Abstract
The objective of this study was to investigate the occurrence of apoptosis of monocytes in an experimental model of multiple trauma and its probable correlation to bacterial translocation. Thirty-two rabbits were applied in three groups: A, controls; B, myotomy of the right femur; and C, myotomy and fracture of the right femur. Blood was sampled for the estimation of endotoxins [lipopolysaccharide (LPS)], tumour necrosis factor (TNF)-α, malondialdehyde (MDA) and isolation of peripheral blood mononuclear cells (PBMCs). PBMCs, derived after centrifugation over Ficoll, were incubated in flasks and apoptosis of non-adherent lymphocytes and adherent monocytes was estimated after staining for Annexin-V and flow cytometry. TNF-α of supernatants of cultured monocytes was also determined. Tissue segments were cultured after death. Median survival of groups A, B and C was > 14, > 14 and 9·00 days, respectively. Apoptosis of lymphocytes in group C was higher than group A at 2, 4 and 48 h and of monocytes in group C higher than group A at 2 and 4 hours. LPS in group C was higher than group A at 2, 4 and 48 h. Apoptosis of lymphocytes and monocytes was correlated positively with serum TNF-α and negatively with TNF-α of monocyte supernatants. Cultures of organ segments of group A were sterile. Pseudomonas aeruginosa was isolated from liver, lung and spleen in five animals in group B (45·45%) and in six in group C (54·54%). Early apoptosis of blood monocytes supervened after multiple trauma; the phenomenon was accompanied by apoptosis of blood lymphocytes and subsequent bacterial translocation.
Keywords: apoptosis, macrophages/monocytes, stress
Introduction
Multiple trauma is a major cause of the systemic inflammatory response syndrome (SIRS) often accompanied by rapid deterioration of the patient and subsequent death [1]. Current evidence suggests that multiple organ failure in the event of multiple trauma might be connected with the advent of bacterial translocation and immunoparalysis due to depletion of lymphocytes [2]. Despite the plethora of information about lymphocyte apoptosis in multiple trauma, no data are available about monocytes that constitute the major determinants of the innate immune response and of the transition of antigenic stimuli to the host’s adaptive immune system [3]. The present study was focused on an experimental model of multiple trauma aiming to define (a) the role of monocyte apoptosis in conjunction to the phenomena of apoptosis of lymphocytes and of bacterial translocation; and (b) the time-frame of these events.
Materials and methods
Animals
A total of 32 white New Zealand male rabbits, mean (± s.d.) weight 3·39 ± 0·33 kg, were used for the study. The study received permission from the Veterinary Directorate of the Perfecture of Athens, according to Greek legislation in conformance to the 160/91 Directive Council of the EU. The animals were housed in single metal cages and had access to tap water and standard balanced rabbit chow ad libitum. Temperature ranged between 18 and 22°C, relative humidity between 55 and 65% and the light/dark cycle was 6 a.m./6 p.m.
Experimental design
Animals were initially sedated by intramuscular injection of 25 mg/kg of ketamine and 5 mg/kg of xylazine. Anaesthesia was maintained by the intravenous administration of 20 mg/kg of sodium thiopental. Through a midline neck incision the left common carotid artery was recognized and catheterized by a 20-gauge catheter that was stabilized by a 3·0 silk suture. The catheter was connected to a multi-channel recorder (Electronics for Medicine, Houston, TX, USA) permitting the recording of systolic and diastolic arterial pressures and heart rate at 15-min intervals for a total period of 4 h. Rectal temperature was also measured at the same time intervals. Resuscitation of animals was achieved by the continuous intravenous infusion of 0·9% normal saline with a catheter inserted under aseptic conditions into the right ear vein.
Animals were divided into three study groups, as follows:
group A (n = 10): controls where no intervention was performed;
group B (n = 11): animals subject to dissection of skin and muscle of the right femur, as described below, followed by closure by layers; and
group C (n = 11): animals subject to multiple trauma by a technique described previously [4]. After a 3-cm longitudinal incision of the anterolateral aspect of the right thigh, the fascia over the vastus lateralis and the fibres of the vastus lateralis were dissected up to the periosteum of the femur. Then, using a bone cutter (Liston), a transverse fracture in the middle of the right femur of each animal was performed. Muscle and skin were then closed by layers.
The degree of trauma of each animal was correlated with that of humans, the latter being expressed by the Injury Severity Score (ISS) [5]. The ISS is defined as the sum of squares of the highest grades of injury in each of the three most severely injured areas of the body. These grades range between 1 and 5, corresponding to 1 for a minor injury, 2 for a moderate injury, 3 for a severe but not life-threatening injury; 4 for a severe and life-threatening injury with survival probable; and 5 for a critical injury with survival uncertain. Simulating that score to the above experimental model, animals of group A have an ISS equal to 1, animals of group B an ISS equal to 4 and animals of group C an ISS equal to 25. Any value above 20 corresponds to high-risk patients [5].
Five ml of blood was sampled after puncture under aseptic conditions of the vein of the left ear at time 0 and thereafter at 2, 4, 24 and 48 h. Two ml of blood was collected into heparinized syringes for the isolation of monocuclear cells; another 3 ml was sampled with a non-heparinized syringe. One was added to blood culture flasks and the remaining to pyrogen-free tubes. After centrifugation serum was kept refrigerated at −70°C until assayed.
After operation, animals were transported to their cages and followed-up daily for a total of 14 days. At death necropsy was performed. Animals remaining alive after 14 days were killed by bolus intravenous administration of sodium thiopental. After a midline abdominal incision, segments of 0·3–0·5 g of liver, spleen and lower lobe of the right lung were cut with different blades; they were put into separate sterile containers and applied for quantitative culture.
Assays for lipopolysaccharide (LPS), tumour necrosis factor (TNF)-α and malondialdehyde (MDA)
For the estimation of LPS, serum samples were diluted 1:10 in sterile and pyrogen-free water (BioWhitaker, Walkersville, MD, USA) and incubated for 5 min at 70°C. The concentration of LPS was then measured by the QCL-1000 Limulus amoebocyte lysate assay (BioWhitaker, lower limit of detection 0·5 EU/ml) using a standard curve created by known concentrations of LPS of Escherichia coli serotype O111:B4. All determinations were performed in duplicate and the mean of two observations was applied.
TNF-α was measured by a bioassay on the L929 fibrosarcoma cell line, as described previously [6,7]. Briefly, confluent cells were washed thoroughly with Hanks’s solution and harvested with 0·25% trypsin/0·02% ethylenediamine tetraacetic acid (EDTA) (Biochrom AG, Berlin, Germany). Cells were centrifuged, re-suspended in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and 2 mM of glutamine (Biochrom AG) and distributed into a 96-well cell culture plate at a density of 1 × 105 cells/well. The final volume of fluid into each well was 0·05 ml. After incubation for 2–3 h at 37°C at 5% CO2, 0·06 ml of serum or of standard dilutions of known concentrations of human TNF-α (Sigma, range 5·75–375·00 pg/ml; St Louis, MO, USA) were added into each well followed by 0·05 ml of a 0·3 mg/ml dilution of cycloheximide (Sigma). Incubation continued overnight; then the supernatant of each well was discarded by aspiration and 0·1 ml of a 0·5 mg/ml methylene blue solution in methanol 99% was added. After 10 min the dye was removed and the wells were washed thoroughly three times with 0·9% sodium chloride. The wells were left to dry and remnants of the dye in each well became soluble by the addition of 0·1 ml of 50% glacial acetic acid (Merck, Darmstadt, Germany). Optical density in each well was read at 495 nm (Hitachi Spectophotometer, Tokyo, Japan) against blank wells and control wells without added serum. Concentrations of TNF-α were estimated by the reduction of the optical density of control wells by unknown samples applying a standard curve generated by standard concentrations. All determinations were performed in quadruplicate. The interday variation of the assay was 13·75%.
Lipid peroxidation was estimated by the concentration of MDA, as described previously [8]. Briefly, a 0·1-ml aliquot of each sample was mixed to 0·9 ml of trichloroacetic acid 20% (Merck) and centrifuged at 12 000 g and 4°C for 10 min. The supernatant was removed and incubated with 2 ml of thiobarbituric acid 0·2% (Merck) for 60 min at 90°C. After centrifugation, a volume of 10 µl of the supernatant was injected into a high-performance liquid chromatography system (HPLC; Agilent 1100 Series, Waldbronn, Germany) with the following elution characteristics: Zorbax Eclipse XDB-C18 (4·6 × 150 mm, 5 µm) column under 37°C; mobile phase consisting of a 50 mm K3PO4 (pH 6·8) buffer and methanol 99% at a 60:40 ratio with a flow rate of 1 ml/min; fluorometric detection with signals of excitation at 515 nm and emission at 535 nm. The retention time of MDA was 3·5 min and was estimated as µmol/l by a standard curve created with 1, 1, 3, 3-tetramethoxy-propane (Merck). All determinations were performed in duplicate.
Blood and tissue cultures
Volumes of 0·5 ml of blood were added into blood culture flasks with 20 ml of thioglycolate medium (Becton Dickinson, Cockeysville, MD, USA) and incubated at 35°C in an automatic incubator. Incubation stopped whenever an automated sign was given reading an increase in optical density of the flask; otherwise incubation was extended to a total period of 7 days. A volume of 1 ml was plated onto McConkey agar and identification of colonies was performed by the API20E and the API20NE systems (bioMérieux, Paris, France).
Tissue processing was carried out on the same day following sampling. Tissue segments were weighted and homogenized; a 0·1 ml aliquot was then diluted 1:10 into sterile sodium chloride four consecutive times. Another aliquot of 0·1 ml of each dilution was plated onto McConkey agar and incubated at 35°C for a total period of 3 days. Plates were incubated at 35°C and the number of viable colonies were counted into each dilution and multiplied by the appropriate dilution factor. Identification of colonies was performed as above. The lower detection limit was 10 colony-forming units (cfu)/g. The number of viable cells was expressed as its log10 value in cfu/g.
In 10 rabbits housed under the same conditions as those applied in the above experiments, stool samples were collected by a sterile swab (Oxoid, London, UK). Swabs were plated onto McConkey agar; plates were incubated for 18 h at 35°C. Identification of colonies was performed as above.
Assay for blood monocytes
For the isolation of blood monocytes, heparinized venous blood was layered over Ficoll Hypaque (Biochrom) and centrifuged. The separated mononuclear cells were washed three times with phosphate-buffered saline (PBS) (pH 7·2) and resuspended in RPMI-1640 supplemented with 10% FBS and 2 mM of glutamine in the presence of 100 U/ml of penicillin G and 0·1 mg/ml of streptomycin (Sigma). After incubation for 1 h at 37°C in 5% CO2, non-adherent cells were removed while adherent monocytes were washed three times with Hanks’s solution. Cells were then harvested by 0·25% trypsin/0·02% EDTA and counted. Non-adherent lymhocytes were centrifuged, washed three times with PBS (pH 7·2) and counted. The rate of apoptosis of lymphocytes and monocytes was estimated after incubation for 15 min in the dark with the protein Annexin-V at the fluorocolour fluorescein isothiocyanate (FITC) (emission 520 nm, Immunotech, Marseille, France) and with propidium iodide (PI) (emission 550 nm, Immunotech). Annexin-V is connected to the phosphatidylserine residue revealed in cell membranes upon initiation of the apoptotic process. PI is connected to dead cells. Cells staining positive for Annexin-V and negative for PI after running through the EPICS XL/MSL flow cytometer (Beckman Coulter Co., Miami, FL, USA) were considered apoptotic.
The remaining half of the monocytes were added into 12-well plates at a volume of 2·4 ml per well with RPMI-1640 supplemented with 10% FBS and 2 mM of glutamine. They were incubated for 18 h at 37°C under 5% CO2. TNF-α of supernatants was estimated as described above and expressed in pg/104 cells.
Statistical analysis
Due to the non-normal distribution of single values, results were represented by their median and range; those of bacteria translocating in tissues were given by their median and 95% confidence intervals of the mean. Comparisons between groups were performed by analysis of variance (anova) for values referring to vital signs of animals according to Bonferroni’s post hoc analysis; other parameters were compared by the Mann–Whitney U-test with a correction according to Bonferroni. Parameters were correlated according to Spearman’s rank order. Comparisons of qualitative data between groups ware performed by Fisher’s exact test. Survival of each group was estimated by Kaplan–Meier analysis; comparisons between different groups were performed by the log-rank test. Any P value below 0·05 was considered significant.
Results
Comparisons of vital signs of the three animal groups over follow-up are shown in Fig. 1. Heart rate of groups B and C was higher than group A (P = 0·045). Systolic and diastolic pressures and rectal temperatures of groups B and C were lower than group A (P < 0·0001).
Fig. 1.
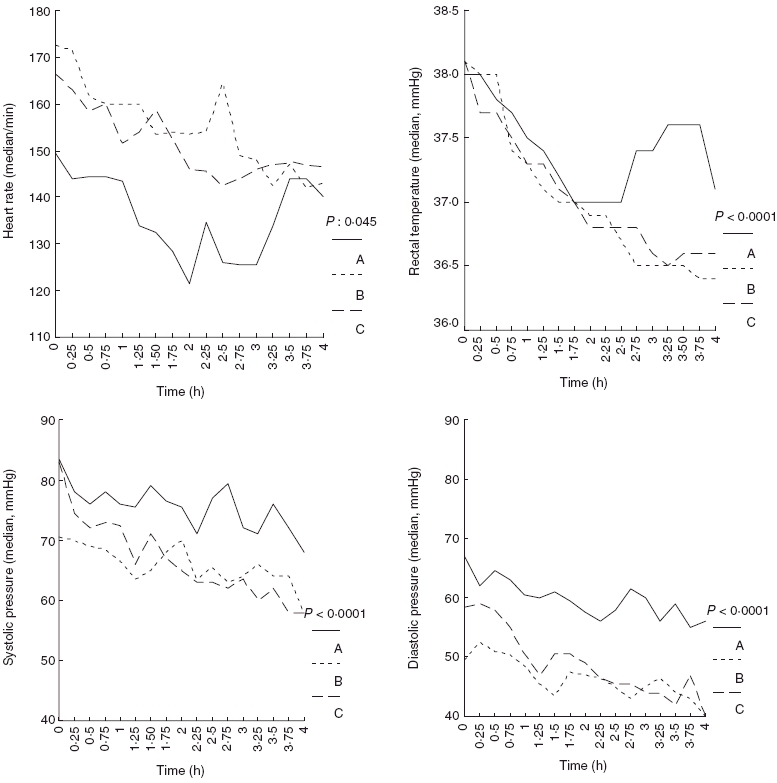
Comparative heart rates, rectal temperatures, systolic and diastolic blood pressures of animals of group A (controls), group B (myotomy of femur) and group C (myotomy and fracture of femur) over the first 4 h of follow-up.
Median survival of group A was more than 14 days; in group B more than 14 days [P not significant (n.s.) compared to group A]; and in group C 9 days (P = 0·0075 compared to group A and P = 0·0133 compared to group B). Survival curves of the treatment groups are shown in Fig. 2.
Fig. 2.
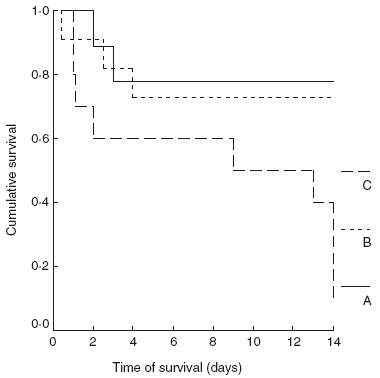
Comparative survival of animals of group A (controls), group B (myotomy of femur) and group C (myotomy and fracture of femur).
The rate of apoptosis of lymphocytes and monocytes and concentrations of TNF-α of supernatants of monocytes in the three study groups are shown in Table 1. Apoptosis of lymphocytes in group C was higher than group A at 2, 4 and 48 h (P of comparisons = 0·012, 0·028 and 0·049, respectively). Apoptosis of lymphocytes in group C was higher than group B at 2 h (P = 0·033) and in group B higher than group A at 4 h (P = 0·048). Apoptosis of monocytes in group C was higher than group A at 2 and 4 h (P of comparisons = 0·006 and < 0·0001, respectively). Apoptosis of monocytes in group C was higher than group B at 2 and 4 h (P of comparisons = 0·006 and 0·017, respectively). TNF-α of monocyte supernatants in group C was lower than group A at 2 h (P = 0·014) and in group C lower than group B at 2 h (P = 0·036). TNF-α of monocyte supernatants in group B was lower than group A at 4 h (P = 0·022).
Table 1.
Comparative rates of apoptosis of lymhocytes and monocytes and concentrations of tumour necrosis factor (TNF)-α of supernatants of monocytes of group A (controls), group B (myotomy of femur) and group C (myotomy and fracture of femur).
| Time (h) | Group A | Group B | Group C |
|---|---|---|---|
| Apoptosis of lymphocytes (median-range, %) | |||
| 2 | 0·54 (0·42–2·16) | 0·51 (0·25–2·68) | 47·14 (1·54–64·13)a,c |
| 4 | 1·41 (0·54–18·18) | 37·30 (0·17–49·36)d | 37·34 (2·52–58·85)a |
| 24 | 13·35 (1·29–31·45) | 9·42 (0·47–52·08) | 9·47 (0·61–57·87) |
| 48 | 1·97 (1·59–2·65) | 1·06 (0·20–52·11) | 8·81 (0·85–23·66)a |
| Apoptosis of monocytes (median-range, %) | |||
| 2 | 11·64 (2·94–23·08) | 4·58 (1·74–21·43) | 50·11 (34·18–78·57)b,c |
| 4 | 26·56 (8·11–31·72) | 28·33 (3·20–65·21) | 57·24 (35·14–81·51)b,c |
| 24 | 17·43 (12·23–36·06) | 39·85 (2·22–68·63) | 34·01 (14·13–54·33) |
| 48 | 55·32 (25·25–79·36) | 19·31 (6·48–75·93) | 27·24 (13·72–43·59) |
| TNF-α of monocyte supernatants (median-range, pg/104 cells) | |||
| 2 | 63·47 (31·54–73·60) | 19·01 (10·04–43·02) | 0·33 (0–45·16)a,c |
| 4 | 155·53 (0–512·45) | 6·80 (0–55·81)d | 25·32 (0–641·53) |
| 24 | 25·49 (0–312·93) | 21·04 (0–119·78) | 20·46 (0–100·07) |
| 48 | 9·11 (2·60–220·80) | 25·65 (0–202·89) | 8·18 (0–84·78) |
Comparisons between groups A and C at the indicated time intervals:
P < 0·050
P < 0·010. Comparisons between groups B and C at the indicated time intervals
P < 0·05. Comparisons between groups A and B at the indicated time intervals
P < 0·05.
Serum concentrations of LPS, TNF-α and MDA of the three study groups are given in Table 2. LPS of group C was higher than group A at 2, 4 and 48 h (P of comparisons between groups = 0·007, 0·049 and 0·031, respectively). MDA of group C was higher than group B at 2 h (P = 0·028).
Table 2.
Comparative concentrations of endotoxins [lipopolysacchariede (LPS)], tumour necrosis factor (TNF)-α and malondialdehyde (MDA) over time of animals of group A (controls), group B (myotomy of femur) and group C (myotomy and fracture of femur).
| Time (h) | Group A | Group B | Group C |
|---|---|---|---|
| LPS (median-range, EU/ml) | |||
| 0 | 0·50 (0·50–1·75) | 0·55 (0·50–1·32) | 0·82 (0·50–11·06) |
| 2 | 0·54 (0·50–2·82) | 0·53 (0·50–11·32) | 3·09 (0·53–13·35)a |
| 4 | 0·50 (0·50–1·80) | 0·52 (0·50–1·36) | 1·72 (0·50–9·63)a |
| 24 | 0·50 (0·50–1·75) | 0·50 (0·50–4·61) | 0·50 (0·50–9·20) |
| 48 | 0·50 (0·50–1·01) | 0·50 (0·50–11·64) | 1·05 (0·50–1·63)a |
| TNF-α (median-range, pg/ml) | |||
| 0 | 95·29 (5·75–333·29) | 96·25 (5·75–663·89) | 66·15 (5·75–852·18) |
| 2 | 99·33 (5·75–939·70) | 240·02 (15·66–1219·82) | 152·21 (5·75–777·61) |
| 4 | 186·70 (11·75–375·44) | 81·35 (11·50–455·18) | 120·60 (5·75–435·31) |
| 24 | 64·45 (11·77–411·98) | 99·97 (11·50–541·83) | 77·52 (5·75–695·86) |
| 48 | 129·21 (5·75–633·89) | 69·77 (5·75–759·64) | 133·97 (11·50–1641·91)b |
| MDA (median-range, pmol/l) | |||
| 0 | 0·34 (0·07–2·81) | 0·25 (0·05–1·93) | 0·84 (0·15–1·04) |
| 2 | 1·02 (0·07–3·22) | 0·29 (0·05–0·79) | 1·26 (0·28–2·29)b |
| 4 | 0·35 (0·14–2·99) | 0·32 (0·06–1·36) | 0·83 (0·13–1·13) |
| 24 | 0·52 (0·12–2·34) | 0·23 (0·09–1·53) | 1·19 (0·15–2·67) |
| 48 | 0·26 (0·07–6·73) | 0·35 (0·17–1·90) | 0·30 (0·23–1·96) |
Comparisons between groups A and C at the indicated time intervals
P < 0·050. Comparisons between groups B and C at the indicated time intervals
P < 0·05.
Apoptosis of lymphocytes was correlated positively with heart rate (rs: + 0·362, P = 0·033) and negatively with rectal temperature (rs: – 0·485, P = 0·003). The rate of apoptosis of lymphocytes in all groups was correlated positively with serum TNF-α (rs: + 0·511, P < 0·0001) and to the rate of apoptosis of monocytes (rs: + 0·629, P < 0·0001). The rate of apoptosis of lymphocytes in all groups was correlated negatively with survival (rs: – 0·521, P = 0·039).
Apoptosis of monocytes was correlated positively with heart rate (rs: + 0·436, P = 0·006) and negatively with rectal temperature (rs: – 0·405, P = 0·012); it was also correlated negatively with systolic blood pressure (rs: – 0·379, P = 0·021). The rate of apoptosis of monocytes in all groups was correlated positively with serum TNF-α (rs: + 0·357, P = 0·002) and correlated negatively with serum MDA (rs: – 0·367, P = 0·006), with TNF-α of monocyte supernatants (rs: – 0·288, P = 0·013) and with survival (rs: – 0·445, P = 0·029).
Blood cultures yielded Pseudomonas aeruginosa in two (20%), four (40%), nil (0%) and nil (0%) animals in group A at 2, 4, 24 and 48 h, respectively. Respective positive blood cultures in group B were found in seven (63·63%), seven (63·63%), one (9·09%) and one (9·09%) animals. Respective positive blood cultures in group C were found in eight (72·72%), eight (72·72%), nil (0%) and nil (0%) animals. Cultures of liver, lung and spleen of all animals in group A were sterile. Evidence of bacterial translocation was found in five animals in group B (45·45%) and in six animals in group C (54·54%). The above rates of bacterial translocation were higher compared to group A (P = 0·019). P. aeruginosa was isolated from liver, lung and spleen of these animals. The bacterial load of liver, lung and spleen for the animals in groups B and C are shown in Fig. 3. All six animals in group C where P. aeruginosa was isolated in tissues eventually died. Death occurred in two of five animals in group B where P. aeruginosa was isolated in tissues.
Fig. 3.
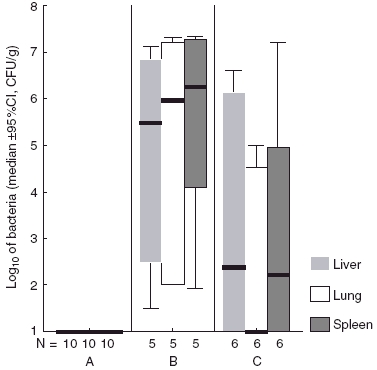
Translocating isolates of Pseudomonas aeruginosa in liver, lower lobe of the right lung and spleen of animals of group A (controls), group B (myotomy of femur) and group C (myotomy and fracture of femur). The number of animals with positive tissue cultures is indicated.
Median (range) apoptosis of lymphocytes of animals without evidence of bacterial translocation in tissues was 1·10% (0·25–64·93%) at 2 h and 10·40% (0·17–58·85%) at 4 h. Respective values of animals with evidence of bacterial translocation was 24·13% (0·51–60·83%) (P n.s. compared to animals without evidence of translocation) and 40·29% (10·41–63·55%) (P = 0·008 compared to animals without evidence of translocation).
Median (range) apoptosis of monocytes of animals without evidence of bacterial translocation in tissues was 11·65% (1·74–50·11%) at 2 h and 30·80% (3·20–42·70%) at 4 h. Respective values of animals with evidence of bacterial translocation was 36·53% (4·58–78·57%) (P n.s. compared to animals without evidence of translocation) and 60·61% (23·50–81·50%) (P = 0·006 compared to animals without evidence of translocation).
Bacterial species isolated from stool samples were Escherichia coli in eight animals (80%), Enterococcus spp. in six animals (60%), P. aeruginosa in three animals (30%) and Enterobacter cloacae in four animals (40%).
Discussion
Multiple trauma is a common cause of SIRS characterized by elevated concentrations of proinflammatory cytokines [2]; bacterial translocation and apoptosis are pathophysiological mechanisms often triggered in patients with polytrauma. Although considerable evidence is available for apoptosis of lymphocytes and of lymphoid tissues in the advent of multiple injuries, data about apoptosis of monocytes are lacking. The present study aimed to define the rate of apoptosis of blood monocytes in an experimental model of multiple trauma and its time sequence in correlation with apoptosis of lymphocytes and to the occurrence of bacterial translocation.
The applied experimental model was accompanied by haemodynamic deterioration of animals leading to death; the phenomenon was proportionate to the level of trauma being less intense in animals with single muscle injury and lethal in animals with bone fractures (Figs 1 and 2). Results revealed a considerable rate of apoptosis of blood monocytes and lymphocytes within the first 2 h after induction of injuries (Table 1). Apoptosis was correlated positively with heart rate and negatively with systolic pressure and rectal temperature. As a consequence, early apoptosis led to tachycardia, hypothermia and hypotension so as to contribute to haemodynamic deterioration in the event of polytrauma. All the apoptotic phenomena were correlated positively with serum TNF-α, as expected, as TNF-α is a trigger of the death pathway [2]. Apoptosis of monocytes was accompanied by low ex vivo release of TNF-α, signifying a decrease of the biosynthetic potency of monocytes. Biosynthetic inertia of monocytes might be considered a consequence of apoptosis. Increased apoptosis of monocytes and lymphocytes could not be demonstrated at 24 and 48 h. This might be attributed to death of cells being apoptotic at 2 and 4 h and of their subsequent disappearance from systemic circulation at 24 and 48 h. Apoptosis of monocytes was increased after 48 h in animals in group A (Table 1). This might be expected in healthy rabbits, where the transit time of monocytes in blood had been reported to be close to 30 h [9].
Early apoptosis of thymus, spleen, intestine, lung and liver has been shown in experimental studies [10] as well as in human subjects. In patients with polytrauma, early apoptosis was detected in lymphoid tissues [11,12]. However, this is the first study, to our knowledge, proving the early induction of the apoptotic mechanism in blood monocytes in the field of multiple trauma. This finding might be of prime importance for the initiation of immunoparalysis, because monocytes are cells linking innate and adaptive immune responses [2].
The applied experimental model elicited early elevation of MDA and TNF-α (Table 2), indicating the initiation of the systemic inflammatory response in accordance with the results of other authors [13]. TNF-α was also detected in sera of animals in group A, although at lower concentrations than in group C. TNF-α detected in the sera of controls might be explained by the impact of operative procedures and anaesthesia [14]. SIRS of animals was accompanied by early bacterial translocation, as evidenced by systemic endotoxaemia and positive tissue cultures (Table 2 and Fig. 3). Bacterial translocation is a common denominator after multiple trauma intensifying SIRS and accelerating progression to death [15–17]. Systemic endotoxaemia complicating multiple injuries occurs early in the clinical course of patients [18]; its advent might be attributed to co-existing apoptosis of intestinal epithelia [10,12].
The described early apoptosis of monocytes and lymphocytes might be responsible for the bacterial translocation of P. aeruginosa observed in the animals studied. Enteric flora assessed in controls consists of enterobacteriaceae as well as of P. aeruginosa. No evidence of translocation was found for enterobacteriaceae. P. aeruginosa is a nosocomial pathogen causing infections in the immunocompromised and debilitated hosts [19]. Severe lymphopenia and monocytopenia resulting from increased apoptosis might explain the bacterial translocation of that species contributing to death. The latter hypothesis is based on the findings of significantly increased apoptosis of lymphocytes and monocytes at the 4-h time interval among animals with microbiological documentation of translocation, as opposed to animals with lack of any evidence of translocation. Although bacteraemia by P. aeruginosa was detected, albeit at a much lower frequency, in controls, that species was not isolated in tissues of controls. This observation intensifies further the need for apoptosis of blood lymphocytes and monocytes for translocation by that species to supervene.
The experimental model presented of multiple trauma revealed the existence of early apoptosis of blood monocytes; the phenomenon was accompanied by apoptosis of blood lymphocytes and subsequent bacterial translocation. Further research is mandatory to elucidate the significance of these findings for the prevention of immunoparalysis in the multiple trauma patient.
Acknowledgments
This study was funded in part by the University of Athens, Research Program ‘Kapodistrias’.
References
- 1.Jiang JX, Tian KL, Chen HS, Zhu PF, Wang ZG. Plasma cytokines and endotoxin levels in patients with severe injury and their relationship with organ damage. Injury. 1997;28:509–13. doi: 10.1016/s0020-1383(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 2.Keel M, Trentz E. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Nakae H, Endo S, Yamada Y, Inada K. Bound and soluble adhesion molecule and cytokine levels in patients with severe burns. Burns. 2000;26:139–14. doi: 10.1016/s0305-4179(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 4.Efstathopoulos N, Bathrellos E, Giamarellos-Bourboulis EJ, et al. N-6 polyunsaturated fatty acids confer hemodynamic stability in an experimental model of multiple trauma. Prostaglandins Leukot Essent Fatty Acids. 2005;72:357–62. doi: 10.1016/j.plefa.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Baker SP, O’Neil B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 6.Engelhard D, Pomernz S, Gallily G, Strauss N, Tuomanen E. Serotype-related differences in inflammatory response to Streptococcus pneumoniae in experimental meningitis. J Infect Dis. 1997;175:979–82. doi: 10.1086/514005. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, et al. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48:93–9. doi: 10.1128/AAC.48.1.93-99.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R, Chase SD. Rapid, fluorometric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr. 2002;775:121–6. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 9.Goto Y, Hogg JC, Suwa T, Quinlan KB, van Eeden SF. A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5′-bromo-2′-deoxyuridine. Am J Physiol Cell Physiol. 2003;285:C253–9. doi: 10.1152/ajpcell.00035.2003. [DOI] [PubMed] [Google Scholar]
- 10.Guan J, Jin D, Jin L, Lu Q. Apoptosis in organs of rats in early stage after polytrauma combined with shock. J Trauma. 2002;52:104–11. doi: 10.1097/00005373-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Schmieg RE, Jr, Swanson PE, et al. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. 2000;28:3207–17. doi: 10.1097/00003246-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Ocal K, Avlan D, Cinel I, et al. The effect of N-acetylcysteine on oxidative stress in intestine and bacterial translocation after thermal injury. Burns. 2004;30:778–84. doi: 10.1016/j.burns.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Iyaoka M, Iwase K, Suzuki R M, et al. Clinical evaluation of circulating interleukin-6 and interleukin-10 levels after surgery-induced inflammation. J Surg Res. 2005;125:144–50. doi: 10.1016/j.jss.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Douzinas E, Giamarellos-Bourboulis EJ, Andrianakis I, Xiarchos A, Roussos C. Effect of intravenous administration of high doses of immunoglibulins on systemic endotoxemia of patients with multiple injuries. Infect Dis Clin Pract. 2005;13:247–9. [Google Scholar]
- 16.Samonte VA, Goto M, Ravindranath TM, et al. Exacerbation of intestinal permeability in rats after a two-hit injury: burn and Enterococcus faecalis infection. Crit Care Med. 2004;32:2267–73. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–53. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 18.Buttenschoen K, Berger D, Strecker W, et al. Association of endotoxemia and production of antibodies against endotoxins after multiple injuries. J Trauma. 2000;48:918–23. doi: 10.1097/00005373-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Annane D, Aegerter P, Jars-Guincestre C, Guidet B. Current epidemiology of septic shock. Am J Resp Crit Care Med. 2003;168:165–72. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]


