Abstract
Cytokines produced by Th2 cells are responsible for the pathogenesis of asthma. Th1-biased immune responses caused by attenuated salmonella have the potential to relieve asthmatic symptoms. We evaluated whether oral administration of attenuated salmonella could modulate allergic responses in a chicken ovalbumin (OVA)-induced asthmatic murine model. Mice were fed with attenuated salmonella SL7207 one dose before and three doses during the induction of an allergic response. Lung histology, percentages of eosinophil in bronchoalveolar lavage fluid, serum levels of OVA-specific antibodies and cytokine production by OVA-activated splenocytes were evaluated in mice with or without the administration of SL7207. A significant reduction in pulmonary eosinophilic infiltration was observed in mice receiving attenuated salmonella. Lower levels of OVA-specific IgG1 but higher titres of OVA-IgG2a in serum were also detected in this group. Splenocytes from salmonella-fed mice produced lower levels of Th2 cytokines upon OVA stimulation. The administration of attenuated salmonella significantly suppressed immunopathological symptoms in OVA-sensitized mice. Inhibition of Th2 responses might explain the potential mechanisms. This study provides some evidence for the feasibility of attenuated salmonella as an effective vaccine for allergic diseases.
Keywords: asthma, salmonella, vaccine, airway inflammation, Th2 cells, cytokines
Introduction
Bronchial asthma has emerged as a worldwide public health problem with increases in incidence, morbidity and mortality during the last two decades [1]. This disease is characterized as a chronic inflammatory disorder of the airways associated with enhanced Th2 responses to inhaled allergens, leading to bronchial eosinophil infiltration, airway-hypersensitivity and elevated serum IgE levels [2–4]. Th2-type cytokines such as interleukin (IL)-4 and IL-13 enhance IgE production [5,6]. IL-5 promotes the differentiation, maturation, activation and prolonged survival of eosinophils [7,8]. Novel effective strategies that have the potential to modulate Th2-type responses might therefore have beneficial effects towards allergic asthma.
Increasing evidence suggests that exposure to bacteria or its components, such as lipopolysaccharides (LPS), might influence the severity of asthma [9–12]. Furthermore, an inverse relationship between allergic disorder and microbial infection has been reported by an epidemiological survey [13]. The ‘hygiene hypothesis’ also proposes that reduced exposure to infectious organisms in childhood, due to cleaner environments, may have increased the prevalence of asthma and other atopic disorders [14]. The protective effect from infectious organisms may be mediated, at least in part, by microbe-induced Th1 cytokines, such as interferon (IFN)-γ, and consequently down-regulated allergic Th2 responses. Moreover, some experiments in mice support the idea that Th1 immune responses inhibit Th2-mediated diseases in a non-antigen-specific manner [15,16]. It may therefore be beneficial to use certain bacterial species as non-specific protective vaccines against allergic diseases such as asthma.
Attenuated Salmonella spp., with its well-understood genetics, known physiology and host Th1-bias immune responses, serves as a potential candidate for an effective vaccine against asthma [17]. Safe attenuated salmonella strains are available and are often applied to farm animals and humans for vaccination [18–20]. Several strains have been tested recently as vehicles of DNA delivery in vivo [21–23]. From those studies, T helper cell responses induced by genetic immunization seemed strongly biased toward the Th1-activity, as indicated by IFN-γ production from antigen-stimulated T cells and high serum antigen-specific IgG2a levels [24,25]. Thus, the goal of this study was to evaluate whether oral administration of the attenuated salmonella, SL7207, could modulate allergic responses in a chicken ovalbumin (OVA)-induced murine asthma model.
Materials and methods
Animals and bacteria
Female BALB/cByJ mice aged 6–8 weeks were purchased from the National Laboratory Animal Centre (Taipei, Taiwan, ROC). They were maintained and handled according to the guidelines of Animal Care Committee of Chang Gung University and NIH Guides for the Care and Use of Laboratory Animals. The auxotrophic Salmonella typhimurium aroA strain SL7207 [21] (S. typhimurium 2337–65 derivative hisG46, DEL407 (aroA::Tn10{Tc-s})) was kindly provided by Dr B. A. D. Stocker, Stanford University, CA, USA. Bacteria were grown in Luria-Bertani medium at 37 °C with vigorous shaking until they reached mid-log phase (regularly 0·5–1 × 108 colony forming units (cfu)/ml).
Sensitization, treatment and challenge
Asthmatic model and salmonella-treated mice were sensitized by four intraperitoneal (i.p) injections of 50 µg OVA (grade V; Sigma, St. Louis, MO, USA) which had been emulsified in 0·8 mg aluminium hydroxide in 200 µl saline (OVA/alum) on days 7, 8, 9 and 20 (Fig. 1). Normal saline-control mice were injected with 0·8 mg aluminium hydroxide in 200 µl saline alone (NS/alum). Mice were challenged on days 20, 23, 27, 30 and 34 by inhalation of either normal saline (NS group) or OVA aerosols (OVA and OVA + SL7207 groups) in an exposure chamber for 20 min. Aerosols were generated by nebulizing 2% OVA solution in saline, or saline alone (NS-control group), with a Pulmo-Aide nebulizer (DeVilbiss, Sunrise Medical Corp., CA, USA). S. typhimurium aroA strain SL7207-treated mice were fed with 108 cfu bacteria in 100 µl PBS with 5% sodium bicarbonate on days 0, 7, 20 and 27 immediately before the i.p. injection of OVA/alum or receiving an OVA aerosol (Fig. 1). None of the mice exhibited any overt signs of illness during the experiment.
Fig. 1.
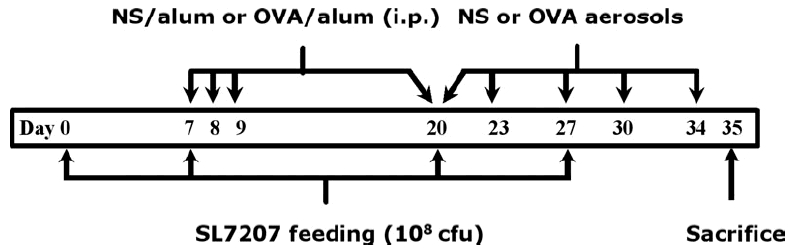
Schematic diagram depicting protocols for SL7207-feeding and OVA-induced allergy protocol. Mice were fed with SL7207, then sensitized and challenged with OVA as described in Materials and methods. NS, normal saline; OVA, ovalbumin.
Bronchoalveolar lavage fluid and lung histology
Twenty-four hours after the final OVA challenge, lungs were lavaged three times with 1 ml of saline. To evaluate different cell types and numbers, cytospin preparations of bronchoalveolar lavage fluid (BALF) cells were stained with Liu stain and differential cell counts were performed on 500 cells, as based on the staining characteristics and morphology. The supernatant from the first lavage was collected and frozen at −70 °C until further analyses. Lungs were removed after BALF collection and embedded in OCT (Tissue-Tek, Sakura Finetek, Torrance, CA, USA) [26]. Multiple 8-µm sections were stained with haematoxylin and eosin (HE) for light microscopy.
Determination of OVA-specific IgG1, IgG2a and IgE
Serum samples were collected 24 h after the last challenge. OVA-specific IgG1, IgG2a and IgE antibody titres were determined by ELISA. Briefly, each microtitre plate well was coated with 10 µg/ml OVA, blocked with 3% bovine serum albumin (BSA, Sigma) in PBS, and incubated with 100 µl of diluted samples. Biotinylated antimouse IgG1, IgG2a or IgE (BD Pharmingen, San Diego, CA, USA) was then added to each well and followed by avidin-horseradish peroxidase (BD Pharmingen). The reaction was developed by the addition of 100 µl/well of substrate containing 0·2 mg/ml of o-orthophenylenediamine dihydrochloride (OPD) substrate (Sigma) and stopped with 3 M H2SO4. Arbitrary units of IgG1 and IgG2a were determined by the conversion of diluted serum with the comparison of a constructed standard curve with the use of pooled serum obtained from multiply OVA-sensitized mice. OD490nm readings of IgE levels were obtained with the 5-fold diluted serum of all samples.
Cytokine production from OVA-stimulated splenocytes
Splenocytes were stimulated in vitro with 100 µg/ml OVA at a density of 5 × 106 cells/ml in RPMI 1640 medium (Gibco-BRL) with 10% fetal calf serum for 6 days. The concentrations of cytokines (IL-4, IL-5, IL-10, IL-13 and IFN-γ) in culture supernatants were evaluated with ELISA kits specific to each cytokine (IL-5 from R & D Systems, Minneapolis, MN, USA; others from BD Pharmingen). Cytokine concentrations were calculated based on standards run in parallel with recombinant cytokines. The limits of detection were: 15·6 pg/ml for IL-4 and IL-5; 31·3 pg/ml for IL-10 and IFN-γ; 39 pg/ml for IL-13.
Statistical analysis
All data are expressed as mean ± SD. All analyses were performed using Student’s t-test. A probability value of P < 0·05 was considered to be significant.
Results
Attenuated Salmonella reduces airway inflammation
To investigate whether the attenuated salmonella were able to modulate OVA-induced airway inflammation in asthmatic animals, we sensitized mice i.p. with OVA in alum, challenged them with repeated doses of OVA aerosol, and fed them with 108 cfu bacteria of log-phase SL7207 one dose before and three doses during the induction of allergic responses. OVA-induced eosinophilic pulmonary infiltration was greatly reduced in SL7207-fed mice after the final OVA challenge (Fig. 2). The percentage of eosinophils in BALF was reduced in the lungs of bacteria-treated mice (29·83 ± 17·86%) compared with that from controls (47·17 ± 18·10%, P = 0·0002). The decrease of eosinophilia was also confirmed histologically with HE staining of lung tissue (Fig. 3). Lung tissue from sensitized control mice showed widespread inflammatory infiltrates mainly in the peribronchiolar and perivascular areas. Lung morphology in SL7207-fed mice, however, was similar to that observed in normal saline control mice.
Fig. 2.
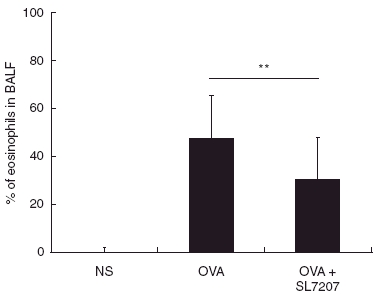
Reduced eosinophil infiltration in the lungs of mice fed with SL7207. Following the final OVA challenge, percentages of eosinophils in BALF cells were counted with the Liu staining. Data are represented as mean percentage ± SD, NS, normal saline control group (n = 22); OVA, OVA-sensitized and challenged group (n = 33) and OVA + SL7207, SL7207-treated group (n = 33). **P < 0·01 when compared with the OVA-sensitized control group.
Fig. 3.
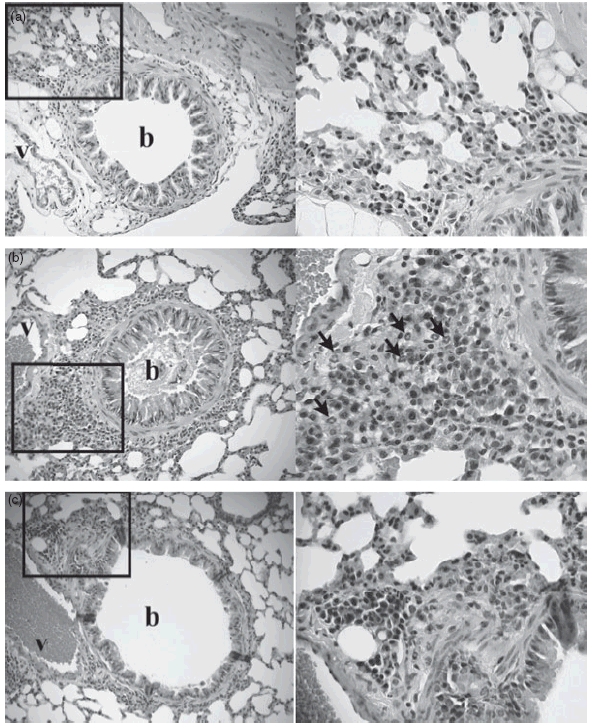
SL7207 inhibits OVA-induced airway inflammation. Lungs were obtained from (a) normal saline control, (b) OVA-sensitized and challenged and (c) SL7207-fed mice groups after BALF collection, and embedded in OCT. Representative HE-stained sections at 200× are shown for each experimental group. Sections at 400× view are also shown for the indicated areas. Eosinophils are indicated by arrows in the perivascular (v) and peribronchial (b) spaces.
Effects of SL7207 administration on cytokine production from splenocytes
To evaluate whether the administration of attenuated salmonella modulated the T-helper cell subpopulations, cytokine profiles were first analysed from the supernatants of spleen cells that were treated with heat-killed SL7207. As previously described [27], higher amount of IFN-γ, but not Th2-cytokines, such as IL-4, IL-5 and IL-13 were produced by splenocytes from SL7207-fed mice (data not shown). We next explored whether attenuated salmonella modulated OVA-specific T-helper cell responses and reduced pulmonary inflammation. Mice were sacrificed on day 35, and spleen cells were removed and stimulated with OVA in vitro for 6 days. Figure 4 shows that bacterial administration significantly down-regulated OVA-specific Th2-cytokine production (control versus SL7207-treated group: IL-4: 537·1 ± 281·8 pg/ml versus 332·7 ± 236·7 pg/ml, P = 0·0126; IL-5: 14·2 ± 4·3 ng/ml versus 8·3 ± 6·3 ng/ml, P = 0·0007; IL-13: 24·0 ± 8·8 ng/ml versus 15·6 ± 12·0 ng/ml, P = 0·0119; IL-10: 4·1 ± 1·6 ng/ml versus 2·3 ± 1·5 ng/ml, P = 0·0003). Conversely, no significant changes in IFN-γ production were detected in any experimental groups.
Fig. 4.
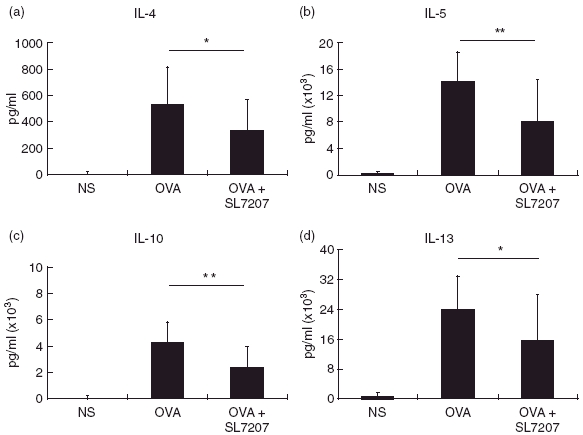
Effects of SL7207 administration on cytokine production. Splenocytes were cultured in vitro with 100 µg/ml OVA in complete RPMI 1640 medium for 6 days. The concentrations of (a) IL-4, (b) IL-5, (c) IL-10 and (d) IL-13 in supernatants were determined with ELISA. Data are presented as mean ± SD, n = 22 for each group. *P < 0·05 and **P < 0·01 when compared to the OVA-sensitized control group.
The administration of SL7207 modulates serum levels of OVA-specific IgG1 and IgG2
OVA sensitization and challenge evoked higher OVA-specific Th2 responses and serum IgG1 and IgE levels. We therefore investigated whether treatment with attenuated salmonella affected serum anti-OVA immunologlobulin levels. When compared with controls, treatment with SL7207 during immunization with OVA notably reduced the level of OVA-specific IgG1 (208·24 ± 70·74 KU/ml in treated mice versus 299·39 ± 85·68 KU/ml in control mice, P = 0·0004), but significantly increased IgG2a production (32·68 ± 34·53 KU/ml in treated mice versus 16·07 ± 14·78 KU/ml in control mice, P = 0·0442) (Fig. 5). However, no significant difference in OVA-specific IgE production was detected following treatment with SL7207 (OD490nm: 0·053 ± 0·003 in NS control mice; 0·612 ± 0·258 in OVA-sensitized control mice; and 0·499 ± 0·257 in SL7207-treated mice; P = 0·185 between the latter two groups).
Fig. 5.
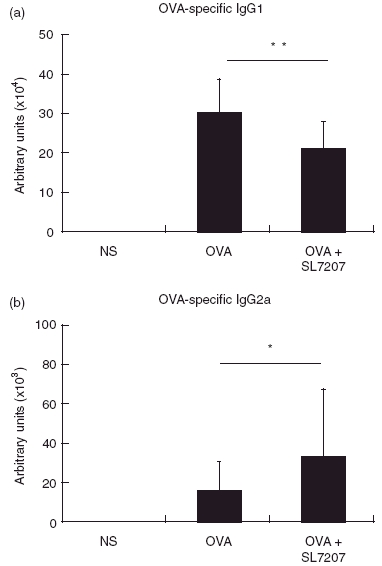
Serum OVA-specific IgG1 (a) and IgG2a (b) levels. OVA-specific antibodies were determined from three groups by ELISA. n.d. not detectable. Data are represented as mean ± SD, n = 22 for each group. *P < 0·05 and **P < 0·01 when compared to the OVA-sensitized and challenged control group.
Discussion
The results demonstrate that oral feeding with attenuated Salmonella typhimurium strain SL7207 reduced important manifestations of atopic asthma in a mouse model. Four doses of salmonella bacteria significantly suppressed eosinophilia in OVA-sensitized BALB/c mice. Production of Th2-type cytokines from OVA-activated splenocytes was also greatly reduced.
Interactions between the host immune system and bacterial pathogens involve specific and non-specific humoral and cellular responses. Th1 responses are induced during most bacterial and viral infections, and have the potential to down-regulate allergic Th2 responses [28]. It was therefore postulated that an approved bacterial vaccine might have the potential to improve allergic asthma. Safe attenuated strains are already available as vaccines against pathogenic salmonella infection and are also commonly used for vaccination in farm animals [18,19]. Several attenuated Salmonella enterica serovar Typhi strains have been developed, and their safety and efficacy as candidate typhoid fever vaccines have been evaluated in clinical trials [20]. In addition, Darji et al. [21] studied the potential use of attenuated S. typhimurium strain SL7207 carrying protective antigen against L. momocytogenes infection. Strong Th1 responses were demonstrated in mice treated with SL7207. Furthermore, other reports of the application of Salmonella demonstrated similar conclusions [29,30]. Although it is important to elicit antigen-specific Th1 responses to allergens in asthmatic individuals, many patients suffer from unknown allergen or more than one allergen [31,32]. Enhanced non-specific Th1 responses may therefore offer therapeutic benefits.
Other bacteria, including Mycobacterium, have been studied for allergy suppressive activity. An inverse association between tuberculin responses and atopic disorder was observed by Shirakawa et al. [17]. In addition, mycobacterial infection suppressed the development of asthma in mice [9,33]. Although strong Th1-biased immune responses do develop with Mycobacterium or BCG administration, intraperitoneal and subcutaneous injections are not particularly favourable clinically. However, oral administration of attenuated Salmonella might enhance the expression of cytokines or other immuno-modulatory genes that might function as adjuvant and enhance the Th1 activity in allergic individuals.
The biased Th1 response induced with attenuated salmonella might provide a potential mechanism for the reduction of Th2 responses in SL7207-fed mice. Following oral administration, Salmonella egress from the gut lumen via the M cells of Peyer’s Patches [34,35], migrate into lymph nodes and spleen, and then induce systemic immune responses with increased Th1 activity. Toll-like receptors (TLR) have recently been described as molecules that link innate and adaptive immunity [36]. TLR-4 appears to be the signalling receptor for Salmonella lipopolysaccharides (LPS) [37] and mediates increases in the expression of pro-inflammatory cytokines, such as TNF-α and IL-12 [38,39]. Therefore, the increased production of IFN-γ might be caused by the interaction between LPS of Salmonella and TLR-4. Indeed, splenocytes of SL7207-fed mice secreted high amounts of IFN-γ upon heat-killed salmonella stimulation, in agreement with previous studies [27]. This provides a possible mechanism for the reduction of Th2 responses in SL7207-fed mice. Whether the administration of Salmonella will modulate the expression of airway remodeling genes requires further investigation.
We observed significant reductions in Th2-type cytokines produced from the OVA-stimulated splenocyte cultures of SL7207-fed mice. Due to the pivotal role of Th2 activity in the pathogenesis of allergic asthma [2–4], higher levels of IL-4, IL-5, IL-10 and IL-13 as well as more severe asthmatic symptoms were demonstrated after sensitization. Some controversy exists about the role of IL-10 as a Th2-cytokine. IL-10 has been considered as one of the major mediators for T regulatory cells, however, high levels of IL-10 were produced from splenocytes of OVA-sensitized mice upon antigen stimulation in our animal model. Furthermore, treatment with SL7207 reduced IL-10 production from OVA-activated splenocytes. Both anti- and pro-inflammatory effects of IL-10 have been demonstrated in asthma [40,41]. The dual role of IL-10 during immune responses has been reviewed by Mocellin et al. [42]. Moreover, the transcription factor Stat6, which is activated by IL-4 signalling and is vital for the development of a Th2 response, is critical for the expression of IL-5, IL-10, and IL-13 [43]. In a similar murine model, the expression of IL-10 as well as IL-4, IL-5, and IL-13 was detected in the Th2 cell population [44]. Thus, IL-10 can be considered as a Th2-cytokine in our model rather than a regulatory cytokine.
In conclusion, the oral administration of attenuated S. typhimurium, strain SL7207, down-regulated allergen-induced airway inflammation and Th2 cytokine secretion. The development of an oral Salmonella vaccine might have the dual benefit of controlling Salmonella infection and allergic asthma in humans.
Acknowledgments
We are grateful to Dr B. A. D. Stocker for kindly providing Salmonella strain SL7207 and advice for the use of the bacteria. The assistance from Dr Christopher G Wallace in the preparation of this manuscript is highly appreciated. This study was supported by grants from National Health Research Institute, grant numbers: NHRI-EX(91–95)-9110SC.
References
- 1.Beasley R, Crane J, Lai CK, Pearce N. Prevalence and etiology of asthma. J Allergy Clin Immunol. 2000;105:466–72. doi: 10.1016/s0091-6749(00)90044-7. [DOI] [PubMed] [Google Scholar]
- 2.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–82. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF, Jr, Asthma N, Engl J. Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Umetsu DT, Mclntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–20. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 5.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–8. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 6.Haas H, Falcone FH, Holland MJ, Schramm G, Haisch K, Gibbs BF, Bufe A, Schlaak M. Early interleukin-4. its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]
- 7.Kay AB, Barata L, Meng O, Durham SR, Ying S. Eosinophils and eosinophil-associated cytokines in allergic inflammation. Int Arch Allergy Immunol. 1997;113:196–9. doi: 10.1159/000237545. [DOI] [PubMed] [Google Scholar]
- 8.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–8. [PubMed] [Google Scholar]
- 9.Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–74. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- 10.Tulic MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;22:604–12. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- 11.Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002;268:2516–22. doi: 10.4049/jimmunol.168.5.2516. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, Vargaftig BB, Russo M. Bacterial lipopolysaccharide signaling through toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol. 2003;171:1001–8. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 13.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–9. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 15.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–9. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis DB. Allergy immunotherapy and inhibition of Th2 immune responses: a sufficient strategy? Curr Opin Immunol. 2002;14:644–51. doi: 10.1016/s0952-7915(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 17.VanCott JL, Chatfield SN, Roberts M, Hone DM. Regulation of host immune responses by modification of salmonella virulence genes. Nat Med. 1998;4:1247–52. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 18.Hassan JO. Effect of vaccination of hens with an avirulent strain of Salmonella typhimurium on immunity of progeny challenged with wild-type Salmonella strains. Infect Immunity. 1996;64:938–44. doi: 10.1128/iai.64.3.938-944.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox BC. Safety and efficacy of an avirulent live Salmonella choleraesuis vaccine for protection of calves against S. dublin infection. Am J Vet Res. 1997;58:265–71. [PubMed] [Google Scholar]
- 20.Garmory HS, Brown KA, Titball RW. Salmonella vaccines for use in human: present and future perspectives. FEMS Microbiol Rev. 2002;26:339–53. doi: 10.1111/j.1574-6976.2002.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 21.Darji A, Guzman CA, Gerstel B, Wachholz P, Timmis KN, Wehland J, Chakraborty T, Weiss S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–75. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 22.Urashima M, Suzuki H, Yuza Y, Akiyama M, Ohno N, Eto Y. An oral CD40 ligand gene therapy against lymphoma using attenuated Salmonella typhimurium. Blood. 2000;95:1258–63. [PubMed] [Google Scholar]
- 23.Li Y, Guo K, Chen H, Xie Y, Song C, Tang X, Ren D. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer. 2001;94:438–43. doi: 10.1002/ijc.1489. [DOI] [PubMed] [Google Scholar]
- 24.Stocker BAD. Aromatic-dependent Salmonella as anti-bacterial vaccines and as presenters of heterologous antigens or of DNA encoding them. J Biotech. 2000;83:45–50. doi: 10.1016/s0168-1656(00)00297-2. [DOI] [PubMed] [Google Scholar]
- 25.Flo J, Tisminetzky S, Baralle F. Oral transgene vaccination mediated by attenuated Salmonella is an effective method to prevent Herpes simplex virus-2 induced disease in mice. Vaccine. 2001;19:1772–82. doi: 10.1016/s0264-410x(00)00375-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen LC, Zhang Z, Myers AC, Huang SK. Altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10 kDa protein. J Immunol. 2001;167:3025–8. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 27.Harrison JA, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche CE. Correlates of protection induced by live Aro- Salmonella typhimurium vaccines in the murine typhoid model. Immunol. 1997;90:618–25. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones BD. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–61. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 29.Thatte J, Rath S, Bal V. Immunization with live versus killed Salmonella typhimurium leads to the generation of an IFN-γ-dominant versus an IL-4-dominant immune response. Int Immunol. 1993;5:1431–6. doi: 10.1093/intimm/5.11.1431. [DOI] [PubMed] [Google Scholar]
- 30.Soo S-S, Villarreal-Ramos B, Khan CMA. Genetic control of immune response to recombinant antigens carried by an attenuated Salmonella typhimurium vaccine strain: Nramp1 influences T-helper subset response and protection against Leishmanial challenge. Inf Immunity. 1998;66:1910–7. doi: 10.1128/iai.66.5.1910-1917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos AB, Chapman MD, Aalberse RC, et al. Cockroach allergens and asthma in Brazil. Identification of tropomyosin as a major allergen with potential cross- reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329–37. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 32.Leung TF, Lam CW, Chan IH, Li AM, Ha G, Tang NL, Fok TF. Inhalant allergens as risk factors for the development and severity of mild-to-moderate asthma in Hong Kong Chinese children. J Asthma. 2002;39:323–30. doi: 10.1081/jas-120002289. [DOI] [PubMed] [Google Scholar]
- 33.Smith JJ, Van Loverent H, Hoekstra MO, Schijf MA, Folkerts G, Nijkamp FP. Mycobacterium vaccine administration during allergen sensitization or challenge suppresses asthmatic features. Clin Exp Allergy. 2003;33:1083–9. doi: 10.1046/j.1365-2222.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 34.Neutra MR, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 35.Siebers A, Finlay BB. M cells and pathogenesis of mucosal and system infection. Trends Microbiol. 1996;4:22–9. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill LAJ, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–9. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 37.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–7. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 38.Modzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 39.Weinmann AS, Mitchell DM, Sanjabi MN, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promotor is a TLR-dependent, rel-independent event. Nat Immunol. 2001;2:51–7. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 40.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 41.Makela MJ, Kanehiro A, Borish L, et al. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA. 2000;97:6007–12. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/s1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 43.Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109:S109–20. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 44.Encinas JA, Janssen EM, Weiner DB, Calarota SA, Nieto D, Moll T, Carlo DJ, Moss RB. Anti-T-cell Ig and mucin domain-containing protein 1 antibody decreases Th2 airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2005;116:1343–9. doi: 10.1016/j.jaci.2005.08.031. [DOI] [PubMed] [Google Scholar]


