Abstract
Fumaric acid esters (FAE) have proven their therapeutic efficacy in psoriasis, a Th1 mediated skin disease. More recently, preliminary data have suggested an activity in multiple sclerosis (MS) as well. To investigate further possible mechanisms of action of these compounds in inflammatory diseases, we studied the FAE methyl hydrogen fumarate (MHF) and dimethyl fumarate (DMF) in chronic experimental autoimmune encephalomyelitis (EAE) induced by immunization of C57BL/6 mice with MOG peptide aa 35–55. Preventive treatment with these FAE was delivered twice a day by oral gavage. Both esters had a significant therapeutic effect on the disease course and histology showed a strongly reduced macrophage inflammation in the spinal cord. Multiparameter cytokine analysis from blood detected an increase of IL-10 in the treated animals. We conclude that the underlying biological activity of FAE in EAE is complex and, to elucidate the molecular mechanisms, further investigation is needed.
Keywords: encephalomyelitis, multiple sclerosis, therapy, inflammation, cytokines
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by the morphological hallmarks of inflammation, demyelination, loss of oligodendrocytes and subsequent axonal/neuronal damage. Experimental autoimmune encephalomyelitis (EAE) is a model disease reflecting some of the typical features of the human disease MS. It is characterized by an ascending paralysis resulting from lymphocytic infiltration of the CNS associated with macrophage and microglia activation [1]. One of the key mechanisms thought to contribute to the progression of autoimmune demyelination are the effector functions of phagocytic cells in the EAE lesion [2,3].
A well described Th1-mediated skin disease is psoriasis [4], which can be effectively treated with Fumaderm®, a mixture of fumaric acid esters (FAE) including dimethyl fumarate (DMF) and ethylhydrogen fumarate [5]. After oral intake, DMF is rapidly hydrolysed to methyl hydrogen fumarate (MHF) with MHF possibly being the active metabolite [6]. The biological half life of MHF is 36 h, and 30% is bound by serum proteins [7]. FAE have been reported to induce a Th1→Th2 shift as part of their treatment effect. However, the clinical relevance of this observation is unclear and the definite molecular mechanisms of DMF and MHF actions are poorly understood. MHF, for example, can induce interleukin (IL)-10, IL-4 and IL-5 expression in PBMC in vitro [8] without changing interferon (IFN)-γ, IL-12 and IL-2 levels. MHF has also been shown to increases the production of IL-4 and IL-5 in T cells in vitro [9]. Tumour necrosis factor (TNF)-α levels are affected by MHF; initially increasing and subsequently decreasing in response to MHF. Other in vitro studies have shown that DMF can inhibit the transcription of many pro-inflammatory cytokines and this inhibition appears to correlate with a blockade of the TNF-induced nuclear translocation of a NF-κB p65. MHF has been reported to inhibit LPS-induced NF-κB activation in dendritic cells (DC) and endothelial cells in vitro [10,11]. Moreover, DC differentiation is inhibited by both DMF and MHF in a dose-dependent manner and the capacity of DC to stimulate lymphocytes in culture is reduced after DMF treatment [12]. However, since therapeutic concentrations of FAE in vivo are unknown and may differ considerably from those used in in vitro experiments, the clinical relevance of all these in vitro results remains to be determined.
Recently FAE have been discussed as therapeutic tools for autoimmune diseases beyond psoriasis. An initial study describes the rather dramatic effect of FAE on magnetic resonance imaging (MRI) inflammation in a small number of MS patients [13]. However, knowledge on the mechanisms in vivo is extremely limited. Our goal was to investigate the action of DMF and MHF given preventively in chronic MOG-induced EAE of the C57BL/6 mouse, a model that resembles many features of progressive neurological destruction in MS. In addition to observing the effects on clinical disease course, multi parameter cytokine profiling of longitudinal blood samples was applied to screen for molecular changes during treatment and histological analysis was used to extend our understanding of in vivo mechanisms.
Materials and methods
Animals
Female C57BL/6 mice were purchased from Harlan Laboratories (Harlan Winkelmann, Borchen, Germany) for all following experiments. Animals were 8–12 weeks old and body weight was in the range 20–30 g. Animals were housed in an IVC facility with controlled light cycle and were given commercial food pellets and water ad libitum. All experiments were approved by the Lower Saxony state authorities for animal experimentation.
Induction and clinical evaluation of EAE
For induction of EAE, mice received s.c. injections in the flanks and tail base of 50 µg MOG 35–55 peptide (synthesized at Charité Berlin, Department for peptide- and protein-chemistry) in PBS emulsified in an equal volume of complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H37RA (Difco, Detroit MI, USA) at a final concentration of 0·5 mg/ml. Two injections of pertussis toxin (List Biological Laboratories Inc., California, USA; 200 ng per mouse i.p) were given on days 0 and 2. Animals were weighed and scored for clinical signs of disease on a daily basis. Disease severity was assessed using a scale ranging from 0 to 10; scores were as follows [14]: 0 = normal; 1 = reduced tone of tail; 2 = limp tail, impaired righting; 3 = absent righting; 4 = gait ataxia; 5 = mild paraparesis of hindlimbs; 6 = moderate paraparesis; 7 = severe paraparesis or paraplegia; 8 = tetraparesis; 9 = moribund; 10 = death. In accordance to Lower Saxony animal protection laws, mice were sacrificed in case of paraplegia (score 7 or higher). Animals that had to be terminated because of paraplegia were consecutively rated as ‘7’ despite their absence in the further experiment.
Treatment
The medication was diluted in 200 µl 0·08% Methocel/H2O as vehicle and administered by oral gavage starting from day 3 post immunization (p.i) until termination. Each treatment group consisted of 8 animals: vehicle alone as a negative control, 5 mg/kg body weight DMF twice a day, 15 mg/kg body weight DMF twice a day, 5 mg/kg body weight MHF twice a day. The compounds were obtained via Fumapharm AG. MHF, which is highly acidic, was given as calcium salt to avoid acidosis. The lower DMF dose and the MHF dose correlated to the dose used in human psoriasis in clinical trials. The threefold higher dosage of DMF was used to compensate for body surface disparity of mice. Oral gavage was used to ensure exact dosing and to avoid compound degradation.
‘Multi-analyte profiling’ (MAP)
Plasma samples (50 µl) were obtained under general anaesthesia from retro-orbital sinus of all mice before immunization, at the peak of the disease (day 11) and in partial remission (day 21). The plasma protein concentration of 60 cytokines and other markers was measured by MAP testing (Rules Based Medicine, Austin, TX, USA; < http://www.rulesbasedmedicine.com>).
Histology
In the early chronic phase, day 27 p.i., animals were deeply anaesthesized with ketamine/xylazine hydrochloride and transcardially perfused with saline followed by 4% of paraformaldehyde. The complete spinal cord was carefully removed and 8–10 axial sections were further processed for routine paraffin embedding [14]. Early terminated animals were excluded from histology due to low comparability of different disease stages.
Paraffin sections were subjected to haematoxylin/eosin (H&E) staining to assess parameters of inflammatory infiltrates. Immunohistochemistry was performed with 5 µm paraffin sections as described previously [14]. If necessary, antigen unmasking was achieved by heat pretreatment of sections for 30 min in 10 mM citric acid buffer in a microwave oven (850 W). After inhibition of unspecific binding with 10% BSA, sections were incubated overnight at 4 °C with the appropriate primary antibody in 1% BSA. Secondary antibodies were used as indicated below. After blockade of endogenous peroxidase with H2O2, the peroxidase-based ABC detection system (DAKO, Hamburg, Germany) was employed with diaminobenzidine tetrahydrochloride (DAB) as the chromogenic substrate. Specificity of staining was confirmed by omitting the primary antibody as a negative control. T cells were labelled with rat anti-CD3 (Serotec; Wiesbaden, Germany; 1: 200) and macrophages with rat anti-mouse Mac-3 (Pharmingen; 1:200), each with a rabbit anti-rat secondary antibody (Vector via Linavis, Wertheim, Germany; 1:200).
Quantitative evaluation of histopathological changes was essentially performed as described earlier [14]. Coded sections from cervical, thoracic and lumbar spinal cord were evaluated by a blinded observer by means of overlaying a stereological grid and counting mean CD3 and Mac-3 positive cells within 3 visual fields (each 0·096 mm2) with the most intense pathology under a 400 fold magnification.
Statistical analysis
Analysis of the clinical course was performed using Two-way anova (GraphPad Prism program, California, USA). The Mann–Whitney U-test was performed for histological analyses (SPSS program, SPSS, Chicago, IL, USA). All data are given as mean ± SEM. P-values were considered significant at P < 0·05, highly significant at P < 0·01 or P < 0·001.
Results
EAE clinical course of FAE treated C57BL/6 mice
EAE was induced in C57BL/6 mice using MOG 35–55. Three days after disease induction, before the first symptoms occurred, mice were treated preventively with either carrier (control), 5 mg/kg DMF, 15 mg/kg DMF or 5 mg/kg MHF twice a day and the progression of clinical disability was ascertained. Since the pilot experiments did not consistently show a robust treatment effect of 5 mg/kg DMF, a higher dose group (15 mg/kg DMF) was included in the subsequent experiments.
Symptoms of EAE started after 12·3 days (mean) p.i. in the control group, after 13·9 days in the 5 mg/kg DMF group, after 14·8 days in the 15 mg/kg DMF group and after 13·1 days in the MHF group (Fig. 1a), the delay of the FAE treated groups was not significant. The results of two independent experiments were pooled regarding clinical scores and histology in order to attain larger group sizes (n = 16 mice per group for control and MHF, n = 15 for DMF 5 mg/kg and DMF 15 mg/kg) (Fig. 1a). There was a dose dependent, highly significant benefit from preventive treatment with DMF, with the 15 mg/kg dose being more effective (P < 0·0001 compared to control, two-way anova) than the 5 mg/kg dose (P < 0·001 compared to the control group). The difference between DMF 5 mg/kg and 15 mg/kg was also significant (P < 0·0001). The most favourable course was seen within the MHF treated group (P < 0·0001 compared to control). The disease incidence and the number of mice reaching the most severe clinical score, where they had to be terminated due to animal protection laws are given in Table 1. Usually one day before the first EAE symptoms occur, animals loose 1–2 g of body weight, which is partly due to cytokine stress. Figure 1b shows the extent of weight loss in the different groups, which resembles, although less sensitively, the clinical course in these animals. There was no significant difference between 5 mg/kg DMF and control mice. The weight difference between control and MHF treated animals was significant (P < 0·001, two-way anova).
Fig. 1.
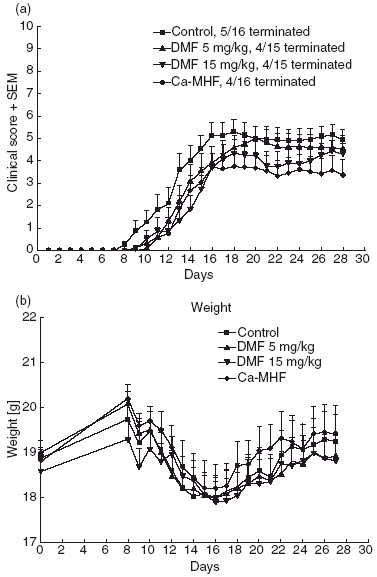
(a) Mean EAE disease scores ± standard error of the mean (SEM) of C57BL/6 mice treated with DMF 5 mg/kg (upright triangle), DMF 15 mg/kg (inverted triangle), MHF (diamond) and carrier alone (square). Preventive treatment was started on day 3. The treatment effect was significant for MHF (P < 0·0001 compared to the control group, two-way anova) and also for DMF, with the 15 mg/kg dose being more effective (P < 0·0001) than the 5 mg/kg dose (P < 0·001). (b) Mean weight and SEM of C57BL/6 mice which were treated with DMF 5 mg/kg (upright triangle), DMF 15 mg/kg (inverted triangle), MHF (diamond) and carrier alone (square) in the experiment shown in (a). DMF 5 mg/kg does not differ much from the control group, whereas the DMF 15 mg/kg group already exhibits lower weight in the beginning. MHF treated animals loose significantly less weight than the control animals (P < 0·001, two-way anova).
Table 1.
Disease incidence and termination rate because of disease severity.
| Control | DMF 5 mg/kg | DMF 15 mg/kg | MHF | |
|---|---|---|---|---|
| Incidence of EAE | ||||
| 1st exp. | 100% | 85% | 100% | 75% |
| 2nd exp. | 88% | 88% | 100% | 75% |
| All | 94% | 87% | 100% | 75% |
| Termination (reaching paraplegia or more) | ||||
| 1st exp. | 13% | 43% | 14% | 38% |
| 2nd exp. | 50% | 13% | 38% | 13% |
| All | 31% | 27% | 27% | 25% |
Values represent percentage of affected animals per group.
The mean termination time point was 13·3 days for control animals, 15·5 days for 5 mg/kg DMF, 15·5 days for 15 mg/kg DMF and 14·3 days for 5 mg/kg MHF, the observed delay is not significant (P > 0·05, Mann–Whitney U-test).
Histology
The beneficial clinical effect of FAE was consistent with the results from histological studies performed on day 27 p.i. (Fig. 2): Infiltration of CD3 positive cells (T-cells) into the spinal cord was slightly reduced at both dosages of DMF (P = 0·14 for the 5 mg/kg group, P = 0·12 for the 15 mg/kg group, Mann–Whitney U-test), and significantly by MHF (P < 0·01). In addition, significantly fewer Mac-3 positive cells (macrophages/microglia) were found in the spinal cord of DMF 15 mg/kg and MHF treated animals (P < 0·01 and P < 0·001, respectively). DMF 5 mg/kg also appeared to reduce the number of Mac-3 positive macrophages in the spinal cords of these mice, however, this effect was not statistically significant (Fig. 3a,b).
Fig. 2.
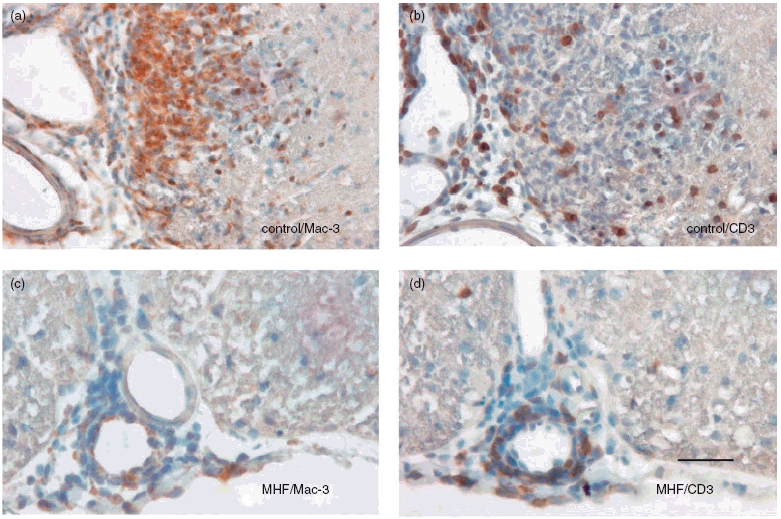
Representative areas from spinal cord cross sections show reduced infiltration of macrophages (a,c) and T cells (b,d) in control animals (a,b) compared to MHF treated animals (c,d) after immunohistochemistry. Nuclei were counterstained with haematoxylin, 5 µm sections.
Fig. 3.

Blinded quantification of inflammation in spinal cord during chronic EAE (27 days p.i). Control n = 11, 5 mg/kg DMF n = 11, 15 mg/kg DMF n = 11, MHF n = 12. Bars show mean cell count per mm2. (a) The infiltration of CD3 + cells (T cells) was nonsignificantly reduced with DMF and significantly reduced with MHF (P < 0·01, Mann–Whitney U-test). (b) The infiltration of Mac-3+ cells (macrophages) was reduced nonsignificantly with 5 mg/kg DMF, significantly with 15 mg/kg DMF (P < 0·01, Mann–Whitney U-test) and highly significantly reduced with MHF (P < 0·001).
Multi-analyte profiling (MAP) results
As an attempt to understand the biology underlying the clinical and histological observations, plasma levels of 60 proteins (including cytokines and other inflammation markers) were determined using MAP. A control experiment was performed for validation of the method, in which plasma samples were taken from 5 healthy mice at 3 time points, the plasma from the last time point was divided and the two fractions were analysed separately. Standard errors were low for over 30 markers, it was more than 10% for eotaxin, growth hormone, IL-1α, IL-2, IL-4, IL-11, IP-10, MIP-1β, myoglobin, SCF, GM-CSF, IFN-γ, IL-12p70, IL-17, IL-3, IL-6, IL-7, MIP-2, OSM, RANTES, TIMP-1 and TNF-α (not shown). Intra-animal variability is shown for IL-5, IL-10, TNF-α and IFN-γ (Fig. 4). SEM was low for IL-10 and IL-5 and rather high for IFN-γ. TNF-α was below the detection level. Therefore while a high reproducibility in most parameters can be assumed, there may be variability in those cytokines listed above.
Fig. 4.

For intrasample validation of cytokine measurement by MAP we divided aliquots of plasma samples from 5 healthy animals. Bars represent aliquots of the same sample. Baseline values of TNF-α (1 tick = 1 ng/ml) were under the detection limit. SEM for IL-10 (1 tick = 100 pg/ml) and IL-5 (1 tick = 100 pg/ml) was low, whereas it was rather high for IFN-γ (1 tick = 1 pg/ml).
In one of the earlier experiments where mice were treated with carrier, 5 mg/kg DMF and 5 mg/kg MHF, samples were taken from each group (8 animals/group) before immunization, at the onset of disease (day 11) and in partial remission (day 21) for MAP analysis. In this experiment, IL-5 (Fig. 5) was significantly increased by DMF in the late phase (P < 0·05, Mann–Whitney U-test) while IFN-γ, TNF-α and IL-4 were below detection level in most cases (not shown). IL-10 (Fig. 6) levels tend to be higher (P > 0·05) in DMF and MHF treated animals.
Fig. 5.
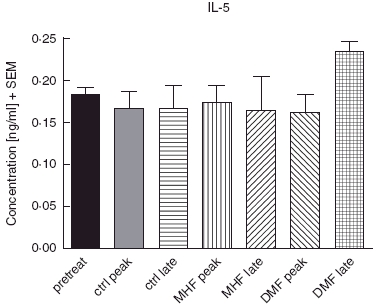
IL-5 levels during EAE with FAE therapy by MAP-analysis.
Fig. 6.
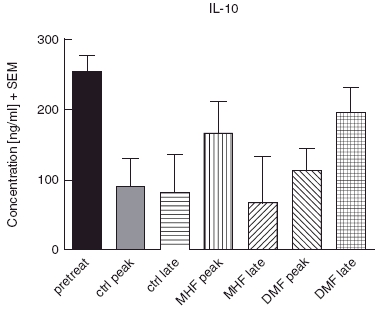
IL-10 levels during EAE with FAE therapy by MAP-analysis. IL-10 was non-significantly elevated in the FAE treated groups. Shown are mean ± SEM.
Discussion
In a chronic mouse model for MS we showed that the fumaric acid esters DMF and MHF given preventively, can effectively reduce disease activity. This finding was further underscored by histological data showing a dramatic decrease in inflammatory cell infliltrates. Our observations in EAE are consistent with the successful use of FAE (a mixture with the main compound DMF) in psoriasis, a classic CD4+ Th1 T cell mediated disease [5]. Similar to psoriasis, the MOG 35–55 induced EAE of the mouse has a predominance of Th1 mechanisms [15] underlying its pathology. This is probably due to CpG motifs in the DNA of Mycobacterium tuberculosis from complete Freund’s adjuvant used for immunization. The infiltrate seen in this model is dominated by an influx of macrophage/microglial cells and secretion of proinflammatory cytokines [16,17].
Symptoms of EAE occurred 10–12 days (mean) after administration of the first FAE dose. The clinical course was ameliorated in treated animals from the beginning and continued until the end of the experiment. An extended observation was not performed in this study, spinal cord inflammation in all remaining animals was analysed in the early chronic phase (day 27). One of the most interesting observations of this study was a clear reduction in macrophage infiltration of the spinal cord in the treated groups, a finding which corroborated the more favourable clinical course of treated animals. As was seen in the clinical course of the disease, this was dose dependent for DMF and most pronounced for MHF. The inhibition of T cell infiltration was less obvious, but still significant in the MHF treated group. In addition to their antigen presenting function, macrophages/microglia cells are active players in myelin destruction in EAE as well as in MS, and phagocytosis of myelin is a marker for ongoing demyelination [18]. Toward this end, activated microglia secrete pro-inflammatory cytokines such as IL-1, IL-6, LT, MIP-1α and others [19] and proteinases which seem to be involved in the breakdown of the blood–brain barrier [20]. It is of note that, so far, the observed clinical effect of FAE in MS is the strong reduction of new Gadolinium enhancing lesions [13]. Histopathological studies made on brain biopsies by Brück et al. [21] have clearly shown that Gadolinium enhancement correlates with macrophage infiltration.
The underlying mechanisms by which FAE exert its biological effects are not yet understood, although there is some data published from in vitro studies and from the use in psoriasis. The key target seems to be immune cells, and it is presumed that a significant aspect of its effect is mediated by a Th2 polarization of CD4+ T cells. Consistent with this assumption is a study that shows that the release of IL-4 and IL-10 by lymphocytes stimulated with herpes simplex virus antigen is increased by treatment with DMF in vitro [22]. Alteration of cell survival has also been postulated as a possible mode of action for FAE. It has been shown that DMF can inhibit the nuclear entry of NF-kappaB [11] and induce apoptosis in lymphocytes and dendritic cells [23].
With Multi Parameter Cytokine Analyses, we could not confirm a relevant change in cytokine expression in plasma samples which would point to one of the known patterns. IL-4 and IFN-γ were generally low or even below the detection limit, IL-5 was only significantly increased by DMF in the chronic phase. In cell culture studies, MHF has been reported reduce the production of IFN-γ by DC in a dose dependent manner [10] however, the concentrations used in these in vitro experiments may not be reached in vivo. IL-2 was not significantly altered by FAE treatment (data not shown). There was also no change in plasma TNF-α levels (data not shown). IL-10 was found to be partly elevated during FAE treatment. This might contribute to the understanding of the anti-inflammatory action of FAE, as IL-10 is a multifunctional immunomodulatory cytokine which has the ability to inhibit the production of pro-inflammatory cytokines in monocytes/macrophages as well as antigen presentation in these cells [24]. It is also secreted by CD4+CD25+ regulatory T cells and is involved in their suppressive function [25], however, it can, on the other hand, induce them. Another source for IL-10 are Th2-polarized T cells. Although these data were gained from blood samples and may not exactly reflect changes in lymphoid tissue or the spinal cord in situ, as they were obtained from animals after in vivo administration of FAE, they may give us insight into more physiological activities of these potentially valuable therapeutics.
Further studies are needed to learn more about the mechanisms involved in the observed clinical effect of DMF and MHF in the EAE model. Moreover, FAE are promising for clinical trials in larger MS cohorts.
Acknowledgments
The technical support by Silvia Seubert and Annette Horn is greatly appreciated. Supported by funds from the Medical Faculty, University of Goettingen, and by research grants from Fumapharm AG and BiogenIdec AG.
References
- 1.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 2.Heppner FL, Greter M, Marino D, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–52. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 3.McQualter JL, Darwiche R, Ewing C, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–82. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 5.Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. German Multicentre Study. Br J Dermatol. 1998;138:456–60. doi: 10.1046/j.1365-2133.1998.02124.x. [DOI] [PubMed] [Google Scholar]
- 6.Werdenberg D, Joshi R, Wolffram S, Merkle HP, Langguth P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm Drug Dispos. 2003;24:259–73. doi: 10.1002/bdd.364. [DOI] [PubMed] [Google Scholar]
- 7.Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol. 1999;141:424–9. doi: 10.1046/j.1365-2133.1999.03034.x. [DOI] [PubMed] [Google Scholar]
- 8.Asadullah K, Schmid H, Friedrich M, et al. Influence of monomethylfumarate on monocytic cytokine formation – explanation for adverse and therapeutic effects in psoriasis? Arch Dermatol Res. 1997;289:623–30. doi: 10.1007/s004030050251. [DOI] [PubMed] [Google Scholar]
- 9.de Jong R, Bezemer AC, Zomerdijk TP, Pouw-Kraan T, Ottenhoff TH, Nibbering PH. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol. 1996;26:2067–74. doi: 10.1002/eji.1830260916. [DOI] [PubMed] [Google Scholar]
- 10.Litjens NH, Rademaker M, Ravensbergen B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004;34:565–75. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- 11.Loewe R, Holnthoner W, Groger M, et al. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol. 2002;168:4781–7. doi: 10.4049/jimmunol.168.9.4781. [DOI] [PubMed] [Google Scholar]
- 12.Zhu K, Mrowietz U. Inhibition of dendritic cell differentiation by fumaric acid esters. J Invest Dermatol. 2001;116:203–8. doi: 10.1046/j.1523-1747.2001.01159.x. [DOI] [PubMed] [Google Scholar]
- 13.Schimrigk S, Brune N, Hellwig K, et al. Oral fumaric acid esters for the treatment of active multiple scierosis: an open-label, baseline controlled study. Eur J Neurol. 2006 doi: 10.1111/j.1468-1331.2006.01292.x. in press. [DOI] [PubMed] [Google Scholar]
- 14.Linker RA, Maurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 15.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 16.Gold R, Hartung HP, Toyka KV. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med. 2000;6:88–91. doi: 10.1016/s1357-4310(99)01639-1. [DOI] [PubMed] [Google Scholar]
- 17.Owens T, Sriram S. The immunology of multiple sclerosis and its animal model, experimental allergic encephalomyelitis. Neurol Clin. 1995;13:51–73. [PubMed] [Google Scholar]
- 18.Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–75. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- 19.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–73. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 20.Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993;35:297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- 21.Bruck W, Bitsch A, Kolenda H, Bruck Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol. 1997;42:783–93. doi: 10.1002/ana.410420515. [DOI] [PubMed] [Google Scholar]
- 22.Heiligenhaus A, Li H, Schmitz A, Wasmuth S, Bauer D. Improvement of herpetic stromal keratitis with fumaric acid derivate is associated with systemic induction of T helper 2 cytokines. Clin Exp Immunol. 2005;142:180–7. doi: 10.1111/j.1365-2249.2005.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121:1383–8. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Koldzic DN, Izikson L, et al. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–56. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]


