Abstract
Anti-DNA autoantibodies were thought to play a major role in the pathogenesis of lupus nephritis (LN). A recent study revealed that affinity-purified anti-DNA antibodies had a cross-reaction with human glomerular mesangial cells (HMC). However, whether the cross-reaction was antigen–antibody-mediated was unclear. The aim of the current study was to investigate the binding of anti-DNA antibodies to HMC membrane proteins and to characterize the target antigens. Affinity-purified IgG anti-DNA antibodies were purified by DNA-cellulose chromatography in sera from nine patients with biopsy-proven active lupus nephritis. In vitro cultured primary HMCs were disrupted by sonication and HMC membranes were obtained by differential centrifugation. The membranes of human umbilical vein endothelial cells (HUVEC), human proximal renal tubular epithelial cell line (HK2) and peripheral mononuclear cells (PMC) were obtained as controls. Binding of anti-DNA antibodies to the membrane proteins was investigated by Western blot analysis using soluble membrane proteins as antigens. Both HMC membrane and affinity-purified anti-DNA antibodies were treated with DNase I to exclude DNA bridging. All nine affinity-purified anti-DNA antibodies could blot the HMC membrane proteins, and there were at least three bands at 74 kDa, 63 kDa and 42 kDa that could be blotted. Among the nine IgG preparations, all nine (100%) could blot the 74 kDa band; eight (88·9%) could recognize 63 kDa and 42 kDa protein bands separately. After DNase treatment, the same bands could still be blotted by most affinity-purified anti-DNA antibodies. Affinity-purified anti-DNA antibodies could also blot similar bands on membrane proteins of other cells, but some bands were different. In conclusion, anti-DNA autoantibodies could cross-react directly with cell membrane proteins of human glomerular mesangial cells and might play an important role in the pathogenetic mechanism in lupus nephritis.
Keywords: anti-DNA antibodies, cross-reaction, lupus nephritis, mesangial cell, membrane proteins
Introduction
Anti-DNA autoantibodies are not only a helpful serological marker for diagnosis of systemic lupus erythematosus (SLE); they have also been shown to be pivotal in the pathogenesis of lupus nephritis [1–3]. However, the mechanisms by which these antibodies participate in the induction of tissue damage and glomerulonephritis remain obscure. Anti-DNA antibodies were thought traditionally to cause glomerulonephritis by forming circulating or in situ immune complexes with DNA [4–6]. Recently, it was believed that anti-dsDNA antibodies were pathogenic to the kidney via cross-reactivity directly or via a nuclear antigen bridge binding to the glomerular structures [7]. Therefore, it was suggested that anti-DNA antibodies might initiate inflammatory processes through immunological cross-reactions with cell-surface and extracellular matrix components [8–10].
Recently, Chan et al. [11] reported that affinity-purified anti-DNA antibodies could bind directly to human glomerular mesangial cells (HMC) without DNA bridge by cellular enzyme-linked immunosorbent assay, flow cytometry and immunohistochemical staining; however, the target antigens on the membrane of human mesangial cells have not yet been identified.
In an effort to characterize the cell-surface components which cross-react with anti-DNA antibodies, we initiated the present study.
Materials and methods
Patients
Serum samples from nine anti-DNA antibody-positive patients with biopsy-proven lupus nephritis were obtained from 2003 to 2004 in Peking University First Hospital; seven were female and two were male, with a mean age at 32·56 ± 15·63 years. All patients fulfilled the 1997 American College of Rheumatology revised criteria for systemic lupus erythematosus (SLE) [12]. Serum samples from healthy subjects (n = 4) with comparable gender and age distributions were collected as controls. All sera were stored at −20°C until use. Informed consents were obtained for blood sampling.
Isolation of anti-DNA antibodies from active SLE sera
Sera (0·5 ml) were diluted with 20 mM Tris-HCl pH 7·2 and applied to a 1 ml protein G (Amersham Biosciences, Uppsala, Sweden) Sepharose affinity column. The bound IgG fraction was eluted with 0·1 M glycine, pH 2·7 and neutralized immediately with 2 M Tris-HCl pH 9·0 and dialysed extensively against phosphate-buffered saline (PBS). The concentration of IgG was measured by a spectrophotometer at OD280 and the purity of the isolated IgG was confirmed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE).
The purified IgG preparations were loaded onto a native DNA-cellulose column (Amersham Biosciences, Piscataway, NJ, USA) [13] equilibrated with 25 mM Tris-HCl pH 8·0, 0·05 M NaCl at a flow rate of 0·5 ml/min. Anti-DNA antibodies were eluted with a linear NaCl gradient. Absorbance at A280 was measured throughout the purification process. Isolated anti-DNA antibodies were desalted and concentrated using polyethylene glycol 20 000 before determination of IgG concentration or use in experiments.
Cell culture
Cell lines
Human proximal renal tubular epithelial cell line (HK2) was purchased from the American Type Culture Collection (ATCC Corp., Mamassas, VA, USA) and cultured according to the supplier’s instructions.
Isolation and culture of primary human glomerular mesangial cells (HMC)
Histologically normal human cortical renal tissue was obtained from kidneys removed for cancer. The isolation and subculture of human glomerular mesangial cells (HMC) were performed as described previously, with some modifications [14]. Briefly, the medulla was dissected away from the cortex and discarded. The cortex was then transferred to a 140-mesh stainless steel sieve. The tissue was forced through the sieve with moderate pressure using the bottom of a pestle and repeated washing with cold 0·85% sodium chloride solution. Material remaining on the screen was discarded. The sieved suspension was then poured through an 80-mesh sieve to retain large tissue fragments, and finally through a 220-mesh sieve. The material retained on the fine sieve was then washed extensively with cold 0·85% sodium chloride until nothing but glomeruli, and possibly tiny tubular fragments attached to glomeruli remained, checked through by phase contrast microscopy. This material was transferred to 50-ml plastic centrifuge tubes centrifuged at 201 g for 5 min, then the glomeruli was digested with collagenase (1 mg/ml) for 3–4 min and plated in T-75 plastic culture flasks in RPMI-1640 medium (Gibco, Invitrogen Corp., NY, USA) supplemented with 300 mg/l l-glutamine, 2·38 g/l N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), 330 mg/l pyruvic acid sodium salt, 2·5 g/l NaHCO3, 60 mg/l penicillin, 135 mg/l streptomycin and 20% fetal calf serum (FCS) (Gibco). Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C and culture media was changed thrice a week. Cells from the third to the fifth passages were used for experiments, and the homogeneous pure HMC were assessed by standard morphology and immunofluorescence staining [15].
Isolation and culture of human umbilical vein endothelial cells (HUVEC)
Endothelial cells were harvested from human umbilical veins by collagenase (0·1%, Gibco) digestion and plated in culture flasks in M199 medium (Gibco) supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 20% FCS (Gibco), 6·25 µ/ml heparin, 2·38 mg/ml HEPES and 20 µg/ml endothelial cell growth factor (ECGF, Roche, Mannheim, Germany) [16]. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C and the culture medium was changed thrice a week. After 5–7 days, monolayer cells were treated with 0·05% trypsin and 0·02% ethylenediamine tetraacetic acid (EDTA) in Ca2+ Mg2+-free Hanks’s balanced salt solution and resuspended in complete medium. Subcultures were performed approximately every 5–7 days at a ratio of 1:2–3. The cells were identified by typical endothelial cell morphology with phase contrast microscopy, von Willebrand factor (vWF) and Weibel-Palade bodies, which were observed by indirect immunofluorescence and immunohistochemistry with rabbit-anti-human vWF antibody (Zymed, San Francisco, CA, USA) and electron microscopy, respectively. The cells were used for isolation of cell membrane and antigen extraction between the third and fifth passages.
Isolation of peripheral blood mononuclear cells (PMC)
Fresh heparinized blood from a normal blood donor was placed into 50 ml conical centrifuge tubes. Using a sterile pipette, an equal volume of PBS was added at room temperature and mixed well. Ficoll-Hypaque solution was layered slowly underneath the blood/PBS mixture, then centrifuged for 30 min at 805 g. Using a sterile pipette, the upper layer that contains the plasma and most of the platelets was removed. Using another pipette, the mononuclear cell layer was transferred to another centrifuge tube. The cells were washed by adding excess PBS (about three times the volume of the cells layer) and centrifuged for 10 min at 340 g until most of the platelets were removed. The cells were counted and the viability of the cells was determined by Trypan blue exclusion.
Preparation and extraction of cell membrane proteins
The membrane fraction was prepared as described [17] with some modification. Ten million (10 × 06) viable cells were washed three times in 0·01 M, pH 7·4 PBS. Cells were suspended in 5 ml of buffer A containing 10 mM Tris/HCl, 10 mM CaCl2 and 0·25 M sucrose adjusted to pH 7·4. The cells were lysed with ultrasonic processor (Sonics & Materials, Inc., Newtown, CT, USA) in the presence of protease inhibitors [phenylmethylsulphonyl fluoride (PMSF) 10 mg/ml, leupeptin 1 mg/ml and aprotinin 1 mg/ml, Sigma, St. Louis, MO, USA]. The whole homogenate was centrifuged at 1200 g for 20 min. The supernatant was incubated with 100 µg/ml DNase I in PBS containing 10 mM MgCl2 for 1 h at 37°C (the efficacy of DNA cleavage was checked by polyacrylamide gel electrophoresis and AgNO3 staining before and after DNase treatment), after which the reaction was stopped by the addition of EDTA (15 mM). The supernatant was then centrifuged at 20 000 g for 60 min at 4°C. The pellet was dissolved in lysing buffer (0·5 ml) containing 1% TritonX-100 and a cocktail of protease inhibitors (0·1 M EDTA, 1 M iodoacetamide, 10 mg/ml PMSF) in PBS at 0°C with gentle vortexing for about 30 min. Non-dissolving material was removed by centrifugation at 12 000 g for 15 min at 4°C. The supernatant was aliquoted and stored at −70°C until use.
Western blot analysis
Cell membrane proteins (200 µl) were prepared in non-reducing conditions. After boiling for 5 min and ultracentrifugation, the sample was loaded into a 10% polyacrylamide gel and electrophoresed at 150 V for about 2 h. Proteins were transferred to a nitrocellulose membrane at 32 mAmp for 70 min. The membrane was blocked in 5% skimmed milk for 1 h at room temperature and then incubated overnight with affinity-purified IgG anti-DNA antibodies at 4°C. The membrane was washed with 0·1% Tween20 in Tris-buffered saline (TBST) three times and then incubated with the alkaline phosphatase-conjugated affinity-purified goat anti-human IgG (γ-chain specific) diluted 1:6000 with TBST with 5% skimmed milk for 1 h at room temperature. Colour was developed by addition of the appropriate substrates: nitro-blue-tetrazoleum (NBT) and 5bromo-4chloro-3indolyl phosphate (BCIP). The reaction was stopped after several minutes with distilled water.
To confirm the specificity of anti-DNA activity, the affinity-purified anti-DNA antibodies were preincubated with calf thymus DNA (Sigma) at a final concentration of 50 µg/ml for 1 h at room temperature. Then, Western blot analysis was performed as described above.
Results
Binding of IgG anti-DNA antibodies to HMC membrane proteins
All nine IgG anti-DNA antibodies from sera of patients with lupus nephritis could recognize the protein(s) of HMC membrane. Among the nine affinity-purified IgG anti-DNA preparations, all nine (100%) could blot the 74 kDa band; eight (88·9%) could recognize 63 kDa and 42 kDa protein bands separately. None of the four normal controls could recognize any of the protein bands (Fig. 1).
Fig. 1.
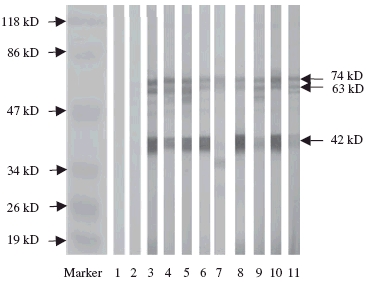
Western blot analysis to reveal the reactivity of IgG anti-DNA antibodies from sera of the patients with lupus nephritis with membrane proteins of human mesangial cells; the membrane proteins were not predigested with DNase I. Lane 1: blank control; lane 2: normal control; lanes 3–11: IgG anti-DNA antibodies from sera of the patients with lupus nephritis.
Binding of IgG anti-DNA antibodies to HMC membrane proteins predigested with DNase I
After the affinity-purified IgG anti-DNA preparations and HMC membrane proteins were predigested with DNase I, the affinity-purified IgG anti-DNA preparations could still recognize the same bands but with less frequency. Among the nine affinity-purified IgG anti-DNA preparations, eight (88·9%) could still recognize the 42 kDa band, six (66·7%) could still recognize the 74 kDa and five (55·6%) could recognize the 63 kDa bands (Fig. 2).
Fig. 2.
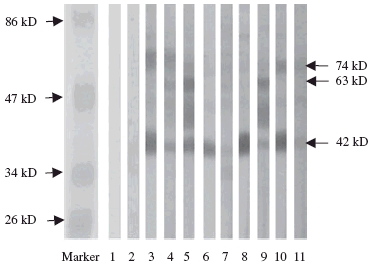
Western blot analysis to reveal the reactivity of IgG anti-DNA antibodies from sera of the patients with lupus nephritis with membrane proteins of human mesangial cells predigested with DNase I. Lane 1: blank control; lane 2: normal control; lanes 3–11: IgG anti-DNA antibodies from sera of the patients with lupus nephritis
Specificity of the affinity-purified anti-DNA IgG preparations
The specificity of the binding was investigated by preincubating the anti-DNA antibodies with dsDNA. This treatment abolished the binding of these antibodies to the blotted proteins completely (Fig. 3).
Fig. 3.
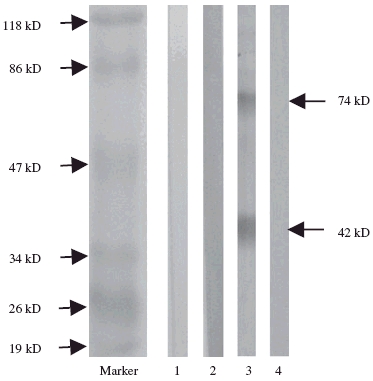
Western blot analysis to reveal the specificity of IgG anti-DNA antibodies from serum of one patient with lupus nephritis. Membrane proteins of human mesangial cells were used as antigens. Lane 1: blank control; lane 2: normal control; lane 3: affinity-purified IgG anti-DNA antibodies; lane 4: affinity-purified IgG anti-DNA antibodies as lane 3 but preincubated with 50 µg/ml dsDNA.
Binding of anti-DNA antibodies to membrane proteins of other primary cells and a cell line
This was carried out to identify whether the antigens of HMC membrane recognized by anti-DNA antibodies were shared by other cells. The membrane proteins of HUVEC, HK2 and PMC were extracted and subjected to immunoblot analysis. The 74 kDa protein could be recognized only by anti-DNA antibodies on the membranes of HMC. The 63 kDa protein could be recognized by anti-DNA antibodies on the membranes of HUVEC, HK2 but not PMC. The 42 kDa could also be blotted onto all the membrane proteins of HUVEC, HK2 and PMC. None of the protein bands was blotted by the normal control (Fig. 4).
Fig. 4.
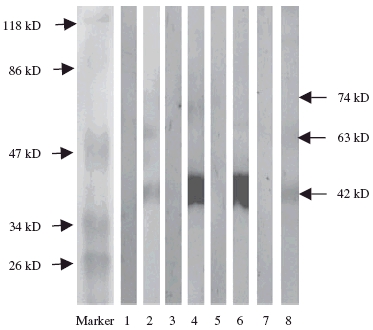
Western blot analysis to reveal the reactivity of IgG anti-DNA antibodies with membrane proteins of four different cells. The membrane proteins of human glomerular mesangial cells (HMC) (lanes 1, 2), human umbilical vein endothelial cell (HUVEC) (lanes 3, 4), human proximal renal tubular epithelial cell line (HK2) (lanes 5, 6) and peripheral mononuclear cells (PMC) (lanes 7, 8) were used as antigens. Lanes 1, 3, 5, 7: affinity-purified IgG from a healthy volunteer as negative control; lanes 2, 4, 6, 8: affinity-purified IgG anti-DNA antibodies from a patient with lupus nephritis.
Discussion
Since their discovery in 1957, anti-DNA antibodies have been suggested to play a crucial role in the pathogenesis of lupus nephritis. Clinically, anti-DNA antibodies could be eluted from kidneys of patients with active nephritis, suggesting that these antibodies might be important in the induction of tissue damage; however, the mechanisms remained obscure. Traditionally, it was thought that tissue injury was mediated by the deposition of circulating immune complexes [4,5] or by in situ formation of immune complexes [6] composed of DNA and anti-DNA antibodies. Studies on lupus nephritis have shown that free DNA and DNA/anti-DNA complexes were not present in circulation, and that intravenous injection of DNA/anti-DNA preparations failed to localize to the glomerulus [18]. There had been no method that rendered DNA immunogenic with the production of antibodies to native DNA until Krishnan and Marion immunized normal mice with mammalian DNA linked to an arginine-rich fusion protein, Fus-1, and induced IgG antibodies to native DNA, suggesting a critical role for nucleic acid binding proteins in the induction of these antibodies [19]. It was suggested that DNA itself might have no role in the pathogenesis of LN and that anti-DNA antibodies might initiate the inflammatory processes through immunological cross-reactions with cell-surface and extracellular matrix components [8–10].
Anti-DNA antibodies eluted from human lupus kidney and lupus-prone mice displayed polyreactive binding properties that might determine the capacity of the antibodies to form immune deposits. An important characteristic of polyreactive antibodies was their ability to bind renal structural antigens, as has been demonstrated in several studies [20,21].
Naparstek [22] reported that when anti-DNA autoantibodies were applied to mesangial cells in culture the antibodies bound to the extracellular matrix (ECM). Investigations to identify the target antigen(s) in ECM recognized by this fraction of anti-DNA autoantibodies revealed that 50–70% of the autoantibodies bound to the α2-laminin component of ECM. The antigenic epitope was narrowed further to the E3 component of α2-laminin, the 5/2 C-terminal globular regions and specifically to a single peptide of 21 amino acids, named TV5100. Cross-reactivity between human anti-DNA autoantibodies and anti-α-laminin antibodies in human sera and possible pathogenicity was studied. Higher titres of autoantibodies to the TV5100 peptide were seen in sera from SLE patients compared with healthy controls. Moreover, there was a statistical correlation between clinical activity and anti-TV5100 autoantibodies titres in sera from the patients and higher titre anti-TV5100 autoantibodies might be associated with the development of diffuse proliferative glomerulonephritis.
Putterman and colleagues [23–25] found that one monoclonal anti-DNA antibody, R4A, was able to bind to the cell surface of MRL mesangial cells and to a 100 kDa band from the mesangial cell lysate, and identified this protein as α-actinin. In animal models of lupus, increased titres of anti-α-actinin antibodies in the serum and kidney eluates of lupus mice with active nephritis were observed significantly. The time-course for development of anti-α-actinin antibodies in these mice paralleled closely that of IgG anti-dsDNA antibodies. In addition, serum anti-dsDNA activity could be inhibited by α-actinin, suggesting that many anti-dsDNA autoantibodies might cross-react with α-actinin. Putterman et al. proposed that an overlap between anti-DNA and anti-α-actinin antibodies might be a determining factor in the pathogenic potential of anti-DNA autoantibodies.
Madaio [26] described a subset of monoclonal anti-DNA autoantibodes derived from lupus-prone MRL lpr/lpr mice that had the ability to localize within the nuclei of cells. Trypsin treatment inhibited cell surface binding, indicating that cell surface proteins were involved in the internalization and an 110 kDa protein identified as myosin I. In cultured cells, the anti-DNA antibodies bound to membrane myosin I and localized into caveolae, and the ability of the anti-DNA autoantibodies to localize to the nucleus was associated with mesangial hypercellularity.
Mesangial cells have been thought to play a central role in immune-mediated glomerular injury as well as in the normal physiology of glomeruli; mesangial cell proliferation and immune deposition in the mesangium are prominent histological characteristics of lupus nephritis. It is known that antigen expression of the target organ might be a factor in determining susceptibility to lupus nephritis, so it is possible that the preferential deposition of immune complexes in glomeruli is likely to be due to the local expression of cognate renal antigens.
In the current study, it was demonstrated that anti-DNA antibodies could bind to the HMC cell surface. Anti-DNA antibodies isolated from sera of patients with active lupus nephritis could recognize 74 kDa, 63 kDa and 42 kDa membrane proteins of mesangial cells which differed with α1-laminin (approximately 400 kDa) [27,28], α-actinin (100 kDa) [23], myosin I (110 kDa) [26] and the histones (from 15 kDa to 23 kDa with five major classes in total) in their molecular weight. The heterogeneity of antigen recognition by the anti-DNA antibodies suggested that some novel unknown autoantigens might be present on mesangial cells. Further identification of the target antigens might be crucial to elucidate the role of anti-DNA antibodies played in the pathogenesis of lupus nephritis. Anti-DNA antibodies could still blot the above three protein bands after DNase treatment of the cell membrane proteins, suggesting that the binding of anti-DNA antibodies to the kidney antigen(s) was direct.
Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells could be inhibited completely by preincubating the affinity-purified anti-DNA IgG preparations with dsDNA, suggesting that membrane proteins might share the same epitope(s) recognized by anti-dsDNA antibodies with dsDNA. More work is needed to investigate whether the anti-DNA antibodies binding to HMC could affect mesangial cell functions.
Lupus nephritis is one of the few renal diseases in which immune deposits can be found in all four renal compartments: glomeruli, tubules, interstitium and blood vessels [29–37] and lupus is a typical multi-system autoimmune disease. It is reasonable to speculate that the anti-DNA autoantibodies against HMC might also recognize other cells. In the current study, the antigens similar to HMC were also recognized by the antibodies on the membrane proteins of HUVEC, HK2 and PMC. The results showed that the membrane antigens recognized by the anti-DNA antibodies on the HMC might not be cell specific, but there were still some differences between different cells. In our previous study, we found that the non-DNA-binding antibodies from the patients with LN could also blot some antigens of the membrane proteins of the HMC. Although the target antigens recognized by anti-DNA antibodies and non-DNA-binding antibodies from the same patients have some degree of overlap, they are not completely the same [38]. Identification of the target antigens was important to investigate further the specificity of both anti-DNA antibodies and non-DNA binding antibodies which could blot the membrane proteins of human mesangial cells.
In conclusion, we found that affinity-purified IgG anti-dsDNA antibodies from patients with lupus nephritis could cross-react with membrane proteins of human mesangial cells directly without DNA as a bridge. Amino acid sequencing of the unknown target antigens might contribute to the elucidation of the possible pathogenesis of the anti-DNA antibody in lupus nephritis.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (30370652) and a Specialized Research Fund for the Doctoral Program of Higher Education (20030001024).
References
- 1.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowith JB, Gilkeson GS. Nephritogenic autoantibodies in lupus: current concepts and continuing controversies. Arthritis Rheum. 1996;39:894–903. doi: 10.1002/art.1780390605. [DOI] [PubMed] [Google Scholar]
- 3.Deocharan B, Qing X, Beger E, Putterman C. The antigenic triggers and molecular targets for anti-double stranded DNA antibodies. Lupus. 2002;11:865–71. doi: 10.1191/0961203302lu308rr. [DOI] [PubMed] [Google Scholar]
- 4.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126:607–24. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert PH, Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968;127:507–22. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izui S, Lambert PH, Miescher PA. In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA–anti-DNA complexes in systemic lupus erythematosus. J Exp Med. 1976;144:428–43. doi: 10.1084/jem.144.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rekvig OP, Kalaaji M, Nossent H. Anti-DNA antibody subpopulations and lupus nephritis. Autoimmun Rev. 2004;3:1–6. doi: 10.1016/S1568-9972(03)00081-8. [Review]. [DOI] [PubMed] [Google Scholar]
- 8.Ben Chetrit E, Dunsky EH, Wollner S, Eilat D. In vivo clearance and tissue uptake of an anti-DNA monoclonal antibody and its complexes with DNA. Clin Exp Immunol. 1985;60:159–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Faaber P, Rijke TP, van de Putte LB, Capel PJ, Berden JH. Cross-reactivity of human and murine anti-DNA antibodies with heparan sulfate: the major glycosaminoglycan in glomerular basement membranes. J Clin Invest. 1986;77:1824–30. doi: 10.1172/JCI112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madaio MP, Carlson J, Cataldo J, Ucci A, Migliorini P, Pankewycz O. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J Immunol. 1987;138:2883–9. [PubMed] [Google Scholar]
- 11.Chan TM, Leung JK, Ho SK, Yung S. Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2002;13:1219–29. doi: 10.1097/01.asn.0000014223.71109.13. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [Letter]. [DOI] [PubMed] [Google Scholar]
- 13.Suenaga R, Evans M, Hatfield M, Abdou NI. Study of anti-DNA antibodies prepared by DNA cellulose or Cibacron blue chromatography. J Immunol Meth. 1986;93:131–40. doi: 10.1016/0022-1759(86)90443-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhao MH, Zhang YK, Li XM, Wang HY. Binding capacity and pathophysiological effects of IgA1 from patients with IgA nephropathy on human glomerular mesangial cells. Clin Exp Immunol. 2004;136:168–75. doi: 10.1111/j.1365-2249.2004.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Striker GE, Killen PD, Farin FM. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980;12(Suppl. 1):88–99. [PubMed] [Google Scholar]
- 16.Yu F, Zhao MH, Zhang YK, Zhang Y, Wang HY. Anti-endothelial cell antibodies (AECA) in patients with propylthiouracil (PTU)-induced ANCA positive vasculitis are associated with disease activity. Clin Exp Immunol. 2005;139:569–74. doi: 10.1111/j.1365-2249.2005.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastinel LN, Pleau JM, Dardenne M, et al. High affinity binding sites on plasma membrane obtained from the lymphoblastoid cultured 1301 cell line for highly radioactive serum thymic factor. Biochim Biophys Acta. 1982;684:117–26. doi: 10.1016/0005-2736(82)90055-4. [DOI] [PubMed] [Google Scholar]
- 18.Amoura Z, Piette JC, Bach JF, Koutouzov S. The key role of nucleosomes in lupus. Arthritis Rheum. 1999;42:833–43. doi: 10.1002/1529-0131(199905)42:5<833::AID-ANR1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan MR, Marion TN. Structural similarity of antibody variable regions from immune and autoimmune anti-DNA antibodies. J Immunol. 1993;150:4948–57. [PubMed] [Google Scholar]
- 20.Pankewycz OG, Migliorini P, Madaio MP. Polyreactive autoantibodies are nephritogenicin murine lupus nephritis. J Immunol. 1987;139:3287–94. [PubMed] [Google Scholar]
- 21.Sabbaga J, Pankewycz OG, Lufft V, Schwartz RS, Madaio MP. Cross-reactivity distinguishes serum and nephritogenic anti-DNA antibodies in human lupus from their natural counterparts in normal serum. J Autoimmun. 1990;3:215–35. doi: 10.1016/0896-8411(90)90142-f. [DOI] [PubMed] [Google Scholar]
- 22.Naparstek Y, Ben-Yehuda A, Cohen IR, Bar-Tana R. Crossreactivity of anti-DNA antibodies. Immunol Today. 1990;11:388–9. doi: 10.1016/0167-5699(90)90152-y. [DOI] [PubMed] [Google Scholar]
- 23.Deocharan B, Qing X, Lichauco J, Putterman C. Alpha-actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J Immunol. 2002;168:3072–8. doi: 10.4049/jimmunol.168.6.3072. [DOI] [PubMed] [Google Scholar]
- 24.Mason LJ, Ravirajan CT, Rahman A, Putterman C, Isenberg DA. Is alpha-actinin a target for pathogenic anti-DNA antibodies in lupus nephritis? Arthritis Rheum. 2004;50:866–70. doi: 10.1002/art.20103. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Weinstein E, Tuzova M, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 26.Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest. 1997;100:25–31. doi: 10.1172/JCI119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Champliaud MF, Virtanen I, Tiger CF, Korhonen M, Burgeson R, Gullberg D. Posttranslational modifications and beta/gamma chain associations of human laminin alpha1 and laminin alpha5 chains: purification of laminin-3 from placenta. Exp Cell Res. 2000;259:326–35. doi: 10.1006/excr.2000.4980. [DOI] [PubMed] [Google Scholar]
- 28.Amital H, Heilweil M, Ulmansky R, et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. J Immunol. 2005;175:5516–23. doi: 10.4049/jimmunol.175.8.5516. [DOI] [PubMed] [Google Scholar]
- 29.Appel GB, D’Agati V. Lupus nephritis. In: Striker GE, Klahr S, Jacobson HR, editors. The principles and practices of nephrology. 2. St Louis: Mosby Yearbook; 1995. pp. 159–68. [Google Scholar]
- 30.D’Agati VD. Renal disease in systemic lupus erythematosus, mixed connective tissue disease, Sjogren’s syndrome, and rheumatoid arthritis. In: Jennette CJ, Olson L, Schwartz MM, Silva F, editors. Pathology of the kidney. 5. Philadelphia: Lippincott-Raven; 1998. pp. 541–624. [Google Scholar]
- 31.Cameron JS. Systemic lupus erythematosus. In: Neilson EG, Couser WG, editors. Immunologic renal diseases. Philadelphia: Lippincott-Raven; 1997. pp. 1055–98. [Google Scholar]
- 32.Appel GB, D’Agati V. Renal involvement in systemic lupus erythematosus. In: Massary S, Glassock R, editors. Textbook of kidney disease. Philadelphia: Williams & Wilkins; 1995. pp. 787–97. [Google Scholar]
- 33.Appel GB, Valeri A. The course and treatment of lupus nephritis. Adv Intern Med. 1994;45:525–37. doi: 10.1146/annurev.med.45.1.525. [DOI] [PubMed] [Google Scholar]
- 34.Appel GB, Silva FG, Pirani CL, et al. Renal involvement in systemic lupus erythematosus: a study involving 56 patients emphasizing histologic classification. Medicine. 1978;57:371–410. doi: 10.1097/00005792-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Cameron JS, Turner DR, Ogg CS, et al. Systemic lupus with nephritis: a long-term study. Q J Med. 1979;48:1. [PubMed] [Google Scholar]
- 36.Hill GS, Hinglais N, Tron F, Bach JF. Systemic lupus erythematosus: morphologic correlations with immunologic and clinical data at the time of renal biopsy. Am J Med. 1978;64:61. doi: 10.1016/0002-9343(78)90180-8. [DOI] [PubMed] [Google Scholar]
- 37.Sinniah R, Feng PH. Lupus nephritis: correlation with light, electron microscopic, and immunofluorescent findings and renal function. Clin Nephrol. 1976;6:340. [PubMed] [Google Scholar]
- 38.Hui Du, Min Chen, Ying Zhang, Minghui Zhao. Non-DNA-binding antibodies in patients with lupus nephritis could recognize membrane proteins of glomerular mesangial cells. J Clin Immunol. 2006 doi: 10.1007/s10875-006-9004-8. [DOI] [PubMed] [Google Scholar]


