Abstract
Little is known about the identities and roles of antigen-presenting cells upon exposure to antigens of respiratory syncytial virus (RSV). Here, we focused on elucidating the importance of alveolar macrophages in conferring protective immunity in mice administered a liposome-encapsulated recombinant fragment of the RSV G protein. Mice were depleted of alveolar macrophages by intranasal inoculation of liposome-encapsulated dichloromethylenediphosphonic acid (DMDP). Mice depleted of alveolar macrophages prior to immunization developed reduced levels of serum RSV-neutralizing antibody and showed dramatically impaired protection against RSV challenge. The severity of interstitial inflammation was also markedly reduced in macrophage-depleted mice. In conclusion, this study demonstrates a pivotal role for alveolar macrophages during exposure to liposome-encapsulated RSV antigen in initiating both protective and histopathological responses against RSV.
Keywords: inflammation in RSV disease, respiratory syncytial viral G protein vaccine, vaccine-enhanced RSV disease
Introduction
Despite more than 40 years of research, a safe and effective vaccine against respiratory syncytial virus (RSV) remains an unrealized goal. Nevertheless, considerable advances have been made in our understanding of the complex immune processes which are activated upon RSV infection or immunization with RSV antigens. The RSV G protein, for example, is known to elicit both protective immunity as well as potent T helper cell (both Th1 and Th2) responses which may contribute to aggravated inflammation in the respiratory tract of immunized individuals subsequently exposed to RSV [1–7].
An unexplored facet of RSV vaccinology is the identity and role of antigen-presenting cells within the respiratory tract [8]. Because immunization strategies which target the respiratory tract offer certain advantages over systemic immunization, there is a need for enhanced understanding of the processes by which antigen is processed and presented to immune cells. Foremost among candidate antigen-presenting cells in the respiratory tract are alveolar macrophages and dendritic cells. The goal of the present study was to gain insights into the role of these cells in mediating vaccine-relevant responses to RSV immunization.
Dichloromethylenediphosphonic acid (DMDP) has been used in various studies for depletion of macrophages [9–21]. Liposome-encapsulated DMDP causes the elimination of specific macrophage populations through different administration routes and has minimal effects on non-phagocytic cells [12]. In free form the drug is not toxic, does not easily cross cell membranes, and its half-life in circulation and body fluids is of short duration. Once phagocytosed, DMDP liposomes disrupt phospholipid bilayers via lysosomal phospholipases causing high concentrations of the drug to accumulate in the cell affecting metabolism, but the exact mechanism of depletion has not been elucidated [22].
Liposome-based vaccines are attractive delivery vehicles and have been shown in mouse studies to be relevant in conferring protection against RSV [23,24]. As noted above, roles have been suggested for dendritic cells and alveolar macrophages at different anatomical sites of the respiratory tract. Alveolar macrophages are known to interact with liposomes [25], although the relative contribution to the immune response is unclear. We therefore set out to investigate the importance of alveolar macrophages in liposomal vaccine-mediated immune protection against RSV.
Materials and methods
Cells, RSV and mice
RSV (long strain) was grown in human laryngeal epidermoid carcinoma HEp-2 cells in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 1% fetal calf serum (Sigma), as described previously [23]. Specific pathogen-free BALB/c female mice were purchased from Charles River Laboratories (Wilmington, MA, USA), and housed at Dalhousie University following institutional guidelines and ethically approved experimental procedures.
RSV neutralization assay
For neutralization assays, serially diluted aliquots of serum from immunized mice were added to a 96-well plate containing aliquots of pre-titered RSV. Following 90 min incubation at room temperature, the remaining infectious virus was quantified by plaque assay on HEp-2 cells.
Preparation of liposomes encapsulating recombinant viral antigen
Liposomes were prepared as described previously [23]. A chloroform solution of dioleoylphosphatidylcholine (DOPC; Sigma, 1 mg/mouse) was dried-down under nitrogen, and the DOPC resuspended in a phosphate-buffered saline (PBS) solution of recombinant protein consisting of a fragment of bacterial thioredoxin (Trx) fused to a fragment (amino acids 128–229) of the RSV G protein (designated Trx-G) [23]. Following lyophilization, liposomes were aspirated in pyrogen-free water and incubated at 4°C for 2 h. Liposomes were washed several times to remove unincorporated protein and resuspended in pyrogen-free PBS.
In vivo depletion of alveolar macrophages
Liposomes containing DMDP (Sigma) were prepared as described [12], with a few modifications. Liposomes were prepared by adding 34·4 mg of DOPC (Sigma) in 2 ml of chloroform, evaporated under a nitrogen stream and resuspended in 0·8 ml of a DMDP solution (189 mg/ml pyrogen-free PBS). The suspension was incubated at room temperature for 2 h, sonicated for 3 min in a water bath sonicator, and incubated for an additional 2 h at room temperature. The liposomes were diluted in pyrogen-free PBS and centrifuged at 100 000 g for 30 min to remove free DMDP, after which the liposomes were resuspended in 0·4 ml of pyrogen-free PBS.
Liposomal DMDP administration prior to immunization and RSV challenge
Female BALB/c mice (6–8 weeks old) were anaesthetized intraperitoneally (i.p.) with ketamine/xylazine. Mice were separated into four groups of five mice in which two groups were administered liposomal DMDP 48 h prior to each of two immunizations [9,11]. Mice were immunized twice intranasally at 14-day intervals with either 100 µl PBS or liposome-encapsulated Trx-G. The remaining two groups, which did not receive DMDP, were also administered PBS or liposome-encapsulated Trx-G. Fourteen days following the second immunization, the mice were challenged with 100 µl of RSV (3 × 107 pfu/ml) and killed 4 days later.
Collection of blood and bronchoalveolar lavage
Following anaesthetization of the mice with sodium pentobarbital, an incision was made extending from the lower abdomen to expose the lungs, the heart and the trachea. Following blood collection by heart puncture an incision in the trachea was made, through which a 25-gauge neonatal catheter was inserted and the lungs were flushed three times each with 1 ml cold PBS. The combined collected fluid was kept on ice for preparation of cytospin slides.
Processing of lung for virus assay and histological analysis
Following collection of bronchoalveolar lavage fluid, the left bronchus was tied off using sterile 4–0 surgical silk. The left lobes were separated from the right lobes and homogenized in RPMI-1640 medium supplemented with 1% fetal calf serum. Aliquots of the homogenates for each mouse were centrifuged at room temperature for 1 min at 6000 g, and the supernatants were used to inoculate confluent HEp-2 cell monolayers for determination of RSV titre by plaque assay.
The remaining lobes of the lung were inflated and immersed in 10% formalin prepared in PBS and stored at 4°C for histological analysis. Thin sectioning and haematoxylin and eosin (H&E) staining was performed and slides examined under light microscopy. Inflammatory cell infiltrates were semiquantified around blood vessels and bronchioles using the scoring system of Murphy et al. [26] and Connors et al. [27].
For immunofluorescence staining of dendritic cells, thin tissue slides were prepared from lung fixed in UMFIX (Sakura Finetek, Torrance, CA, USA) and embedded in paraffin blocks. Slides were de-parafinized, rehydrated, blocked with 10% normal goat serum in PBS and incubated overnight with the primary antibody, 2A1 (a gift from Dr Ralph Steinman, Rockefeller University, NY, USA; 1:20 in PBS; 2A1 is a monoclonal antibody which reacts with granules within the cytoplasm of dendritic cells [28,29]. Slides were washed in PBS and incubated for 1 h with secondary antibody, CY3-conjugated goat anti-rat antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA; 1:1000 in PBS). Washed slides were fitted with cover slips using fluorescent mounting medium (DakoCytomation, Carpinteria, CA, USA).
For immunostaining of macrophages with MOMA-2 antibody (Cedarlane Laboratories, Hornby, ON, Canada), thin tissue slides were prepared from lung frozen in OCT (optimal cutting temperature) compound (Sakura) with 20% sucrose, and the slides were stored at −20°C. Slides were fixed in cold (− 20°C) acetone for 10 min, washed in PBS and placed in freshly prepared paraformaldehyde for 2 min. Following washing in PBS, slides were placed in a lysine–glycine bath for 15 min, washed in PBS and blocked for 1 h with 10% normal goat serum in PBS. Slides were incubated overnight with MOMA-2 antibody (1:20 in PBS), washed in PBS and incubated with biotinylated rabbit anti-rat antibody (Vector Laboratories, Burlington, ON, Canada; 1:400 in PBS). Washed slides were incubated for 1 h with streptavidin-horseradish peroxidase (Dako), washed in PBS and treated with an endogenous peroxidase block, 2% H2O2, for 15 min. Following washing, slides were incubated for 15 min with 3-amino-9-ethylcarbazole (AEC) substrate chromagen (Dako), then washed with tap water and counterstained with haematoxylin for 3 min. Slides were washed and fitted with coverslips using glycerol–gelatin mounting medium (Sigma).
Analysis of leucocyte differential in bronchoaveolar lavage fluids
Analysis of the bronchoalveolar lavage fluid was performed by cytocentrifuging aliquots onto microscope slides using a Cytospin 3 cytocentrifuge (Shandon, Pittsburgh, PA, USA). Cells were stained with the May–Grunwald–Giemsa stain and the leucocyte differential was determined using a light microscope.
Statistical analysis
Data were analysed using the GraphPad Instat software package (San Diego, CA, USA) using analysis of variance (anova) by the Kruskal–Wallis test and the Mann–Whitney test for individual column comparisons. Bonferroni tests were also performed for multiple column comparisons.
Results
Treatment of mice with liposome-encapsulated DMDP reduces alveolar macrophages but increases lung neutrophils
In order to investigate the importance of the role of alveolar macrophages in antigen presentation or its interaction with other cell types regarding antigen presentation, alveolar macrophages were depleted from the lungs of BALB/c mice using liposome-encapsulated DMDP. To ensure that DMDP reduced macrophage numbers, we performed bronchoalveolar lavage on mice administered DMDP as well as PBS and counted a minimum of 500 cells per slide. Macrophages expressed as a percentage of cells in bronchoalveolar lavage decreased significantly in the lungs of mice treated with increasing doses of DMDP (Fig. 1). The macrophage population decreased to approximately 25% with 12 µg of DMDP and less than 10% with a dose of 24 µg and 48 µg of DMDP (Fig. 1). Total numbers of macrophages recovered in the lung lavage were: 72 452 ± 6320 per mouse in the control PBS group, 12 158 ± 4170 in the DMDP (12 µg) group, 2646 ± 691 in the DMDP (24 µg) group and 1030 ± 513 in the DMDP (48 µg) group (macrophages showed more dramatic decreases when expressed as total numbers rather than percentage cells, due to the influx of neutrophils described below).
Fig. 1.
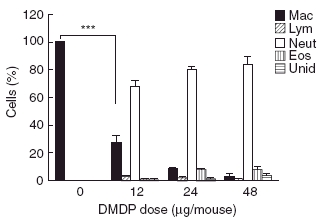
Leucocyte differential in bronchoalveolar lavage fluid of mice administered liposomal dichloromethylenediphosphonic acid (DMDP). Mice were killed 2 days after intranasal administration of varying doses of liposomal DMDP. From left to right, cells are macrophages, lymphocytes, neutrophils, eosinophils and unidentified. Data are represented as mean ± standard deviation and statistical analysis was performed using the Bonferroni multiple comparison test (***P < 0·001) for comparison of selected columns where n = 5 for each group.
Furthermore, mice administered liposomal DMDP showed an increase in the percentage of neutrophils found in the bronchoalveolar lavage (Fig. 1). This neutrophilia was related to the dose of DMDP administered intranasally to the mice. The neutrophil population increased from 0% in the PBS-treated mice to approximately 70% in mice treated with a dose of only 12 µg of DMDP. A dose of 24 µg and 48 µg of DMDP led to almost complete repopulation of the lung lavage with neutrophils. Also, noted in mice administered DMDP, some of the neutrophils had a swollen appearance (data not shown), possibly suggestive of phagocytic activity by the neutrophils [30].
Examination of lung tissue sections using antibodies specific for macrophages (MOMA-2) and dendritic cells (2A1) was also performed. The results (Fig. 2) showed that liposomal DMDP treatment had lesser effects on the interstitial counts of either macrophages or dendritic cells. Interstitial macrophages were reduced by about half, while dendritic cells were essentially unchanged after 48 h of liposomal DMDP treatment.
Fig. 2.
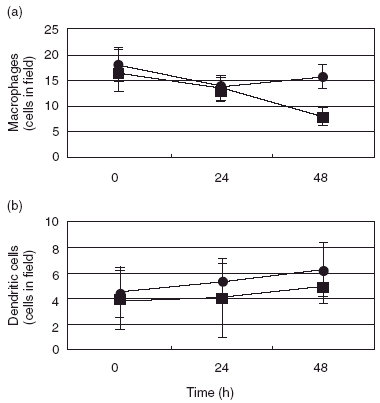
Effects of liposomal dichloromethylenediphosphonic acid (DMDP) treatment on interstitial macrophages (a) or dendritic cells (b). Mice were killed at the times indicated following intranasal administration of 24 µg/mouse of liposome-encapsulated DMDP (closed squares) or liposomes alone (closed circles). Lung tissue sections were stained with cell-specific antibodies and examined by light microscopy.
Depletion of alveolar macrophages prior to immunization dramatically reduces liposomal vaccine-mediated protection against RSV
Normal and alveolar macrophage-depleted mice were administered PBS or Trx-G-encapsulated liposomes twice at 14 days intervals followed by RSV challenge. Pilot experiments had shown that numbers of alveolar macrophages were restored to about 62% of their pre-DMDP treatment levels by the time of RSV challenge, 16 days after DMDP treatment (data not shown). Four days post-infection mice were killed and RSV titres in the lungs were determined by plaque assay. The greatest protection against RSV replication in mice was provided by administration of Trx-G-encapsulated liposomes without depletion of alveolar macrophages. As expected, a large (76-fold) decrease in viral titres was observed in non-depleted mice immunized with Trx-G-encapsulated liposomes, compared to non-depleted mice administered PBS (i.e. no vaccine) (Fig. 3a). In contrast, only a 2·3-fold decrease in RSV titre was observed in macrophage-depleted mice immunized with Trx-G-encapsulated liposomes compared to the macrophage-depleted PBS control group. Of greatest interest, an approximately 100-fold decrease in RSV lung titres was observed between macrophage-depleted and non-depleted mice, following immunization and RSV challenge (Fig. 3a).
Fig. 3.
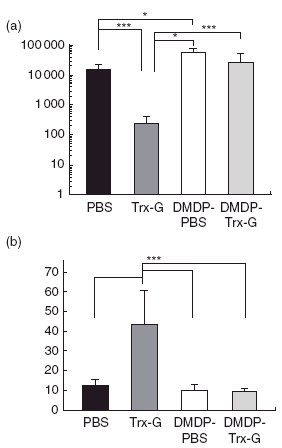
Protection against respiratory syncytial virus (RSV) challenge in alveolar macrophage-depleted and non-depleted mice immunized with RSV G liposomal vaccine. (a) Lung RSV titres 4 days after RSV challenge in alveolar macrophage-depleted and non-depleted BALB/c mice using dichloromethylenediphosphonic acid (DMDP) (12 µg/mouse) followed by two intranasal immunizations at 14-day intervals with phosphate-buffered saline (PBS) or thioredoxin (Trx)-G-encapsulated liposomes. (b) RSV neutralizing antibody titres in the sera of alveolar macrophage-depleted and normal lung population in mice immunized intranasally with liposomes encapsulating Trx-G or inoculated with PBS followed by RSV challenge. The plaque reduction neutralization titre50 (PRNT50) is the reciprocal dilution of sera required to neutralize 50% of RSV plaques on HEp-2 cells. Data are represented as mean ± standard deviation and the Mann–Whitney test (**P = 0·0079; *P = 0·0159) was performed for statistical analysis where n = 5 for each group.
Alveolar macrophage-depleted mice develop low serum RSV-neutralization titres after immunization with Trx-G containing liposomes
Neutralization assays were performed to investigate the effect of alveolar macrophage depletion on serum RSV neutralization titres. As expected, the neutralization assay correlated with the data indicating that greatest protection was provided by Trx-G-encapsulated liposomes in normal, i.e. non DMDP-treated mice (Fig. 3b). Significantly higher (P = 0·0079) RSV neutralizing activity was observed in the sera of normal versus DMDP-treated mice administered Trx-G-encapsulated liposomes.
Depletion of alveolar macrophages does not change the pattern of cellular infiltration into the bronchoalveolar lavage following Trx-G immunization and RSV challenge
We determined the cellular content of bronchoalveolar lavage from normal and alveolar macrophage-depleted mice immunized twice with PBS or Trx-G-encapsulated liposomes followed by RSV challenge. It should be noted that RSV challenge was performed 16 days after the last DMDP treatment thus allowing normal macrophage repopulation of the lung [9,10,12,22]. In both macrophage-depleted and non-depleted mice, similar patterns of cellular infiltration were observed following immunization and RSV challenge.
The most dramatic change associated with liposomal Trx-G immunization followed by RSV challenge was an increase in bronchoalveolar neutrophils. Similar increases in bronchoalveolar lavage neutrophils were observed in macrophage-depleted and non-depleted mice following immunization and RSV challenge. For both groups neutrophils showed a significant percentage increase, compared to PBS controls (normal mice P < 0·001; macrophage-depleted mice P < 0·01) (Fig. 4).
Fig. 4.
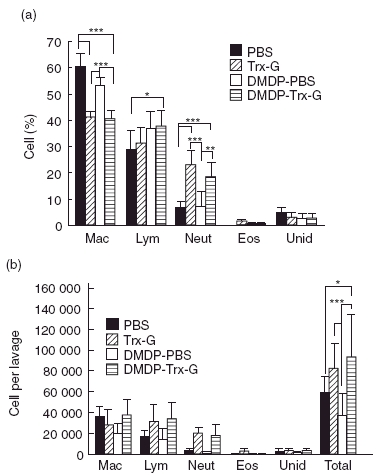
Leucocyte differential (a) and total numbers of recovered cells (B) in the bronchoalveolar lavage fluid of respiratory syncytial virus (RSV) challenged alveolar macrophage-depleted and non-depleted mice administered RSV G liposomal vaccine. Mice were administered dichloromethylenediphosphonic acid (DMDP) intranasally 2 days before two sessions of intranasal immunization occurring at 14-day intervals, followed by RSV challenge. Both alveolar macrophage-depleted and non-depleted mice were administered PBS or thioredoxin (Trx)-G-encapsulated liposomes intranasally. Mac, macrophages; Lym, lymphocytes; Neut, neutrophils; Eos, eosinophils; Unid, unidentified. Data are represented as mean ± standard deviation and selected columns were statistically analysed using the Bonferroni multiple comparisons test (***P < 0·001; **P < 0·01; *P < 0·05) where n = 5 for each group.
Reduction of lung cellular infiltration in alveolar macrophage-depleted mice immunized with Trx-G liposomes followed by RSV challenge
Normal immunized (i.e. non-macrophage-depleted) mice showed a statistically significant (P < 0·01) higher degree of cellular infiltration around the blood vessels compared to all other groups (Fig. 5). Similarly, normal immunized mice had statistically (P < 0·01) higher levels of infiltration around the bronchioles compared to normal and macrophage-depleted mice administed PBS, and immunized macrophage depleted mice (P < 0·001). Macrophage depletion drastically reduced the amount of cellular infiltration normally induced by immunization with Trx-G liposomes followed by RSV challenge. As expected, little to no infiltration was observed in the lungs of mice mock-immunized with PBS, followed by RSV challenge, regardless of whether they were depleted of alveolar macrophages.
Fig. 5.
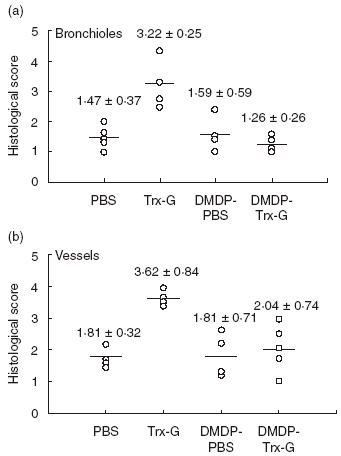
Cellular infiltration surrounding bronchioles and vessels of alveolar macrophage-depleted and non-depleted mice immunized with respiratory syncytial virus (RSV) G liposomal vaccine, followed by RSV challenge. Alveolar macrophage-depleted and non-depleted mice were intranasally administered phosphate-buffered saline (PBS) and thioredoxin (Trx)-G-encapsulated liposomes twice at 14-day intervals and challenged with RSV. Lung sections were prepared as previously described and cellular infiltration surrounding bronchioles (a) and vessels (b) were determined using the semiquantitative scoring systems defined by Murphy et al. [26] and Connors et al. [27]. Data are represented as mean ± standard deviation and selected columns were analysed statistically using the Bonferroni multiple comparisons test (***P < 0·001; **P < 0·01; *P < 0·05).
Discussion
Despite widespread interest in liposomes as vaccine carriers, few studies have focused on their fate in different anatomical locations and their interaction with dendritic cells or alveolar macrophages. To our knowledge, this is the first study to examine the role of alveolar macrophages in RSV infection or in vaccine-mediated immunological processes. The results highlight a dramatic requirement for alveolar macrophages at the time of intranasal immunization in order to achieve an optimal protective response.
Depletion of alveolar macrophages could have marked effects on vaccination and/or exposure to pathogens [31]. In our study, we used intranasal administration of DMDP liposomes in mice [9,11,12,22], a procedure which selectively depletes alveolar macrophages [13–21]. Using this regimen, depletion of alveolar macrophages is attained by 24 h, while repopulation begins by day 5 post-DMDP-administration and is complete by day 18 [9,10,12,22]. Our results confirmed alveolar macrophage depletion in mice administered liposomal DMDP, as well as highlighting a dramatic increase in neutrophils. In contrast to the effects of DMDP liposomes on alveolar macrophages, our results reveal little effect of liposomal DMDP treatment on interstitial macrophages or on dendritic cells.
In addition to depleting alveolar macrophages, liposomal DMDP treatment provoked an early influx (24 h) of neutrophils (Fig. 1), similar to that described in rats by Berg et al. [18], but in apparent contrast to the lack of neutrophilia reported in DMDP-treated CD-1 mice [17]. In our study, airway neutrophils showed a swollen appearance suggesting that they may have engulfed debris from dying macrophages because other studies have shown neutrophils clearing the lung of cellular debris [14,30]. Alternatively, the influx of neutrophils may have occurred due to activation of macrophages phagocytosing the liposomes, provoking the release of cytokines and chemokines, such as interleukin (IL)-8 [16,31,32].
Alveolar macrophages differentiate under the influence of local factors such as type II alveolar epithelial cells, bronchial epithelium, cytokines and surfactants [32]. As a consequence, following activation alveolar macrophages can have a role in antigen uptake and presentation, but play a greater role in the secretion of pro- [e.g. tumour necrosis factor (TNF)-α, IL-1, IL-6] and anti-inflammatory cytokines and factors (e.g. leukotrienes) which orchestrate host cellular defence, as well as cytokines involved in cell recruitment. It is generally accepted that dendritic cells, the principal population in the parenchymal tissue of the lung, are more effective at migrating to the lymph nodes for antigen presentation [11,31,33–36]. Class II major histocompatibility complex (MHC) antigen-bearing dendritic cells activate memory and effector T cells, as do macrophages, but more importantly dendritic cells constitutively express B7 to activate naive T cells in the lymph nodes [9,37].
Although a small population of dendritic cells (immature and precursors) can be found in the alveoli, their ability to efficiently activate naive T cells may conflict with their having a protective role in the alveoli, as cell-mediated immune responses in the alveoli could result in symptoms of inflammatory lung disease [32,38]. It has been suggested that a cytokine environment may be created by alveolar macrophages upon contact with antigens regulating the activity of surrounding dendritic cells [31]. The suppressive effect of alveolar macrophages can be amplified further in the presence of TNF-α, macrophage nitric oxide production, the secretion of soluble suppressive factors such as prostaglandins, or through direct cell–cell contact [11,32]. In fact, in the absence of alveolar macrophages, enhanced plasma cell activity in the parenchyma of the peripheral lung can lead to increases in the humoral response due presumably to the increased activity of dendritic cells [10]. For interest, it may be noted that depletion of alveolar macrophages in rats has been reported not to increase significantly inflammatory cytokine expression in the lung [39].
Alveolar macrophages are generally poor antigen-presenting cells [11,40–42]. However, they do possess superior phagocytic activity and may be able to transfer phagocytosed antigen to dendritic cells for subsequent processing and presentation to T lymphocytes (reviewed in Stumbles et al. [43]).
Although liposomal DMDP treatment results in an influx of neutrophils into the airways, this is temporary and is reversed by day 20 (compare neutrophils in PBS and DMDP–PBS groups in Fig. 4). Mock-immunized mice depleted of alveolar macrophages by liposomal DMDP treatment showed levels of RSV replication similar to (and even higher than) those of control mice (Fig. 3), ruling out any inhibitory effect (inflammatory or otherwise) of liposomal DMDP treatment on RSV infection.
The increased numbers of airway neutrophils triggered by liposomal DMDP treatment raises the question of possible neutrophil involvement in antigen presentation of liposome-encapsulated RSV G protein. However, while neutrophils as well as alveolar macrophages are able to transport antigen to draining lymph nodes, they lack the ability to stimulate immune responses (cited in [44]). The results of the present study (showing low RSV-neutralizing antibody responses in liposomal DMDP-treated mice with elevated airway neutrophil counts) argue similarly against a role for DMDP-induced neutrophils in functional RSV G protein antigen presentation. Moreover, RSV replication often persists in the face of neutrophil or eosinophil inflammation (reviewed in [1,45–47]), suggesting a greater role for these cells in immunopathological reactions rather than anti-viral defence. A contrary role for neutrophils in scavenging liposomal RSV G protein, rendering it unavailable for uptake by antigen-presenting cells, is conceivable, although unprecedented to our knowledge.
In conclusion, our study provides the first evidence for an important role of alveolar macrophages in the induction of protective as well as inflammatory responses against intranasally administered RSV antigens. It should be emphasized that a role for pulmonary dendritic cells is not excluded by the present results, as no method currently exists to selectively deplete dendritic cells. Further studies will determine whether alveolar macrophages fulfil a primary role in antigen presentation to lymphocytes or whether alveolar macrophages function to initiate antigen processing followed by transfer of antigen products to more specialized antigen presenting cells, such as dendritic cells or B lymphocytes [38,48–50].
Acknowledgments
This work was supported by the Canadian Institutes of Health Research. We are grateful to Pat Colp for skilled histological assistance.
References
- 1.Graham BS, Rutigliano JA, Johnson TR. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 2.Openshaw PJ, Culley FJ, Olszewska W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine. 2001;20(Suppl. 1):S27–31. doi: 10.1016/s0264-410x(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 3.Sparer TE, Matthews S, Hussell T, et al. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–6. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikiatkhachorn A, Chang W, Braciale TJ. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J Virol. 1999;73:6590–7. doi: 10.1128/jvi.73.8.6590-6597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–72. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol. 2000;165:6487–95. doi: 10.4049/jimmunol.165.11.6487. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Anderson R. Modulation of protective immunity, eosinophilia, and cytokine responses by selective mutagenesis of a recombinant G protein vaccine against respiratory syncytial virus. J Virol. 2005;79:4527–32. doi: 10.1128/JVI.79.7.4527-4532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris J, Werling D. Binding and entry of respiratory syncytial virus into host cells and initiation of the innate immune response. Cell Microbiol. 2003;5:671–80. doi: 10.1046/j.1462-5822.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 9.Thepen T, van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thepen T, McMenamin C, Oliver J, Kraal G, Holt PG. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur J Immunol. 1991;21:2845–50. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- 11.Holt PG, Oliver J, Bilyk N, et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang-Hoover J, Sutton A, van Rooijen N, Stein-Streilein J. A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis. Immunology. 2000;101:501–11. doi: 10.1046/j.1365-2567.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp S, Leemans JC, Florquin S, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–9. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 15.van Iwaarden JF, Claassen E, Jeurissen SH, Haagsman HP, Kraal G. Alveolar macrophages, surfactant lipids, and surfactant protein B regulate the induction of immune responses via the airways. Am J Respir Cell Mol Biol. 2001;24:452–8. doi: 10.1165/ajrcmb.24.4.4239. [DOI] [PubMed] [Google Scholar]
- 16.Rubins JB. Alveolar macrophages. wielding the double-edged sword of inflammation. Am J Respir Crit Care Med. 2003;167:103–4. doi: 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- 17.Kooguchi K, Hashimoto S, Kobayashi A, et al. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–9. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg JT, Lee ST, Thepen T, Lee CY, Tsan MF. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–9. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 19.Cheung DO, Halsey K, Speert DP. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect Immun. 2000;68:4585–92. doi: 10.1128/iai.68.8.4585-4592.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broug-Holub E, Toews GB, van Iwaarden JF, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–46. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang C, Inman MD, van Rooijen N, et al. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol. 2001;166:1471–81. doi: 10.4049/jimmunol.166.3.1471. [DOI] [PubMed] [Google Scholar]
- 22.van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J Immunol Meth. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 23.Mader D, Huang Y, Wang C, et al. Liposome encapsulation of a soluble recombinant fragment of the respiratory syncytial virus (RSV) G protein enhances immune protection and reduces lung eosinophilia associated with virus challenge. Vaccine. 2000;18:1110–7. doi: 10.1016/s0264-410x(99)00373-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Anderson R. Enhanced immune protection by a liposome-encapsulated recombinant respiratory syncytial virus (RSV) vaccine using immunogenic lipids from Deinococcus radiodurans. Vaccine. 2002;20:1586–92. doi: 10.1016/s0264-410x(01)00487-x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Rothi RJ, Straub L, Cacace JL, Schreier H. Liposomes and pulmonary alveolar macrophages. functional and morphologic interactions. Exp Lung Res. 1991;17:687–705. doi: 10.3109/01902149109062873. [DOI] [PubMed] [Google Scholar]
- 26.Murphy BR, Sotnikov A, Paradiso PR, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–40. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 27.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186:665–72. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba K, Steinman RM, Pack MW, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157–67. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viuff B, Tjornehoj K, Larsen LE, et al. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol. 2002;161:2195–207. doi: 10.1016/S0002-9440(10)64496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicod LP, Cochand L, Dreher D. Antigen presentation in the lung. dendritic cells and macrophages. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:246–55. [PubMed] [Google Scholar]
- 32.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Holt PG, Degebrodt A, O’Leary C, Krska K, Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985;62:586–93. [PMC free article] [PubMed] [Google Scholar]
- 34.Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986;163:436–51. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt PG, Schon-Hegrad MA, Phillips MJ, McMenamin PG. Ia-positive dendritic cells form a tightly meshed network within the human airway epithelium. Clin Exp Allergy. 1989;19:597–601. doi: 10.1111/j.1365-2222.1989.tb02752.x. [DOI] [PubMed] [Google Scholar]
- 36.Kradin RL, McCarthy KM, Xia WJ, Lazarus D, Schneeberger EE. Accessory cells of the lung. I. Interferon-gamma increases Ia+ dendritic cells in the lung without augmenting their accessory activities. Am J Respir Cell Mol Biol. 1991;4:210–18. doi: 10.1165/ajrcmb/4.3.210. [DOI] [PubMed] [Google Scholar]
- 37.Lehnert BE, Valdez YE, Stewart CC. Translocation of particles to the tracheobronchial lymph nodes after lung deposition: kinetics and particle–cell relationships. Exp Lung Res. 1986;10:245–66. doi: 10.3109/01902148609061496. [DOI] [PubMed] [Google Scholar]
- 38.Vu Q, McCarthy KM, McCormack JM, Schneeberger EE. Lung dendritic cells are primed by inhaled particulate antigens, and retain MHC class II/antigenic peptide complexes in hilar lymph nodes for a prolonged period of time. Immunology. 2002;105:488–98. doi: 10.1046/j.1365-2567.2002.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elder A, Johnston C, Gelein R, et al. Lung inflammation induced by endotoxin is enhanced in rats depleted of alveolar macrophages with aerosolized clodronate. Exp Lung Res. 2005;31:527–46. doi: 10.1080/019021490944223. [DOI] [PubMed] [Google Scholar]
- 40.Lyons CR, Lipscomb MF. Alveolar macrophages in pulmonary immune responses. I. Role in the initiation of primary immune responses and in the selective recruitment of T lymphocytes to the lung. J Immunol. 1983;130:1113–19. [PubMed] [Google Scholar]
- 41.Toews GB, Vial WC, Dunn MM, et al. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J Immunol. 1984;132:181–6. [PubMed] [Google Scholar]
- 42.Shellito J, Caldwell JL, Kaltreider HB. Immune functions of murine alveolar macrophages: binding of lymphocytes and support of lymphocyte proliferation. Exp Lung Res. 1983;4:93–107. doi: 10.3109/01902148309055007. [DOI] [PubMed] [Google Scholar]
- 43.Stumbles PA, Upham JW, Holt PG. Airway dendritic cells: co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS. 2003;111:741–55. doi: 10.1034/j.1600-0463.2003.11107806.x. [DOI] [PubMed] [Google Scholar]
- 44.Pollard AM, Lipscomb MF. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–67. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SZ, Forsyth KD. The interaction of neutrophils with respiratory epithelial cells in viral infection. Respirology. 2000;5:1–10. doi: 10.1046/j.1440-1843.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 46.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domachowske JB, Bonville CA, Rosenberg HF. Animal models for studying respiratory syncytial virus infection and its long term effects on lung function. Pediatr Infect Dis J. 2004;23:S228–34. doi: 10.1097/01.inf.0000144672.81955.a4. [DOI] [PubMed] [Google Scholar]
- 48.Holt PG. Antigen presentation in the lung. Am J Respir Crit Care Med. 2000;162:S151–S156. doi: 10.1164/ajrccm.162.supplement_3.15tac2. [DOI] [PubMed] [Google Scholar]
- 49.van Rooijen N. Macrophages as accessory cells in the in vivo humoral immune response: from processing of particulate antigens to regulation by suppression. Semin Immunol. 1992;4:237–45. [PubMed] [Google Scholar]
- 50.Gong JL, McCarthy KM, Rogers RA, Schneeberger EE. Interstitial lung macrophages interact with dendritic cells to present antigenic peptides derived from particulate antigens to T cells. Immunology. 1994;81:343–51. [PMC free article] [PubMed] [Google Scholar]


