD6-2462) using a sandwich enzyme-linked immunosorbent assay (ELISA). The resulting optical density was read on a microtitre plate reader (ELX-808, BioTek Instruments, Winooski, VT, USA) with 540 nm wavelength correction.
Statistical analyses
In vivo experiments were conducted using four to five mice per group. Data from all experiments were analysed with the GraphPad Prism graphical analysis software (version 4·02, GraphPad, Inc., San Diego, CA, USA). Plots are represented as mean values ± s.e.m. Comparisons between groups were made by two-way anova using Bonferroni post-tests. P-values equal to or less than 0·05 were considered to be significant.
Results
TIM-1 antibodies enhance antigen-specific proliferation and IFN-γ production
Previous reports have indicated that interference with the signalling pathways for TIM-1 and its ligand TIM-4 has a strong effect on the regulation of immune responses [10,14]. For example, immunization with antigen in combination with TIM-1 antibody and complete Freund’s adjuvant results in increased in vitro cellular proliferation and IFN-γ and IL-4 production [10]. In order to determine whether TIM-1 antibody can act as an adjuvant in combination with influenza virus in a vaccination model, BALB/c mice were injected with 10 µg whole inactivated Beijing H1N1 in the presence of 100 µg of TIM-1 antibody. After 21 days, splenocytes from immunized mice were isolated and cultured in vitro in the presence of homologous antigen for 96 h. Splenocytes from mice immunized with inactivated Beijing virus and TIM-1 antibody showed a significant increase in homologous antigen-dependent proliferation (Fig. 1a, P < 0·05). The proliferation was both in vitro dose-dependent and antigen-specific, as stimulation using an irrelevant antigen, ovalbumin, did not induce proliferation (data not shown). Proliferation in mice that were vaccinated with virus plus isotype control was not significantly different from PBS controls (Fig. 1a). A general trend of lymphocyte proliferation was observed for all groups, which may be attributed to the mitogenic effects of HA [15]. In order to determine whether antigen stimulation produced Th1 or Th2 cytokines from these cells, supernatants were harvested and tested in both an IFN-γ or IL-4 cytokine ELISA. As shown in Fig. 1b, significant amounts of IFN-γ (6000 pg/ml, P < 0·001) were secreted by cells upon Beijing antigen stimulation. Isotype or naive PBS groups showed only 1000 pg/ml IFN-γ at the highest concentration of antigen. IL-4 production in all groups was very low, and at the limit of detection of the cytokine ELISA ( < 100 pg/ml).
Fig. 1.
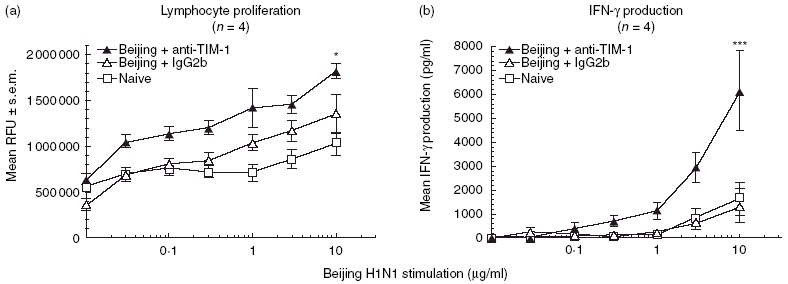
T cell immunoglobulin mucin-1 (TIM-1) antibodies enhance antigen-specific cellular proliferation and interferon (IFN)-γ production. BALB/c mice (four per group) were vaccinated intraperitoneally (i.p.) with 10 µg whole inactivated Beijing influenza virus mixed with 100 µg TIM-1 antibody (α-TIM-1; black triangles), isotype control (IgG2b; open triangles) or phosphate-buffered saline (PBS) (naive; open squares). After 21 days, splenocytes were harvested and cultured in vitro with increasing amounts of whole inactivated Beijing influenza virus for 96 h and analysed for proliferation (a). Supernatants were removed for ELISA analysis of IFN-γ production (b). Data represent the mean ± s.e.m. Statistical significance is denoted by asterisk for values compared to isotype control. *P < 0·05; **P < 0·001.
The proliferative effects of TIM-1 antibodies are due mainly to CD4+ T cells
To characterize further the cellular nature of the antigen-specific proliferative response observed, T cell proliferation blocking anti-CD4 and anti-CD8 monoclonal antibodies were co-incubated with some of the experimental wells to determine whether the proliferative effect observed was due to a particular T cell subpopulation. We found that the majority of the T cell response was due to CD4+ T cells, as blockade with anti-CD4 antibodies greatly reduced cellular proliferation (Fig. 2a, black triangles). In the absence of in vitro anti-CD4 antibodies (Fig. 2a, open triangles), proliferation in response to antigen is enhanced significantly in the animals vaccinated in the presence of TIM-1 antibodies (P < 0·001). However, in the presence of blocking anti-CD4 antibodies, antigen-dependent proliferation is lost (black triangles). In contrast, anti-CD8 blocking antibodies had little or no effect on proliferation in these experiments (Fig. 2b, open circles versus black circles). Analysis of the corresponding cytokine production yielded similar results. Anti-CD4 antibodies resulted in a significant reduction of IFN-γ production, while anti-CD8 blockade had no effect on the amount of IFN-γ produced (data not shown).
Fig. 2.
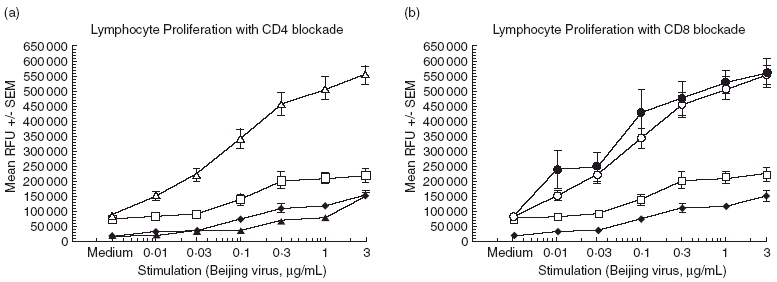
Proliferation in response to antigen is CD4 T cell-dependent. BALB/c mice (six per group) were immunized with 10 µg Beijing influenza virus and 100 µg T cell immunoglobulin mucin-1 (TIM-1) antibody or isotype control. After 21 days, the splenocytes were harvested and cultured as described. Antigen-stimulated proliferation of lymphocytes in the presence of anti-CD4 or anti-CD8 antibodies was compared to lymphocytes stimulated in the absence of blocking antibodies. Antibodies specific for CD4 or CD8 were added to wells containing lymphocytes to a final concentration of 10 µg/ml and allowed to incubate for 96 h in culture prior to analysis. (a) Open triangles, stimulation in the absence of anti-CD4; black triangles, with anti-CD4. (b) Open circles, stimulation in the absence of anti-CD8; black circles, with anti-CD8. Control groups for both (a) and (b): open squares, phosphate-buffered saline (PBS) vaccination; black diamonds, Beijing virus vaccination only. Data represent the mean ± s.e.m. Statistical significance is denoted by asterisk for values compared to stimulation in the presence of blocking antibodies. ***P < 0·001.
TIM-1 antibodies generate cross-strain immune responses
To determine if the Beijing (H1N1)-dependent response was influenza serotype-specific, the splenocytes from Beijing/H1N1-immunized mice were stimulated in vitro with a heterologous inactivated Kiev influenza, an H3N2 virus. While in some experiments high Kiev viral antigen concentrations resulted in cellular toxicity, we still observed that splenocytes from mice vaccinated with Beijing/H1N1, when immunized in the presence of TIM-1 antibody, proliferated in response to Kiev/H3N2 virus stimulation (Fig. 3, P < 0·001). No cross-strain response was observed in the isotype or PBS control groups, suggesting that the TIM-1 antibody had a direct effect on the priming against conserved viral antigens that could possibly include the matrix protein (MP) and nucleoproteins (NP). IFN-γ production in response to Kiev antigen stimulation was also increased (Fig. 4a). As with the homologous challenge, the IL-4 levels were less than 100 pg/ml for all groups.
Fig. 3.
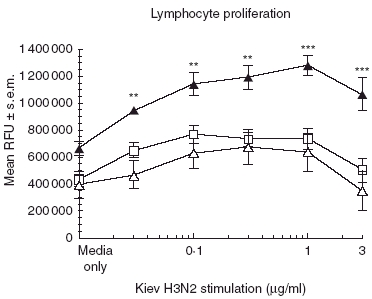
T cell immunoglobulin mucin-1 (TIM-1) antibodies enhance cellular proliferation upon stimulation with a heterologous antigenBALB/c mice (four per group) were vaccinated intraperitoneally (i.p.) with 10 µg whole inactivated Beijing influenza virus mixed with 100 µg TIM-1 antibody (α-TIM-1; black triangles), isotype control (IgG2b; open triangles) or phosphate-buffered saline (PBS) (naive; open squares). After 21 days, splenocytes were harvested and cultured in vitro with increasing amounts of whole inactivated Kiev (H3N2) influenza virus for 96 h and analysed for proliferation. Data represent the mean ± s.e.m. Statistical significance is denoted by asterisk for values compared to isotype control. **P < 0·01; ***P < 0·001.
Fig. 4.
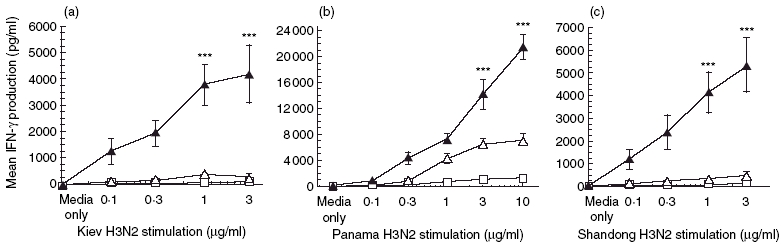
Vaccination with antigen and T cell immunoglobulin mucin-1 (TIM-1) antibodies induces cross-strain reactivity. BALB/c mice (four per group) were immunized with 10 µg Beijing influenza virus and 100 µg TIM-1 antibody (black triangles), isotype control antibody (open triangles) or phosphate-buffered saline (PBS) (open squares). After 21 days splenocytes were harvested and cultured in vitro with increasing amounts of whole inactivated influenza viruses (a, Kiev; b, Panama; c, Shandong) for 96 h. Culture supernatants were collected after the incubation period and assayed for the presence of interferon (IFN)-γ by enzyme-linked immunosorbent assay (ELISA). Interleukin (IL-4) was not detectable in these experiments. Data represent means of duplicates ± s.e.m. Statistical significance is indicated by asterisk for values compared to the isotype antibody control. ***P < 0·001.
TIM-1 antibodies generate heterosubtypic responses
Moran et al. demonstrated that increased IFN-γ facilitated recovery of mice from challenges with a serologically distinct viral strain [16]. In light of our data showing increased T cell proliferation and IFN-γ production using TIM-1 antibody, we wanted to determine whether vaccination with one serotype of influenza virus, e.g. Beijing H1N1, in combination with TIM-1 antibodies, would also result in enhanced IFN-γ production when stimulated by a panel of viruses representing different serotypes. As shown in Fig. 4a, we find that splenocytes from mice vaccinated with Beijing H1N1 in the presence of anti-TIM-1 produce strongly elevated (17-fold) amounts of IFN-γ after restimulation with Kiev H3N2 (P < 0·001), but not control antibody. The response was largely Th1 in character, as IL-4 production was low to undetectable for all groups ( < 50 pg/ml; data not shown). Furthermore, mice immunized with the inactivated Beijing/H1N1 strain not only mount an immune response against Beijing (Fig. 1) and against the Kiev H3N2 strain (Fig. 4a), but also against other influenza strains belonging to the H3N2 serotype (A/Panama/2007/99, Fig. 4b; A/Shandong/9/93, Fig. 4c). The spleens from mice vaccinated with Beijing virus + TIM-1 antibody were found to express IFN-γ at levels of up to 22 000 pg/ml when stimulated with the Panama-inactivated influenza virus isolate (threefold, P < 0·001) and 5000 pg/ml when stimulated with Shandong (11-fold, P < 0·001).
Discussion
It has been well documented that Th1 cytokines such as IFN-γ are required for the clearance of viral infections and, more importantly, for immunity against distinct serological strains of influenza virus [16]. In a successful response to certain viral infections, such as influenza, increased production of IFN-γ, together with cell-mediated immunity, is largely responsible for viral clearance. Accordingly, lack of IFN-γ has been found to correlate with higher influenza viral titres and decreased CTL activity [17]. In line with these observations, we show that TIM-1 antibodies not only stimulate anti-viral antigen-specific cellular proliferation and IFN-γ production against the immunization antigen, but also stimulate cross-strain reactivity against distinct influenza subtypes. Mice immunized with whole inactivated Beijing H1N1 virus mixed with an isotype-matched control antibody responded with a poor IFN-γ response when stimulated with Beijing H1N1 in vitro. In comparison, mice vaccinated with antigen and TIM-1 antibody consistently showed a three- to 17-fold higher dose-dependent IFN-γ response against not only the immunization antigen, H1N1, but also against H3N2 serotypes. The response to the H3N2 virus, A/Panama/2007/99, was particularly high with respect to the absolute amounts of IFN-γ produced, generating 22 000 pg/ml. Similarly, the response to other H3N2 viruses such as Kiev and Shandong, produced significant amounts of IFN-γ that were approximately 10-fold higher than the isotype-matched control groups. Despite the generation of influenza cellular cross-strain immunity, the immune responses generated were none the less antigen-dependent, as vaccination with inactivated virus did not stimulate immune responses against unrelated antigens, such as ovalbumin or other viruses (data not shown).
Our results indicate that the major population of cells responsible for the observed increased cellular immunity were CD4+ T cells, as blocking anti-CD4 but not blocking anti-CD8 antibodies strongly inhibited TIM-1 antibody-mediated cellular proliferation in response to antigen. Lymphocytes were stained for CD3, CD4, CD8 and CD69 and examined by flow cytometry. No significant differences in populations were observed between treatment groups. In addition, preliminary analyses of influenza virus-specific antibody responses suggest that TIM-1 antibodies do not affect B cell responses negatively after vaccination (data not shown), further supporting the T cell stimulatory activity of TIM-1 antibodies and indicating that the use of TIM-1 antibodies as a vaccine adjuvant in vivo does not affect the generation of neutralizing antibodies, an essential component of the activity of available commercial vaccines.
Our results are consistent with the recently reported observation that TIM-1 plays an important role in the co-stimulation of T cells [10]. Umetsu et al. suggest that TIM-1 is activated by homodimerization of the receptor through binding of bivalent TIM-1 antibodies, as monomeric TIM-1 antibody Fabs do not stimulate T cell proliferation in their system. Intriguingly, dimeric TIM-4/Fc is also capable of stimulating T cell proliferation [14], suggesting that binding of either the natural ligand TIM-4 or TIM-1 antibody leads to dimerization of TIM-1, signalling through tyrosine kinase-mediated signal transduction pathways [18] and subsequent antigen-dependent T cell proliferation and IFN-γ production. The results reported herein clearly support this notion.
It is probable that the majority of the cross-strain responsiveness observed is against conserved antigens unrelated to HA or NA, such as MP or NP. Preliminary experiments conducted using commercial vaccines such as Fluzone™ and Fluvirin™ in combination with TIM-1 antibody have generated similar increases of lymphocyte proliferation and IFN-γ production, as reported here for recombinant inactivated complete influenza viruses (data not shown). These vaccines are subunit vaccines containing HA and NA proteins from inactivated influenza strains. In addition, they contain considerable amounts of NP and MP as identified by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and ELISA (data not shown). When administered in the presence of TIM-1 antibody, it is conceivable that these nuclear and matrix components may serve as additional immunogens. In other experiments conducted in our laboratory, TIM-1 antibodies have also enhanced the efficacy of the hepatitis B vaccine, Engerix™, as well as recombinant HIV p24 protein (manuscript in preparation).
This study represents the first report of the use of TIM-1 antibody to enhance T cell immune responses against a known infectious disease pathogen, influenza virus. We recognize that it will be important to determine whether the increase in lymphocyte proliferation and IFN-γ production is sufficient to protect mice from a live challenge using homologous, or even heterologous, viruses. However, the initial data presented here suggest that TIM-1 antibodies are attractive candidates in the search for improved vaccine adjuvants.
Acknowledgments
The authors thank Christina Lu, Elena Prokopenko and Edda Ogami, who rendered technical assistance for this work. We also thank Jennifer Brandt for her assistance in the preparation of this manuscript. The authors gratefully acknowledge the generous support of Mr Randall L. Pittman and Mr James W. F. Brooks.
References
- 1.World Health Organization (WHO). Influenza Fact Sheet. Available at: http://wwwwhoint/mediacentre/factsheets/fs211/en/ accessed October 2005.
- 2.Orr P. National Advisory Committee on Immunization (NACI). Statement on influenza vaccination for the 2003–04 season. Advisory committee statement (ACS) Can Commun Dis Rep. 2003. pp. 1–20. [PubMed]
- 3.Orr P. Statement on influenza vaccination for the 2004–05 season. Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI) Can Commun Dis Rep. 2004;30:1–32. [PubMed] [Google Scholar]
- 4.Oxford JS, Lambkin R, Sefton A, et al. A hypothesis: the conjunction of soldiers, gas, pigs, ducks, geese and horses in Northern France during the Great War provided the conditions for the emergence of the ‘Spanish’ influenza pandemic of 1918–19. Vaccine. 2005;23:940–5. doi: 10.1016/j.vaccine.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Oxford JS. The so-called Great Spanish Influenza Pandemic of 1918 may have originated in France in 1916. Phil Trans R Soc Lond B Biol Sci. 2001;356:1857–9. doi: 10.1098/rstb.2001.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salk J, Salk D. Control of influenza and poliomyelitis with killed virus vaccines. Science. 1977;195:834–47. doi: 10.1126/science.320661. [DOI] [PubMed] [Google Scholar]
- 7.Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity. cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997;71:2715–21. doi: 10.1128/jvi.71.4.2715-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylor NW, Egan W, Richman P. Aluminum salts in vaccine − US perspective. Vaccine. 2002;20(Suppl. 3):S18–23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 9.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–45. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 10.Umetsu SE, Lee WL, McIntire JJ, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–54. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Laboratory Aninal Research, Commission on Life Sciences, National Research Council. ILAR Publication. Washington: National Academy Press; 1996. The Guide for Care and Use of Laboratory Aninals. [Google Scholar]
- 12.Wilde DB, Marrack P, Kappler J, Dialynas DP, Fitch FW. Evidence implicating L3T4 in class II MHC antigen reactivity; monocloncal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983;131:2178–83. [PubMed] [Google Scholar]
- 13.Takahashi K, Nakata M, Tanaka T, et al. CD4 and CD8 regulate interleukin 2 responses of T cells. Proc Natl Acad Sci USA. 1992;89:5557–61. doi: 10.1073/pnas.89.12.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers JH, Chakravarti S, Schlesinger D, et al. TIM-4 is the ligand for TIM-1, and the TIM-1–TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–64. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 15.Anders EM, Scalzo AA, White DO. Mitogenic activity of influenza virus and haemagglutinin. Vaccine. 1985;3:241–4. doi: 10.1016/0264-410x(85)90115-x. [DOI] [PubMed] [Google Scholar]
- 16.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 17.Mbawuike IN, Acuna C, Caballero D, et al. Reversal of age-related deficient influenza virus-specific CTL responses and IFN-gamma production by monophosphoryl lipid A. Cell Immunol. 1996;173:64–78. doi: 10.1006/cimm.1996.0252. [DOI] [PubMed] [Google Scholar]
- 18.De Souza AJ, Oriss TB, O’Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a co-stimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–18. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]


