Abstract
Paediatric studies may provide important insights into the immunopathology of Helicobacter pylori-associated gastritis, as mucosal changes reflect different stages of the immunoinflammatory response. We characterized, by quantitative immunohistochemistry, gastric mucosal lymphocyte phenotype and HLA-DR antigen expression and evaluated correlation with histopathology, in H. pylori-infected (Hp+ve) and uninfected children (Hp–ve). In the infected group, lamina propria CD3+ and IgA plasmocyte cell numbers were significantly higher and a trend for predominance of CD8+ over CD4+ was observed both in epithelium and lamina propria. A correlation of inflammation score with lamina propria CD3+ and CD4+ cell numbers and of CD45RO+ T lymphocytes with density of colonization was observed. The proportion of epithelial cells expressing HLA-DR antigen was significantly higher in the Hp+ve group and furthermore, glandular HLA-DR expression correlated with lamina propria CD3+ cell numbers, emphasizing the potential role of epithelial cells as antigen-presenting cells at this stage of infection.
Keywords: Helicobacter pylori, paediatric, gastritis, HLA-DR antigen expression, lymphocyte subsets
Introduction
Helicobacter pylori (H. pylori) infection is one of the most common gastrointestinal infections worldwide, and the main cause of chronic gastritis, gastric mucosal atrophy, peptic ulcer and some forms of gastric cancer [1–3]. Although there is consensus that acquisition of infection usually occurs very early in life [4,5], major complications will develop only in a minority of infected subjects, predominantly in the adult host [2,6,7], thus suggesting that the gastric mucosal damage is progressive, through childhood until adulthood.
Despite the recognition that infection during childhood is seldom associated with severe disease [8–10], the mechanisms underlying the differences in histopathology and clinical outcome as compared to adults, are still poorly documented [11,12]. After H. pylori acquisition, the nature of the host immune responses, bacterial determinants and potential environmental exposures, all appear to influence outcome. However, so far, a poor correlation between clinical severity and H. pylori virulence factors has been shown in children [13–15]. Differences in H. pylori-induced immune responses, possibly age-dependent, as well as duration and density of infection, could explain the different histopathology and clinical outcome in this age group, compared to adults.
H. pylori induces a strong and complex immune response in the gastric mucosa, both at humoral and cellular level [16,17], which nevertheless fails to clear the infection and may even contribute to immunopathology [2,16]. The cellular response includes innate nonspecific response represented mainly by polymorphonuclear neutrophils and macrophages, as well as adaptative response, dominated by both intraepithelial and stromal T cell. The T cell response is believed to play an important role in H. pylori infection and has been implicated in both protection against disease and the damaging effects of the infection. Chronic H. pylori infection in the adult is characterized by the infiltration of mononuclear cells and the up-regulation of proinflammatory cytokines and the chemokine interleukin-8 (IL-8) [18–21], with a predominant Th1 type response [22–26]. Conflicting results have been reported concerning cell immunophenotype in H. pylori-infected gastric mucosa from adults during H. pylori infection [27–31]. Most consistently, an involvement of T lymphocytes has been documented, with predominance of CD4+ T cell subset in lamina propria and of CD8+ T cell in the epithelium.
There is a paucity of information regarding local immune responses in the paediatric age group. Differently from adults, in which neutrophil infiltrate predominates, H. pylori-associated gastritis in children, is usually mild and superficial, with a predominantly mononuclear infiltrate, scarcity of neutrophil infiltration and a higher degree of lymphoid follicular hyperplasia [8,12,32–35]. In addition to the overall limitation of data including detailed histopathology reports of H. pylori gastritis in children, the characteristics of childhood infection in populations with high prevalence of infection and high cancer risk, remain largely unknown [36]. Although some paediatric studies have evaluated cytokine profile in H. pylori infection-associated gastritis [37–41], mucosal cell infiltrates in children have not been fully characterized [36,40,42]. Thus, the background characterization of the phenotype of gastric mucosal lymphocytes in children with H. pylori infection from different populations is a major research goal [10], as it may improve our understanding of immunopathogenesis. We therefore aimed to characterize by quantitative immunohistochemistry the gastric mucosal B and T cell phenotype and distribution pattern of mucosal lymphocytes and HLA-DR antigen expression in gastric mucosa from H. pylori-infected and uninfected children, and to evaluate their relationship with gastric histopathology.
Patients and methods
Clinical samples
Twenty-eight children and adolescents (15/28 boys, mean age 8·7 years, range 0·7–15·7 years) of European origin (caucasian) referred for endoscopy due to upper gastrointestinal symptoms (mostly recurrent abdominal pain), were included in the study. Informed consent from the parents and approval from local Ethics Commitee, were obtained. Exclusion criteria were treatment with antisecretory, antimicrobial or anti-inflammatory medication, for the three months preceeding the endoscopy. Subjects with peptic ulcer or other chronic diseases were also excluded. None of these children had any other gastrointestinal underlying disease, including evidence for food allergy, giardiasis, inflammatory bowel disease and coeliac disease.
Sampling of mucosal biopsies, specimen collection and evaluation
Endoscopy was performed by the same investigator. Biopsies were systematically taken from gastric antrum (three or four) and gastric body (one or two). Two antral biopsies were used for rapid urease test and culture; the other biopsy specimens were fixed in buffered formalin and paraffin-embedded. Serial 3–4 µm-thick sections were obtained for histological (at least one corpus and one antral biopsy) and immunohistochemical examination (one antral biopsy). Sections were stained with haematoxylin and eosin for conventional histological examination. A modified Giemsa staining was used for H. pylori identification and gastritis was evaluated according to the updated Sydney system [43] by an experienced histopathologist who was unaware of the patient’s H. pylori status or clinical condition. Accordingly, the chronic inflammation score (mononuclear cell infiltration), the activity score (polymorphonuclear cell infiltration) and H. pylori density score, were determined separately and graded from 0 to 3 (for none, mild, moderate and severe, respectively). Whenever more than one biopsy was available from each site (antrum or corpus), a mean score was calculated. The antral biopsy specimens for culture were put into sterile saline solution and processed within 3 h, according to a protocol previously described [44]. Briefly, biopsies were ground with a tissue homogeneizer (Ultra Turax, Labo Moderne, France) and inoculated onto a selective medium (bioMérieux) and a nonselective medium, Mueller-Hinton agar (Oxoid, Basingstoke, UK), supplemented with 10% horse blood. Plates were incubated at 37 °C in a microaerobic atmosphere obtained with a gas-generating system (CampyGen CN 35, Oxoid) for up to 14 days of incubation. Identification of H. pylori was performed according to conventional tests: colony and Gram stain morphology, catalase, oxidase and hydrolysis of urea.
Serology
Sera obtained at time of endoscopy were stored at −20°C until assayed. For determination of anti-H. pylori specific IgG antibodies, a commercial enzyme-linked immunosorbent assay (ELISA, Cobas Core, Roche, Switzerland) was used, with a cut-off of 6 U/ml.
Diagnosis of H. pylori infection
H. pylori status was assessed according to conventional biopsy-based criteria plus serology. Allocation to H. pylori positive (Hp+ve) or H. pylori negative (Hp–ve) group, was based, respectively, on positivity of a urease test, histology, culture and serology or on negativity of all four tests. The H. pylori-infected subjects included in the study were all positive by urease test, culture, serology and histology, and all uninfected patients were negative for H. pylori in all four tests.
Quantitative immunohistochemistry
Immunohistochemistry was performed on 3–4 µm serial sections (slides treated with APES) from well-orientated antral biopsy specimens with adequate tissue representation, by using an indirect immunoperoxidase technique in a threestage procedure at room temperature. Briefly, endogenous peroxidase was blocked by incubation in H2O2/methanol for 10 min. Serial sections were incubated with primary monoclonal antibodies (mouse anti-human antibodies) to the human leucocyte antigens listed in Table 1. Sections were then reincubated with secondary Biotynilated anti-mouse IgG (Biogenex cod: LP000-UL (Biogenex, San Ramon, CA, USA)) at a 1:50 dilution for 30 min, followed by tertiary peroxidase-labelled Streptavidin (Biogenex cod: LP000-UL) at a 1:50 dilution for 30 min. An easily detectable blackish-brown end product was obtained by development with diaminobenzidine hydrochloride (DAB) H2O2. Sections were counterstained with Mayer’s haemalum.
Table 1.
Immunohistochemistry – antibody panel.
| Specificity | Description | mAb dilution | Reference |
|---|---|---|---|
| CD3 | PAN T | 1: 100 | Dako |
| CD20 | PAN B | 1: 600 | Zymed Laboratory |
| CD4 | T cells | 1: 40 | Novocastra Laboratory |
| CD8 | T cells | 1: 200 | Novocastra Laboratory |
| IgA(policlonal) | IgA plasmocytes | 1: 20 | Dako |
| IgG | IgG plasmocytes | 1: 100 | Dako |
| CD45RO | T cells | 1: 1100 | Dako |
| HLA-DR | 1: 50 | Dako |
Intraepithelial (foveolar epithelium) lymphocyte (IEL) density was determined per 100 epithelial cells by manual counting (after counting at least 500 epithelial cells). Lymphocyte and plasmocyte subsets in the lamina propria were enumerated by applying a calibrated eyepiece graticule on a Zeiss microscope (Zeiss, Oberkochen, Germany) under ×400 magnification and results expressed as the number of positive cells/mm2 of lamina propria. Each grid covered 0·0625 mm2, and at least 5 consecutive grids per section were counted. Inadequate or insufficient biopsy specimens were excluded from immunohistochemistry analysis. HLA-DR expression was assessed separately in the surface/foveolar epithelium and in glandular epithelium, semiquantitatively, using a three-point system. It was based on the percentages of epithelium staining positively (0: no staining; 1: less than 10%; 2: 10–50%; 3: > 50%). The positive staining of the histiocytes/lymphocytes in the lamina propria provided the necessary positive control while a biopsy specimen from the normal gastric mucosa acted as negative control. Lymphoid follicles were excluded from analysis, since their random distribution in the tissue specimen might otherwise generate less consistent results. All evaluations were performed by the same observer, who was unaware of the respective H. pylori status and histology findings.
Statistics
Calculation of the mean, standard deviation, median and range was performed for all quantitative variables; t-Student test or the nonparametric Mann–Whitney U-test (if distribution not normal) were used for statistical comparisons between numerical variables; the χ2 test was used to study associations between categorial variables. Spearman’s rank correlation coefficient was calculated to evaluate correlations between numerical variables. Probability values P < 0·05 were considered statistically significant. All statistical tests were two-sided. Statistical analysis was performed using a SPSS version 9·0 program.
Results
Patients
Twenty-eight patients studied included 15 Hp+ve (9 males) and 13 Hp–ve (6 males) subjects, with a median age of 8·7 years (range 0·7–15·7 years). Mean age of Hp+ve cases, 9·4 years (range 3·5–15·7 years) was similar to that of Hp–ve cases, 8·0 years (range 0·7–15·0 years) (P > 0·05). No patient had previously been treated for H. pylori infection.
Histopathological evaluation
In some patients, not all markers could be assessed, due to the small size of biopsies, thus the total number of cases analysed for different cell markers was variable. Most Hp+ve patients showed a slight to moderate chronic gastritis (Table 2). Antrum and corpus inflammation scores were higher in Hp+ve cases (median antrum score 2, range 1–3; median corpus score 2, 0–3) as compared to Hp–ve cases (median antrum score 1, range 0–2; median corpus score 0, range 0–2), with a statistically significant difference in both antrum inflammation (P = 0·015) and corpus inflammation (P = 0·009). Similarly, Hp+ve cases showed significantly higher degrees of activity than Hp–ve cases both in antrum (median Hp+ve antrum scores 1·0, range 0–2; median Hp–ve antrum scores 0, range 0–1, P = 0·015) and in corpus (median Hp+ve corpus score 1·0, range 0–2; median Hp–ve corpus score 0, range 0–1, P = 0·015).
Table 2.
Histopathology (Sydney) scores in Hp+ve and Hp–ve subjects*.
| Hp+ve (n = 15) | Hp–ve (n = 13) | |||||||
|---|---|---|---|---|---|---|---|---|
| Score | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Antrum | ||||||||
| Inflammation | 0·0 | 46·7 | 33·3 | 20·0 | 23·1 | 69·2 | 7·7 | 0·0 |
| Activity | 26·7 | 60·0 | 13·3 | 0·0 | 76·9 | 23·1 | 0·0 | 0·0 |
| H. pylori | 6·0 | 60·0 | 33·0 | 0·0 | ||||
| Corpus | ||||||||
| Inflammation | 18·2 | 27·3 | 45·5 | 9·1 | 69·2 | 23·1 | 7·7 | 0·0 |
| Activity | 36·4 | 45·5 | 18·2 | 0·0 | 92·3 | 7·7 | 0·0 | 0·0 |
| H. pylori | 27·3 | 45·5 | 27·3 | 0·0 | ||||
Results are expressed as a percentage of the total number of cases in each group (%)
In Hp+ve cases, median H. pylori density scores were 1 in the antrum (range 0–2) and 1 in the corpus (range 0–2). No positive correlation was found between H. pylori density scores and chronic inflammation or activity scores, in either antrum or corpus.
Lymphoid follicles were present in 8/15 (53·3%) of Hp+ve and in 2/13 (15·3%) of Hp–ve cases (6/15 Hp+ve and in 1/13 Hp–ve cases in the antrum; in 2/15 Hp+ve and in 1/13 Hp–ve cases in the corpus). In one Hp+ve case, hyperplastic and regenerative features of superficial epithelium were observed in the antrum. As inflammation and activity scores from antrum and corpus were similar within each group (Hp+ve or Hp–ve group), only antrum gastritis scores were considered for subsequent correlation analysis between cell markers expression and histology scores. Age or sex did not influence histology findings.
Lymphocyte and plasmocyte subsets and correlation with age and histology
Concerning immunohistochemical expression of the lamina propria cell infiltrate, although all cell populations were increased in the Hp+ve as compared to the Hp–ve cases, a significant difference between the two groups was only observed in the T cell CD3+ population (median Hp+ve 466·6, range 155·5–766·6; median Hp–ve 233·3, range 122·0–997·0, P = 0·015), and in the IgA plasmocyte population (median Hp+ve 481·4, range 132·8–1422·0; median Hp–ve 349·5, range 0–610·6, P = 0·042) (Figs 1 and 2).
Fig. 1.
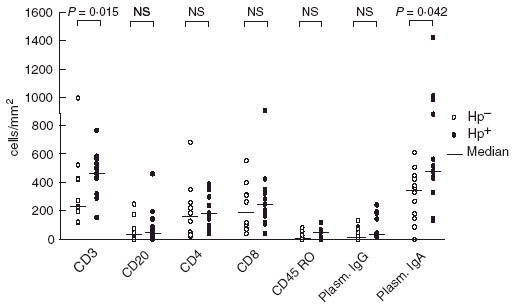
Distribution of lamina propria cell populations in the antrum from H. pylori-infected (•) and uninfected (○) children. – Median. NS, not significant.
Fig. 2.
Microphotographs showing immunohistochemical staining of cell surface markers in antral tissue sections from H. pylori-infected (Hp+ve) children. Representative examples of mucosal staining of (a) CD4+ cells; (b) CD8+ cells; (c) CD3+ cells; (d) CD45RO+ cells; (e) IgA plasmocytes (original magnification ×40); epithelial HLA-DR expression at (f) ×10 and (g,h) at ×20 magnifications.
In both groups, the most abundant cells in lamina propria were also T cells (CD3+) and IgA plasmocytes. Concerning specific T subsets, lamina propria CD8 predominated slightly over CD4 in the Hp+ve group, though without statistical significance. IgG and IgA plasmocyte populations did not correlate with B or T cell subsets.
In the present study, the number of activated T cells (CD45RO+) in the lamina propria was relatively low, and although an increased the number of cells observed in the Hp+ve relative to the Hp–ve group, this did not reach statistical significance (Figs 1 and 2).
IEL were all CD3+ cells, and CD8+ clearly predominated over CD4+ cells in both the Hp+ve and Hp–ve groups (Figs 2 and 3). There was a trend toward a greater increase in intraepithelial CD8+ in the Hp+ve group compared to the Hp–ve group.
Fig. 3.
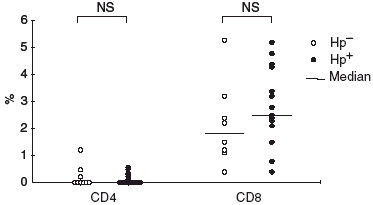
Distribution of intraepithelial lymphocyte subsets in the antrum from H. pylori-infected (•) and uninfected (○) children. – Median. NS, not significant.
None of the lamina propria and epithelial cell populations was associated with age group (considering two age groups, respectively, under and over 10 years old).
Within the lamina propria compartment, a significant positive correlation was observed between the increasing grade of gastritis (inflammation) and CD3+ (P = 0·019, r = 0·458) and CD4+ T cell subsets (P = 0·001, r = 0·611), respectively. Only CD45RO+ correlated significantly with density of bacterial colonization (P = 0·001, r = 0·529). No correlation was found with activity.
HLA-DR expression and correlation with histology and other markers
Results of HLA-DR expression are depicted in Fig. 4. Median HLA-DR expression in epithelium (both foveolar and glandular) was significantly higher (P = 0·02) in the Hp+ve group (median 2, range 1–2, P = 0·02), comparatively to the Hp–ve group (median 1, range 0–2). However, these differences were due to the contribution of differential expression in glandular, but not in foveolar epithelium (Figs 2 and 4).
Fig. 4.
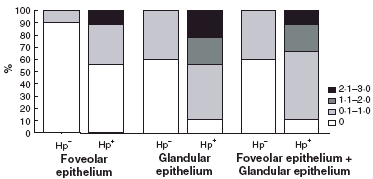
Epithelial HLA-DR expression according to H. pylori status (relative percentages of scores 0–3). □ 0;  0.1–1.0;
0.1–1.0;  1.1–2.0; ▪ 2.1–3.0.
1.1–2.0; ▪ 2.1–3.0.
It is worthy noting that in a few Hp+ve cases no HLA-DR expression was detected, both in foveolar (5/9) and in glandular (1/9) epithelium. In contrast, most Hp–ve cases showed no HLA-DR expression in foveolar epithelium (9/10), or glandular epithelium (6/10). All these cases without HLA expression had either histologically normal mucosa or a slight degree of gastritis. Interestingly, HLA expression was absent in the one available case of the three Hp–ve cases with normal mucosa.
A positive correlation was found between the epithelial expression of glandular HLA-DR and lamina propria density of T cells CD3+ (P = 0·007, r = 0·609) (Fig. 3), but with no other cell populations. Also, there was no correlation between foveolar HLA-DR expression and subsets of the intraepithelial CD4+ and CD8+ lymphocytes (P = 0·283, r = 0·268 and P = 0·344, r = 0·237).
Discussion
Marked differences from adults, regarding clinical course and gastric mucosal histopathology, are recognized in children with H. pylori infection. Studies of paediatric aged subjects may provide important insights into the immunopathology of H. pylori-associated gastric disease, as gastric mucosal changes reflect a different stage of the immunoinflammatory response and furthermore, children are not submitted to adult gastric mucosal noxas, such as alcohol, tobacco and anti-inflammatory medication. Thus, we would anticipate a different immunopathology from the adult host, where gastric mucosal immunoinflammatory events, lasting for decades, may represent mostly secondary phenomena.
At present, very little information exists on gastric mucosa lymphocyte phenotype of H. pylori-infected and uninfected children [34,36,42]. Adult studies reporting mucosal cell population in H. pylori-associated gastritis have shown conflicting results [27–31], partially attributed to methodological differences; however, most consistently a predominant pattern of CD4+ lymphocytes in the lamina propria and of CD8+ lymphocytes in the epithelium has been described.
In the present study strict criteria were used for allocation of H. pylori status. This is particularly relevant in children, in whom negative culture and histology do not exclude a past infection. The obvious ethical constraints to include a healthy control group with a normal histology, justified the presence of Hp–ve cases with some degree of gastritis, possibly secondary to other aetiologies, including viral infections, common at paediatric age. A paediatric study using immunohistochemistry [42], has included a ‘healthy control’ group, selected on the basis of clinical criteria. However, this has also some limitations, as absence of symptoms does not guarantee the exclusion of common pathology at this age group, as a normal endoscopy does not exclude the presence of histopathological changes, since seven of 13 ‘healthy controls’ in this study had gastritis. On the other hand, serology has not been included and thus exclusion of past H. pylori infection in ‘H. pylori negative’ cases was not guaranted.
In addition to differences in patient selection and duration of infection, histopathological heterogeneity between paediatric studies from different geographical areas may also reflect host/strain characteristics. For example, the study by Bedoya et al. [36], of a population at high cancer risk, has shown that older children display higher degrees of neutrophil and lymphocyte infiltrates and of H. pylori colonization density than younger children. In the study of Krauss-Etschman et al. [42], an unexpectedly high prevalence of mucosal atrophy, usually considered a rare event in children, was reported, including those in the control group.
Our results showing a predominance of lamina propria T cells (CD3+) and plasmocytes and a relative paucity of B cells (CD20+) in gastric mucosa, were expected, and agree with morphometric data from Ashorn et al. [34]. A local H. pylori-specific humoral response has been shown both in children and adults [45,46], the latter study reporting significantly higher frequencies of IgA and IgM secreting-cells in gastric mucosa from infected subjects. Interestingly, an acute T-cell and antibody immune response to H. pylori infection has been recently demonstrated in an experimental human study, showing an increase of mucosal CD3, and both CD4 and CD8 T cell numbers, as well as the presence of systemic H. pylori specific IgM responses [47].
In our study, the lack of influence of H. pylori positive status on lymphocyte subsets (both in lamina propria and epithelium) might be at least partially related to the effect of some potentially ‘biasing’ factors. In fact, Hp–ve cases also had some degree of gastritis, potentially due to undetermined aetiologies other than H. pylori. Furthermore, host (population selection, duration and intensity of gastritis)- or strain (density of colonization, strains of lower virulence)-related factors could also be involved. Moreover, admitting an age association of immune response, the relatively young age of our cohort should also be taken into account (age-related immunological immaturity). Although these drawbacks are difficult to overcome, if such findings are further confirmed, it would be plausible to admit that the low grade H. pylori-associated gastritis observed in children may be related to a more attenuated cellular immune response as compared to the adult. Another possibility is that at this stage of the infection, differences in local immune responses are subtle and predominantly qualitative, suggesting the potential interest of functional studies.
In the present study, the relatively low representation of CD45RO+ cells may reflect the earlier stage of infection. However, in the absence of information concerning the relative frequence of CD45RO+ T cells in gastrointestinal mucosa from healthy children, it should be emphasized that compared to adults, they display a much lower proportion of CD45RO+ T cell subset versus total T cells in peripheral blood [48], and thus, an age dependent variation on lymphocyte subsets should also be taken into account. Further work is required to assess the role of both CD4+ and CD8+ cells and their interaction with humoral immune response, in the regulation of the response to H. pylori at this stage of infection.
Concerning HLA-DR (class II) expression, it is accepted that class II antigens are not expressed in the epithelial cells of normal gastric mucosa, and different degrees of enhanced expression have been observed in the inflamed mucosa of chronic gastritis of different aetiologies [49–55], correlating with the degree of gastritis and the number of intraepithelial and lamina propria T cells. There is increasing evidence that epithelial cells can function as antigen-presenting cells for CD4+ cells [56] and H. pylori has also been shown to up-regulate expression of MHC class II on gastric epithelial cells [57]. It has been proposed that this up-regulation is mediated by activation of lamina propria T cells and macrophages, with subsequent release of interferon-γ and other cytokines. Such a connection between lamina propria and epithelial cells is further suggested in our study by the finding of a positive correlation between HLA epithelial expression and number of lamina propria lymphocyte T cell. These findings are consistent with our recent data on cytokine expression in gastric mucosa of H. pylori-infected children [41], reporting a considerable contribution of gastric epithelium to the antral cytokine response.
In conclusion, we have described the lymphocyte immunophenotype of H. pylori-associated gastritis in a paediatric population, showing an increase in lamina propria T cells (both CD4+ and CD8+ subsets) and of IgA plasmocyte cells, accompanied by a selective accumulation of CD8+ T cells in the epithelium. Moreover, an increased HLA-DR epithelial expression was shown in Hp+ve cases, emphasizing the potential role of epithelial cells as antigen-presenting cells at this stage of infection. These findings are consistent with a parallel involvement of the lamina propria and of the epithelium in the local immune response at paediatric stages of the natural history of H. pylori infection.
Acknowledgments
This study was financially supported by a grant from Comissão de Fomento da Investigação em Cuidados de Saúde, Ministério da Saúde, Portugal.
References
- 1.Farthing MJ. Helicobacter pylori infection: an overview. Br Med Bull. 1998;54:6. doi: 10.1093/oxfordjournals.bmb.a011661. [DOI] [PubMed] [Google Scholar]
- 2.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–40. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Go MF. Review article. natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(Suppl. 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JE, Dale A, Harding M, et al. Helicobacter pylori colonization in early life. Pediatr Res. 1999;45:218–23. doi: 10.1203/00006450-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Goodman KJ, Correia P. Transmission of Helicobacter pylori among siblings. The Lancet. 2000;9201:361–4. doi: 10.1016/S0140-6736(99)05273-3. [DOI] [PubMed] [Google Scholar]
- 6.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9:59–69. [PubMed] [Google Scholar]
- 7.Kuipers EJ. Review article: relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12:25–36. doi: 10.1111/j.1365-2036.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- 8.Ashorn M. What are the specific features of Helicobacter pylori gastritis in children. Ann Med. 1995;27:617–20. doi: 10.3109/07853899509002480. [DOI] [PubMed] [Google Scholar]
- 9.Drumm B. Helicobacter pylori in the pediatric patient. Gastrenterol Clin North Am. 1993;22:169–82. [PubMed] [Google Scholar]
- 10.Drumm B, Day AS, Gold B, et al. Helicobacter pylori and peptic ulcer: Working Group Report of the Second World Congress of Pediatric Gastrenterology, Hepatology and Nutrition. J Pediatr Gastrenterol Nutr. 2004;39:S626–31. doi: 10.1097/00005176-200406002-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ernst PB, Crowe SE, Reyes VE. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastrenterology. 1997;113:S35–50. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 12.Ernst PB, Gold BD. Helicobacter pylori in childhood: new insights into the immunopathogenesis of gastric disease and implications for managing infection in children. J Pediatr Gastrenterol Nutr. 1999;28:462–73. doi: 10.1097/00005176-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon T, Domingo D, Martinez MJ, et al. cagA gene and vacA alleles in Spanish Helicobacter pylori clinical isolates from patients at different ages. FEMS Immunol Med Microbiol. 1999;24:215–9. doi: 10.1111/j.1574-695X.1999.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 14.Oleastro M, Gerhard M, Lopes AI, et al. Helicobacter pylori virulence genotypes in Portuguese children and adults with gastroduodenal pathology. Eur J Clin Microbiol Infect Dis. 2003;22:85–91. doi: 10.1007/s10096-002-0865-3. [DOI] [PubMed] [Google Scholar]
- 15.de Gusmão VR, Nogueira Mendes E, De Magalhães Queiroz DM, et al. VacA genotypes in Helicobacter pylori strains isolated from children with and without duodenal ulcer in Brazil. J Clin Microbiol. 2000;38:2853–7. doi: 10.1128/jcm.38.8.2853-2857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Aliment Pharmacol Ther. 1996;10(Suppl. 1):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 17.Rathbone BJ, Wyatt JI, Worsley BW, et al. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut. 1986;27:642–7. doi: 10.1136/gut.27.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree JE, Wyatt JI, Trejdosiewicz LK, et al. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–6. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noah LA, Bosma NB, Jansen J, et al. Mucosal tumor necrosis factor-alpha, interleukin-1 beta and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–9. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 20.Ando T, Kusugami K, Ohsuga M, et al. Interleukin-8 activity correlates with histological severity in Helicobacter pylori- associated antral gastritis. Am J Gastroenterol. 1996;91:1150–6. [PubMed] [Google Scholar]
- 21.Crowe SE, Alvarez L, Dytoc M. Expression of interleukin-8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 22.Kartunnen R, Kartunnen T, Ekre HP, et al. Interferon gamma and interleukin-4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindholm C, Quiding-Jarbrink M, Lonroth H, et al. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–71. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamford K, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 25.D’Elios MM, Manghetti M, Almerigogna F, et al. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–5. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 26.Holck S, Norgaard A, Bennedsen M, et al. Gastric mucosal cytokine responses in Helicobacter pylori-infected patients with gastritis and peptic ulcers. Association with inflammatory parameters and bacteria load. FEMS Immunol Med Microbiol. 2003;36:175–80. doi: 10.1016/S0928-8244(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 27.Fan XJ, Chua A, Shahi CN, et al. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–84. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifarth C, Deusch K, Reich K, et al. Local cellular immune response in Helicobacter pylori associated type B gastritis – selective increase of CD4þ but not gamma delta T-cells in the immune response to H. pylori antigens. Z Gastroenterol. 1996;34:215–24. [PubMed] [Google Scholar]
- 29.Hatz RA, Meimarakis G, Bayerdorffer E, et al. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1996;31:222–8. doi: 10.3109/00365529609004870. [DOI] [PubMed] [Google Scholar]
- 30.Agnihotri N, Bhasin DK, Vohra H, et al. Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand J Gastroenterol. 1998;33:704–9. doi: 10.1080/00365529850171639. [DOI] [PubMed] [Google Scholar]
- 31.Sommer F, Faller G, Konturek P, et al. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–6. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadranel S, Goossens HDE, Boeck M, et al. Campylobacter pyloridis in children. Lancet. 1986;1:735–6. doi: 10.1016/s0140-6736(86)91120-7. [DOI] [PubMed] [Google Scholar]
- 33.Hassal E, Dimmick JE. Unique features of Helicobacter pylori disease in children. Dig Dis Sci. 1991;36:417–23. doi: 10.1007/BF01298868. [DOI] [PubMed] [Google Scholar]
- 34.Ashorn M, Ruuska T, Karikoski R, et al. Gastric mucosal cell densities in Helicobacter pylori-positive and negative dyspeptic children and healthy controls. J Pediatr Gastrenterol Nutr. 1994;18:146–51. doi: 10.1097/00005176-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Whitney AE, Guarner J, Hutwagner L, et al. Helicobacter pylori gastritis in children and adults: a comparative histopathologic study. Ann Dign Pathol. 2000;4:279–85. doi: 10.1053/adpa.2000.17871. [DOI] [PubMed] [Google Scholar]
- 36.Bedoya A, Garay J, Sanzon F, et al. Histopathology of gastritis in Helicobacter pylori-infected children from populations at high and low gastric cancer risk. Hum Pathol. 2003;34:206–13. doi: 10.1053/hupa.2003.43. [DOI] [PubMed] [Google Scholar]
- 37.Luzza F, Parrello T, Sebkova L, et al. Expression of proinflammatory and Th1 but not Th2 cytokines is enhanced in gastric mucosa of Helicobacter pylori infected children. Dig Liver Dis. 2001;33:14–20. doi: 10.1016/s1590-8658(01)80130-4. [DOI] [PubMed] [Google Scholar]
- 38.Guiraldes E, Duarte I, Pena A, et al. 2001. Proinflammatory cytokine expression in gastric tissue from children with Helicobacter pylori-associated gastritis. J Pediatr Gastrenterol Nutr. 2001;33:127–32. doi: 10.1097/00005176-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Camorlinga-Ponce M, Aviles-Jimenez F, Cabrera L, et al. Intensity of inflammation,density of colonization and interleukin-8 response in the gastric mucosa of children infected with Helicobacter pylori. Helicobacter. 2003;8:554–60. doi: 10.1046/j.1523-5378.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 40.Bomtems P, Robert F, Van Gossum A, et al. Helicobacter pylori modulation of gastric and duodenal mucosa T cell cytokine secretions in children compared with adults. Helicobacter. 2003;8:216–26. doi: 10.1046/j.1523-5378.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 41.Lopes AI, Quiding-Jarbrink M, Palha A, et al. A. Cytokine expression in pediatric H.pylori infection. CDLI. 2005;12:994–1002. doi: 10.1128/CDLI.12.8.994-1002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krauss-Etschmann S, Gruber R, Plikat K, et al. Increase of antigen-presenting cells in the gastric mucosa of Helicobacter pylori-infected children. Helicobacter. 2005;10:214–22. doi: 10.1111/j.1523-5378.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 43.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Mégraud F, Lehn N, Lind T, et al. Antimicrobial susceptibility testing of H. pylori in large multicenter trial: the MACH2 study. Antimicrob Agents Chemother. 1999;43:2747–52. doi: 10.1128/aac.43.11.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanchard TG, Nedrud JG, Czinn SH. Local and systemic antibody responses in humans with Helicobacter pylori infection. Can J Gastroenterol. 1999;13:591–4. doi: 10.1155/1999/142457. [DOI] [PubMed] [Google Scholar]
- 46.Mattsson A, Quiding-Jarbrink M, Lonroth H, et al. Antibody-secreting cells in the stomachs of symptomatic ans asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705–12. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurgalieva ZZ, Conner ME, Opekun AR, et al. B-cell and T-cell immune responses to experimental Helicobacter infection in humans. Infect Immun. 2005;73:2999–3006. doi: 10.1128/IAI.73.5.2999-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirruccello SJ, Collins M, Wilson JE, et al. Age-related changes in naive and memory CD4+ T cells in healthy human children. Clin Immunol Immunopathol. 1989;52:341–5. doi: 10.1016/0090-1229(89)90185-2. [DOI] [PubMed] [Google Scholar]
- 49.Papadimitriou CS, Ioachim-Velogianni EE, Tsianos EB, et al. Epithelial HLA-DR expression and lymphocyte subsets in gastric mucosa in type B chronic gastritis. Virchows Arch. 1988;A413:197–204. doi: 10.1007/BF00718611. [DOI] [PubMed] [Google Scholar]
- 50.Engstrand L, Scheynius A, Pahlson C, et al. Association of Campylobacter pylori with induced expression of class II transplantation antigens on gastric epithelial cells. Infect Immun. 1989;57:827–3250. doi: 10.1128/iai.57.3.827-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valnes K, Huitfeldt HS, Brandtzaeg P. Relation between T cell number and epithelial HLA class II expression quantified by image analysis in normal and inflamed human gastric mucosa. Gut. 1990;31:647–52. doi: 10.1136/gut.31.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheynius A, Engstrand L. Gastric epithelial cells in Helicobacter pylori-associated gastritis express HLA-Dr but not ICAM-1. Scand J Immunol. 1991;33:237–41. doi: 10.1111/j.1365-3083.1991.tb03755.x. [DOI] [PubMed] [Google Scholar]
- 53.Lowes JR, Radwan JD, Priddle JD, et al. Characterization and quantification of mucosal cytokine that induces epithelial histocompatibility locus antigen-DR expression in inflammatory bowel disease. Gut. 1992;33:315–949. doi: 10.1136/gut.33.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wee A, The M, Kang JY. Association of Helicobacter pylori with HLA-DR antigen expression in gastritis. J Clin Pathol. 1992;45:30–3. doi: 10.1136/jcp.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archimandritis A, Sougioultzis S, Foukas PG, et al. Expression of HLA-DR, costimulatory molecules B7–1, B7–2, intercellular adhesion molecule−1 (ICAM−1) and Fas ligand (FasL) on gastric epithelial cells in Helicobacter pylori gastritis; influence of H. pylori eradication. Clin Exp Immunol. 2000;119:464–71. doi: 10.1046/j.1365-2249.2000.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoang P, Crotty B, Dalton HR, et al. Epithelial cells bearing class II molecules stimulate allogeneic human colonic intraepithelial lymphocytes. Gut. 1992;33:1089–93. doi: 10.1136/gut.33.8.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan X, Crowe SE, Behar S, et al. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–69. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]


