Abstract
Mucous membrane pemphigoid (MMP) (also known as cicatricial pemphigoid) is a rare autoimmune mucocutaneous blistering disease that affects mucous membranes derived from stratified squamous epithelium and the skin. A subset of MMP affects only the oral cavity and is referred to as the oral pemphigoid (OP). MMP and OP are characterized by subepithelial vesicles on histology and in vivo deposition of immunoglobulins and complement at the basement membrane zone (BMZ) on immunopathology. Previous studies have shown that sera of patients with MMP bind to human integrin β4, while sera of patients with oral pemphigoid bind to the integrin α6 component of the heterodimer. The prognosis in MMP is grave but excellent in OP. In this study we compare the binding of sera from patients with OP from Boston, MA, USA to Naples, Italy, and attempt to identify an epitope to which the anti-integrin α6 human autoantibody binds. Our results indicate that the sera from Boston and Naples are identical in their reactivity. They recognize a fragment I (AA 23–462) and its subfragment IB (AA 217–462) only, in the human integrin α6 molecule. Blocking studies, immunoprecipitation and immunoabsorbtion studies confirm the presence of this single 245 AA region. Antibodies to subfragment IB cause BMZ separation in organ culture using normal human oral mucosa as substrate. This preliminary study indicates that patients on both continents may have similar reactivity and suggests that an intercontinental study group could be established to advance our understanding of the pathogenesis of OP and the biology of anti-α6 integrin autoantibodies.
Keywords: anti-integrin α6 subunit antibody, epitope binding, Italian and US patients, mucous membrane pemphigoid, oral pemphigoid
Introduction
Mucous membrane pemphigoid (MMP) is an autoimmune mucocutaneous blistering disease, which predominantly affects mucosal tissues derived from stratified squamous epithelium and the skin [1]. The autoantibodies in such patients are present against various components in the basement membrane zone (BMZ). These molecules play a critical role in binding the basal epithelial cells to the underlying basement membrane, submucosa or dermis [2].
Oral pemphigoid (OP) is a subset of MMP that affects only oral mucosa [3], and in most patients heals without scarring [4].
A subepithelial vesicle in the oral mucosa characterizes OP. Sera of patients with OP contain autoantibodies directed against the integrin alpha 6 (α6) subunit, which is a component of the hemidesmosome [5]. The binding of integrin α6β4 to its ligand plays an important role in the integrity of the basement membrane [6,7]. Hence it would appear reasonable to presume that antibodies to the integrin α6 subunit could interfere with cell-basement membrane adhesion and possibly influence BMZ separation, which could lead to subepithelial blister formation.
It has been shown recently that autoantibodies to desmoglein3 that cause pemphigus vulgaris (PV) can initiate intracellular phosphorylation events [8]. The binding of autoantibodies to human BP-180 on the hemidesmosome can trigger secretion of interleukin (IL)-6 and IL-8 from human keratinocytes [9]. These interleukins and other cytokines facilitate the recruitment of inflammatory cells to the site of pathology [10]. The identification of epitope(s) within the integrin α6 subunit, to which the pathogenic autoantibodies bind, is important for understanding the pathophysiology of OP. Such binding could initiate and promote events that eventually cause BMZ separation.
In this study we describe a region within the integrin α6 molecules recognized by autoantibodies present in the sera of OP patients. Our purpose was to determine if sera from OP patients in the United States and Italy recognize integrin α6 and, if so, the same epitope(s) within the molecule. Cloned fragments of the integrin α6 subunit identified the binding site of the OP autoantibody. The ability of antibodies to this region to cause BMZ separation in organ culture of oral mucosa suggests the importance of this region in the pathogenesis of OP in patients in North America and Europe.
Materials and methods
Patient sera
Sera used in this study were obtained from four untreated patients with active OP in Naples, Italy. Sera from four untreated patients with severe widespread active disease in Boston, MA, USA were also studied. The extent and severity of disease was identical in both groups. These patients had the pemphigoid disease process limited only to the oral cavity. This was based on a minimum of 3 years’ follow-up, after immunopathological diagnosis. Routine histology showed a subepithelial vesicle with mixed cell infiltrate in the submucosa. Direct immunofluorescence (DIF) showed the deposition of IgG and C3 at the BMZ of perilesional tissues in all patients. During the follow-up period patients were examined carefully, diligently and deliberately for ocular, nasal, upper airway, oesophageal, vaginal, penile or anal involvement. The clinical diagnosis of OP was established by routine histology and confirmed by direct immunofluorescence. The eight patients reported in this study have not been reported in any of our earlier publications. Control sera were obtained from five healthy individuals, five patients with PV, five with MMP, five with bullous pemphigoid (BP) and five with ocular cicatricial pemphigoid (OCP). Blood samples were collected after informed consent, and the Institutional Review Board approved the study.
Antibodies
Polyclonal antibodies to cloned fragments of the integrin α6 subunit were produced in rabbits (Sigma Genosys, The Woodlands, TX, USA). New Zealand rabbits were immunized subcutaneously (s.c.) with 100 µg of purified protein fragments. Pre- and post-immunization sera were collected from respective rabbits.
The control antibodies BQ16 (Ancell Corp., Bayport, MN, USA) and GoH3 (R&D Systems, Minneapolis, MN, USA) against the integrin α6 subunit, and His-Tag (EMD Biosciences, San Diego, CA, USA) were used.
Analysis of antigenic determinants
The protein sequence of the integrin α6 subunit was analysed for antigenicity, flexibility and beta turn with pcgene software. Peak values were assigned for each criterion. Regions of the molecule in which the three criteria were simultaneously present and where peak values for the three criteria were greater than 1·0 were selected for designing of cloned fragments.
Cloning of fragments of the integrin α6 subunit
Fragments representing different parts of the sequence of the human integrin α6 subunit were generated from the frill-length molecule, a generous gift from Professor Arnoud Sonnenberg (the Netherlands Cancer Institute, Amsterdam, the Netherlands).
The fragments representing the extracellular domain are designated as I (23aa−462aa) and II (463aa−1011aa). Fragments IA (23aa−131aa) and 1B (217aa−62aa) are subfragments of the extracellular fragment I. Fragment III (857aa−1073aa) represents part of the extracellular portion and the complete transmembrane and intracellular portion, and fragment IV (l0l2aa−1073aa) represents the complete intracellular portion of the integrin α6 molecule. The fragments we used in this study are represented schematically in Fig. 1. Fragments II, III and III, IV overlap each other.
Fig. 1.
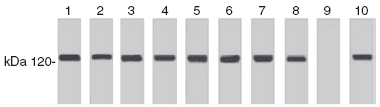
Identification of oral pemphigoid (OP) antigen by OP sera. Immunoblot analysis of four sera from Boston (lanes 1–4) and four sera from Naples, Italy (lanes 5–8). Lane 9 is normal human serum. Lane 10 demonstrates binding of monoclonal antibody GoH3 to DU 145 cell lysate. Note the presence of 120 kDa band indicating binding of sera to human integrin α6.
The polymerase chain reaction (PCR) products were ligated into a Gateway entry vector pENTR IA (Invitrogen, Carlsbad, CA, USA). The restriction sites for Sail and EcoRV were created in the 5′ and 3′ primers, respectively, to facilitate the subcloning. The plasmid isolated and purified from the positive clones was fused with Gateway destination vector pDEST14 (Invitrogen) by LR recombination reaction with clonase enzyme (Invitrogen). To facilitate protein purification, a His-Tag sequence was fused to the N-terminus of the cloned fragments. The correctness of the sequence was verified by sequencing the fragments after cloning them in gateway entry and destination vectors.
Expression and purification of the fragments of the integrin α6 subunit
BL21λDE3plysS strain of Esherichia coli was used for protein expression. The proteins expressed formed inclusion bodies. The inclusion bodies were solubilized in 8 M urea, 20 mM phosphate buffer pH 7·8 and 500 mM NaC1 and passed through an Ni-NTA column equilibrated in the same buffer. Contaminating proteins were washed twice with 4 vols of 8 M urea, 20 mM phosphate buffer pH 6·0 and 500 mM NaC1. The column was then washed twice with 5 vols of 50 mM phosphate buffer pH 8·0 500 mM NaC1 and 20 mM imidazole. Proteins containing His-Tag were finally eluted in 0·5 ml fractions with 50 mM phosphate buffer pH 8·0, 500 mM NaC1 and 250 mM imidazole.
Characterization of OP sera and antibodies against cloned fragments of integrin α6 subunit by immunoblot using DU145 cell lysates
The OP autoantibodies and polyclonal antibodies raised in rabbits against fragments of integrin α6 subunit were tested by Western blot using DU145 cell lysate as described previously [11].
Characterization of OP sera and antibodies against cloned fragments of integrin α6 by immunoprecipitation
OP sera and antibodies against cloned fragments of integrin α6 were characterized further by standard immunoprecipitation technique, as described previously [12].
Characterization of integrin α6 fragments and binding with OP patient serum by Western blot analysis
Characterization of the purified integrin α6 fragments was performed by standard Western blot analysis. Fragments of integrin α6 were separated using semipreparative 4–20% acrylamide gel and transferred to nitrocellulose membrane under the conditions described above. After transfer of the proteins the membranes were cut into 5 mm-wide strips. After blocking with 1% alkali-soluble casein for 1 h the strips were incubated with OP patient sera at 1:100 dilution, then probed with horseradish peroxidase (HRP)-conjugated goat anti-human IgG.
Blocking of OP antibody epitope in fragment IB
Fragment IB was transferred onto a nitrocellulose membrane and the specificity of the OP autoantibodies was tested further for fragment IB of integrin α6. The membrane was blocked with 1% alkali-soluble casein and incubated with rabbit antibodies to fragment IB (1:500). The membrane was washed and treated with OP patient serum (1:100) then reacted with HRP-conjugated goat anti-human IgG. As control, another blot of fragment TB was reacted with serum from the same patient with OP and rabbit antibodies to fragment IB; the blot was probed by HRP-conjugated goat anti-human and goat anti-rabbit IgG, respectively.
Characterization of antigen binding site (epitope) on fragment IB
To purify and to test the reactivity of OP antibodies and to test the presence of additional epitopes in fragment IB of the integrin α6 subunit, OP patient serum was passed through a column of integrin α6 subfragments IB coupled to CNBr-activated Sepharose 4B. The unbound proteins (flow-through) and eluted antibodies were tested for their reactivity with fragment I and IB by Western blot.
Characterization of binding of antibodies to cloned fragments of integrin α6 subunit in oral BMZ
Sections of 4 µ thickness each of normal human oral mucosa were incubated with (i) OP sera, (ii) immunoaffinity purified OP sera, (iii) antibodies to cloned fragments of the integrin α6 subunit and (iv) monoclonal antibodies GoH3 and BQ16. The sections were stained by immunoperoxidase reaction and viewed under a microscope. Normal human sera were the negative control.
Effect of OP antibodies on oral mucosa in organ culture
In order to determine the ability of test antibodies to cause BMZ separation in human oral mucosa, we used an in vitro culture model with normal human oral mucosa, as described previously [5]. Patient serum, immunoaffinity purified OP serum and antibodies to fragments of the integrin α6 subunit and monoclonal antibodies BQ16 and GoH3 were tested.
Results
Identification of OP autoantigen
The autoantigen for OP was identified by immunoblotting and immunoprecipitating the proteins present in the cell lysate of DU145 cells by sera from OP patients. The OP patient sera (n = 8) detected a ∼120 kDa protein, identified as the integrin α6 subunit by monoclonal antibodies GoH3 (Fig. 1).
Expression and purification of extracellular and intracellular fragments of integrin α6 subunit
Four potential antigenic regions were identified on the frill-length integrin α6 subunit molecule by pcgene software analysis. Hence the molecule was divided into two extracellular fragments (I and II); fragment III is partly extracellular and intracellular and fragment IV is an intracellular portion (Fig. 2a). The approximate size of the fragments were: I, 1370 base pairs (bp); IA, 327 bp; IB, 738 bp; II, 1647 bp; III, 651 bp; and IV, 84 bp. The sequence analysis of the cloned fragments matched the integrin cz6 sequence in the NLM pubmed database sequence.
Fig. 2.

(a) Schematic representation of subcloning of various fragments of the human integrin α6 molecule. The human integrin α6 subunit was cloned into four fragments (I–IV). Fragment I was subcloned into two subfragments, IA and IB. (b) Expression and purification of various fragments and subfragments of the human integrin α6. Immunoblot analysis of fragments I–IV and subfragments IA and IB, demonstrating the sizes of the various cloned proteins.
The protein fragments expressed were of the following sizes: T, ∼80 kDa; TA, ∼33 kDa; TB, ∼40·2 kDa; TT, ∼82·5 kDa; TTT, ∼40·8 kDa; and TV, ∼7 kDa (Fig. 2b). The monoclonal antibody BQ16 detected fragment II by immunoblot, while GoH3 detected fragments I and TB by immunoprecipitation and immunoblot (Fig. 3).
Fig. 3.

Binding of oral pemphigoid (OP) sera to fragment IB by immunoprecipitation. The subfragment was immunoprecipitated by four OP sera from Boston, MA, USA and four OP sera from Naples, Italy. Note the presence of the 41 kDa band depicting fragment TB.
Recognition of cloned fragments of integrin α6 subunit by sera from OP patients
All the OP sera (n = 8) demonstrated binding to fragment I. None of the OP sera bound to fragments II, III and IV. There was no difference in the binding pattern of the sera from the patients in Boston, MA and Naples, Italy. Normal human sera (n = 5) samples and sera of OCP, MMP, BP and PV patients did not demonstrate detectable levels of binding to any of the cloned fragments of the integrin α6 molecule. Western blot results of one representative patient are shown in Fig. 4.
Fig. 4.
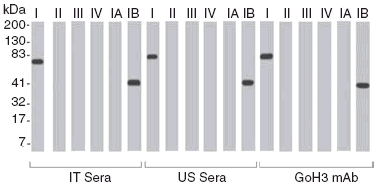
Recognition of cloned fragments and subfragments of the human integrin α6 subunit by oral pemphigoid sera. Immunoblot ananlysis of one representative sample from Naples, Italy (IT sera) and one representative sample from Boston, MA, USA (US sera) demonstrates binding to cloned fragments I and IB. The positive control monoclonal antibody GoH3 binds to fragments I and IB.
The sera from patients with OP were subsequently evaluated for binding with subfragments IA and TB of fragment I by Western blot. All the test sera (n = 8) bound to the subfragment TB (Fig. 4). None of the patient sera bound to IA. The four sera from patients in Boston, MA and the four sera from patients in Italy demonstrated identical binding to subfragment TB. No additional bands were observed.
Antibody reactivity of subfragment lB
The purpose of this experiment was to determine if the sera from different OP patients recognize different binding site(s) within the fragment TB. The fragment TB was immobilized on a CNBr-activated Sepharose 4B column and OP sera passed through the column. The unbound OP serum was tested for reactivity with the fragment TB. The unbound serum fraction did not show any reactivity with the fragments TB. Binding was observed with fragments I and TB (Fig. 5). This phenomenon was observed with all the OP sera tested obtained from Boston and Naples. The absorbed sera were subsequently incubated with nitrocellulose membrane with DU145 cell lysate. The absorbed sera did not demonstrate binding to the integrin α6 subunit on the immunoblot. Unabsorbed sera GoH3 and BQ16 demonstrated binding to a ∼120 kDa protein. These observations would suggest that the sera tested in this experiment recognized only one epitope in the subfragment TB in the α6 subunit.
Fig. 5.

Blocking of epitope in subfragment IB by oral pemphigoid (OP) sera and rabbit antibodies to subfragment IB. (a) Note that reaction of subfragment TB with Boston sera prohibits binding of Naples sera. (b) Reaction with Naples sera blocks binding with Boston sera. (c) Blocking with Boston sera blocks binding of rabbit antibody. (d) Binding with Naples sera blocks binding with rabbit antibody. Binding of OP sera to subfragment IB is demonstrated by a 41 kDa band.
Blocking of OP epitope in subfragment IB by rabbit antibodies
The purpose of this experiment was to determine if serum from an OP patient and rabbit anti-serum bind to the same region within fragment IB. Fragment IB was transferred onto nitrocellulose membrane, then incubated with rabbit antibodies to fragment IB and reacted subsequently with sera from a patient with OP. Binding of human OP serum was not observed (Fig. 6a). This indicated that rabbit antibody to fragment IB blocked the binding of human OP serum to a region of fragment IB. Rabbit antibodies, when incubated separately with subfragment IB to fragment IB and OP serum, demonstrated appropriate binding, as seen in Fig. 6b,c, respectively, and served as controls for this experiment. Similarly, when a 4 µ thick cryostat section of normal human oral mucosa is reacted first with rabbit antibodies to subfragment TB and subsequently with human OP serum, no binding is observed. In a reverse experiment, when the sections were first incubated with OP sera and then reacted with rabbit antibodies to TB, no binding was observed (Fig. 5). These experiments demonstrate that rabbit antibodies to subfragment IB and human OP sera recognize the same region within of the integrin α6 subunit molecule.
Fig. 6.

Reactivity of oral pemphigoid (OP) sera and rabbit antibodies to the integrin α6 subunit in organ culture using normal human oral mucosa. Separation of the epithelium from underlying submucosa is observed when normal human oral mucosa is incubated with OP sera (A), immunoaffinity purified OP sera (B), rabbit antibody to fragment I (C), monoclonal antibody GoH3 (D) and rabbit antibody to fragment TB (E). No basement membrane zone separation is noted when oral mucosa is incubated with normal human sera (F).
Detection of integrin α6 in the BMZ of normal human mucosa by antibodies to integrin α6 fragments
Smooth linear homogeneous staining of BMZ of normal human oral mucosa was observed on immunoperoxidase staining when reacted with OP sera, immunoaffinity purified OP antibodies and rabbit antibodies to cloned fragments (I, IA, IB, II, III and IV) of the integrin α6 subunit, GoH3 and BQ16 (data not shown). No binding was seen with normal human sera.
Effect of OP patient sera and rabbit antibodies to fragments of integrin α6 subunit on normal human oral mucosa in organ culture
BMZ separation in the normal human oral mucosa was observed in sections incubated with human OP serum, immunoaffinity purified OP patient’s serum and rabbit anti-serum to fragment I and IB and monoclonal antibody GoH3. PV serum caused typical acantholysis of epithelial cells and served as positive control. Normal human serum (NHS), BP sera, rabbit anti-serum to fragment II and monoclonal antibodies BQ16 did not cause BMZ separation (Fig. 6). These experiments indicate that antibodies to a region within the subfragment IB of integrin α6 are involved in the process of producing BMZ separation.
Discussion
Our previous studies identified the integrin α6 subunit, a ∼120 kDa protein, present in the BMZ of oral mucosa as the target antigen, to which the sera of OP patients bind [5].
In this study we have identified the region within the molecule to which the OP autoantibodies bind and are capable of producing BMZ separation in vitro. The OP autoantibody binding region lies in the extracellular domain between the amino acid residues 217–462 (subfragment IB).
Immunoblot analysis of sera from eight patients with active OP showed specific binding with the native frill-length integrin α6 subunit in DU145 cell lysate. The sera from patients of other subsets of MMP did not show binding with the integrin α6 subunit, indicating antigen specificity in this group of autoimmune diseases. Similar results were observed for cloned fragments of the integrin α6 subunit. The OP sera bound to the cloned fragment I and no binding was observed with the other three fragments of the integrin α6 subunit. Fragment I was divided into subfragments IA and IB. Subfragment IB reacted with all the OP patients tested, while no binding was observed with subfragment IA. The NHS and sera of patients of other MMP subsets did not react with either of the subfragments of fragment T of the integrin α6 subunit. Monoclonal antibody GoH3 bound to fragments I and IB, while monoclonal antibody BQ16 bound to fragment II. Blocking of subfragment IB by rabbit antibodies against it and then incubating with OP patient serum prevented the binding of OP autoantibodies from reacting with subfragment IB. This experiment further confirms the binding specificity of OP autoantibodies with subfragment IB of the integrin α6 subunit molecule.
The sera of four patients from Boston, MA, USA and four patients from Naples, Italy demonstrated identical binding to fragments I and IB of only the human integrin α6 molecule. Binding to other portion(s) of integrin α6 was not observed. While the small number of sera examined is a limitation of the study, the authors would like to demonstrate that sera from both cities and countries had an identical binding pattern. A larger group from multiple cities on both continents will need to be studied before definitive conclusions can be drawn. If these preliminary observations are confirmed, this would have a significant impact on the study of OP. OP is rare; sharing patients’ sera, cells and other biological specimens would advance significantly our better understanding of this disease and the role of antibodies to human integrin α6.
Immunoperoxidase staining demonstrated that the BMZ of normal human oral mucosa bind rabbit antibodies to cloned fragments and subfragmetns of integrin α6 in a manner identical to that observed with antibodies from patients with OP, immunoaffinity purified OP antibodies and monoclonal antibodies GoH3 and BQ16.
Organ culture studies demonstrate that sera from OP patients and immunoaffinity purified OP antibodies produce BMZ separation in normal human oral mucosa. Similar BMZ separation is observed with rabbit antibodies to fragment I and subfragment IB, but not to other fragments of integrin α6 molecules. Normal human sera and BP sera did not produce BMZ separation. Pemphigus vulgaris sera produced acantholysis of oral epithelium. Thus, the cumulative evidence suggests that antibodies directed against the IB region of the integrin α6 molecule can cause BMZ separation and possibly play a role in blister formation. In organ culture studies we observed that the monoclonal antibody GoH3 caused BMZ separation, while the monoclonal antibody BQ16 did not cause BMZ separation. It has been reported previously that the monoclonal antibody GoH3 in vitro causes epithelial cells to detach from laminin, while BQ16 does not. The difference between the two monoclonal antibodies to integrin α6 could be due to several reasons; one reason could be the difference in their binding sites. The phenomenon of BMZ separation and cell detachment by OP autoantibodies, monoclonal antibodies GoH3 and rabbit antibodies against fragments I and IB, and not by monoclonal antibodies BQ16 and rabbit antibodies against fragment II, indicates the specific role of epitope(s) in subfragment IB: a process that may eventually cause the basal epithelial cells of the oral mucosa to separate from underlying structures, resulting in separation of the BMZ.
The α6 subunit has been shown to form a heterodimer with either β1 or β4 [13,14]. The α6 integrin subunit has two variants, α6A and α6B [15]. Most normal tissues appear to express only one of the two variants [16]. The α6A molecule has two minor variants, α6A and α6A′. The heterodimer in the skin is usually α6Aβ4 [15], although during embryogenesis α6B may be expressed with β4 in human skin (personal communication, Dr A. Sonnenberg) In the oral cavity it is usually α6A that is associated with the β4 integrin subunit in the α6β4 heterodimer (personal communication, Dr A. Sonnenberg). Because the α6β4 heterodimer, present in the skin and oral mucosa, is the same, then what could be the reason(s) that patients with OP do not have skin lesions? One possible explanation is that the variants of α6A subunit (α6A and α6A′) may have a different distribution between the oral mucosa and skin. This molecular difference between α6A in the skin and oral tissues may be sufficient for antigen specificity and antibody binding. A second possibility is that while the α6 subunit in the normal oral mucosa maybe the same as the skin, it may change from one subunit to the other (e.g. α6A to α6A′) in only diseased tissues during the process of pathogenesis by a variety of mechanisms, such as viral transformation, mutation, etc. Such a local change in biochemical structure could explain why the disease occurs at certain sites and not others within the oral cavity. These hypothetical studies could be confirmed in future experimental studies.
The deposition of complement components (C3) at the BMZ is observed in more than 95% of DIF studies on oral tissue in patients with OP [17]. We made a similar observation in our patients. The significance of the binding of complement to the BMZ in OP is probably similar to that in MMP or BP, at least to the extent of blister formation [18]. The binding of complement results in migration and activation of leucocytes, mainly neutrophils, to the site of pathology, resulting in the release of cytokines and proteolytic enzymes that cause detachment of epithelial cells from BMZ [19]. Several molecules may be involved. Some of these include regulated upon activation normal T cell expressed and secreted (RANTES), several interleukins (IL-6, IL-8), tumour necrosis factor (TNF)-α, TNF-β, gamma interferon and ecotaxin [20]. Recent observations by several investigators indicate that the final pathway that usually results in blister formation may require release of granular protein with proteolytic activity from the eosinophils [21], and collagenases and neutrophil elastases by neutrophils and eosinopils [22,23].
Recent studies have shown that laminin binds to integrin α6 between fibrinogen repeats III and IV [24]. This region of the integrin α6 subunit is within subfragment IB. Hence it can be presumed that antibodies to integrin α6 in the sera of OP patients may influence the binding of integrin α6β4 to laminin. This binding could send a dual signal that affects the extracellular and intracellular environment of the basal epithelial cell. The extracellular signal may influence processes such as steric hindrance or biochemical changes that influence functional binding of the ligand laminin to the integrin α6β4 adhesion receptor. The intracellular signal could generate the production of cytokines and chemokines such as IL-6 and IL-8, which results in local recruitment of leucocytes and other inflammatory cells. It is possible that this combined effect is one of many other plausible mechanisms that lead eventually to BMZ separation.
This study has distinct limitations, first in the limited number of OP sera tested. OP is a rare disease. A larger sample size could be collected only by a multi-centre international study. Creating an international OP study group could achieve that goal. Secondly, the subfragment IB needs to be subcloned into smaller fragments to define the antibody binding site(s) more effectively. Such studies are in progress. It is possible that once smaller peptides are created and a larger cohort of multi-racial sera is tested, other binding sites may be detected. Thirdly, an in vivo passive transfer study in neonatal mice has not been conducted. The oral cavity of the neonatal BALB/c mouse is very small, and will not provide sufficient tissue or visibility to perform mechanistic studies. To study the in vivo capabilities of human autoantibodies it would be essential to find an animal model that has the features and capability of addressing the pertinent questions. Furthermore, it should be noted that the quantity of the pathogenic integrin α6 subunit autoantibody in the sera of OP patients is very small. Hence, it would be necessary to obtain a large volume of sera from a single patient to conduct such studies. Pooling sera from multiple patients will cloud the data. In our opinion, creating a hybridoma or pathogenic human monoclonal antibody would be a better source for such an autoantibody. In spite of these limitations, there is significant benefit from publishing our present data. This information is important for investigators studying integrin biology, autoimmunity and mucosal biology. Integrins, specifically the α6 subunit, play a critical role in cancer. These observations could benefit investigators studying cancer cell motility and metastasis. The observations in this study reinforce the important role of the integrin α6β4 heterodimer in the integrity of epithelial basement membranes in mucosal tissues and the skin.
Acknowledgments
The authors are grateful to Dr Hakan Gurcan for his technical assistance, Christopher Vaillancourt and Olga Lyczmanenko for the preparation of the manuscript and Dr Arnoud Sonnenberg for his assistance and advice.
References
- 1.Fleming TE, Korman NJ. Cicatricial pemphigoid. J Am Acad Dermatol. 2000;43:571–91. doi: 10.1067/mjd.2000.107248. [DOI] [PubMed] [Google Scholar]
- 2.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–18. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Mobini N, Nagarwalla N, Ahmed AR. Oral pemphigoid: subset of cicatricial pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:37–43. doi: 10.1016/s1079-2104(98)90395-x. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher G, Shklar G. Oral involvement in mucous membrane pemphigoid. Clin Dermatol. 1987;5:18–27. doi: 10.1016/0738-081x(87)90045-9. [DOI] [PubMed] [Google Scholar]
- 5.Bhol KC, Goss L, Kumari S, Colon JE, Ahmed AR. Autoantibodies to human α6 integrin patients with oral pemphigoid. J Dent Res. 2001;80:1711–15. doi: 10.1177/00220345010800080601. [DOI] [PubMed] [Google Scholar]
- 6.Adams JC, Watt FM. Expression of β1, β3, β4 and β5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991;115:829–41. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercurio AM, Rabinovitz I, Shaw LM. The alpha6 beta4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–5. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz P, Hu P, Liu Z, et al. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–84. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt E, Wehr B, Tabengwa EM, Reimer S, Brocker EB, Zillikens D. Elevated expression and release of tissue-type, but not urokinase-type, plasminogen activator after binding of autoantibodies to bullous pemphigoid antigen 180 in cultured human keratinocytes. Clin Exp Immunol. 2004;135:497–504. doi: 10.1111/j.1365-2249.2004.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engineer L, Ahmed AR. Emerging treatment for epidermolysis bullosa acquisita. Jam Acad Dermatol. 2001;44:18–28. doi: 10.1067/mjd.2001.113693. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen: partial sequence and biochemical characterization. Proc Natl Acad Sci USA. 1996;10:14714–19. doi: 10.1073/pnas.93.25.14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid KA, Hevi S, Chen Y, Le Caherec F, Chuck SL. A proteomic approach identifies proteins in hepatocytes that bind nascent apolipoprotein B. J Biol Chem. 2002;14(220):10–17. doi: 10.1074/jbc.M112448200. [DOI] [PubMed] [Google Scholar]
- 13.Hemler ME, Crouse C, Takada Y, Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988;263:7660–5. [PubMed] [Google Scholar]
- 14.Kajiji S, Tamura RN, Quaranta V. A novel integrin (alpha 6 beta 4) from human epithelial cells suggests a fourth family of integrin adhesion. EMBO J. 1989;8:673–80. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogervorst F, Admiraal LG, Niessen C, et al. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993;121:179–91. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura RN, Cooper HM, Collo G, Quaranta V. Cell type-specific integrin variants with alternative α chain cytoplasmic domains. Proc Natl Acad Sci USA. 1991;88:10183–7. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels TE, Quadra-White C. Direct immunofluorescence in oral mucosal disease: a diagnostic analysis of 130 cases. Oral Surg Oral Med Oral Pathol. 1981;51:38–47. doi: 10.1016/0030-4220(81)90124-9. [DOI] [PubMed] [Google Scholar]
- 18.Bagan J, Lo Muzio L, Scully C. Mucosal disease series. Number 3. Mucous membrane pemphigoid. Oral Dis. 2005;11:197–218. doi: 10.1111/j.1601-0825.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 19.Eversole LR. Immunopathology of oral mucosal ulcerative, desquamative, and bullous diseases. Selective review of the literature. Oral Surg Oral Med Oral Pathol. 1994;77:555–71. doi: 10.1016/0030-4220(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 20.Verdolini R, Cerio R. Autoimmune subepidermal bullous skin diseases: the impact of recent findings for the dermatopathologist. Virchows Arch. 2003;443:184–93. doi: 10.1007/s00428-003-0776-4. [DOI] [PubMed] [Google Scholar]
- 21.Borrego L, Maynard B, Peterson EA, et al. Deposition of eosinophil granule proteins precedes blister formation in bullous pemphigoid. Comparison with neutrophil and mast cell granule proteins. Am J Pathol. 1996;148:897–909. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Shapiro SD, Zhou X, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105:113–23. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Zhou X, Shapiro SD, et al. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–55. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 24.Demetriou MC, Cress AE. Integrin clipping: a novel adhesion switch? J Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]


