Abstract
CD4+ CD25bright regulatory T (Treg) cells have been identified as a principle regulator of tolerance during pregnancy. In the setting of pre-eclampsia, however, little is known about the dynamics of these cells. In the current study, we determined CD4+ CD25bright Treg cells in the peripheral blood using flow cytometry and forkhead box P3 (FoxP3+) cells at the placental bed using immunohistochemical staining. Peripheral blood mononuclear cells (PBMC) of 38 pre-eclamptic cases (17 cases Japanese, 21 cases Polish), 40 normal late pregnancy subjects (20 subjects Japanese, 20 subjects Polish), and 21 non-pregnant healthy controls (10 subjects Japanese, 11 subjects Polish) were included. We found the percentage of CD25bright cells within the CD4+ T cell population in PBMC was reduced significantly in both Japanese and Polish pre-eclamptic cases than in normal pregnancy subjects (P < 0·001) and non-pregnant healthy controls (P < 0·001). Also, the percentage of FoxP3+ cells within CD3+ T cells in the placental bed biopsy samples of pre-eclamptic cases were decreased compared to those in normal pregnancy subjects. These findings suggest that a decreased number of Treg cells was present in pre-eclampsia, and these changes might break the maternal tolerance to the fetus.
Keywords: placental bed biopsy, pre-eclampsia, pregnancy, regulatory T cells, tolerance
Introduction
Pre-eclampsia occurs in 3–5% of pregnancies and is a major cause of maternal mortality and a leading cause of iatrogenic preterm birth and fetal growth restriction. Women are at increased risk during their first conception [1] and/or when conception is with a new partner [2, 3], when conception occurs very shortly after the beginning of their sexual relationship [4] and when conception occurs after donated embryo transfer [5]. These epidemiological findings suggest that a soluble human leucocyte antigen (HLA) class I molecule in the seminal plasma induces specific tolerance against HLA class I molecules of the male partner, and when this tolerance is not induced enough they become pre-eclamptic [6]. This is supported by the fact that soluble HLA class I molecules can induce specific tolerance via the induction of apoptosis in alloreactive T cells [7]. It is well known that continuous oral exposure to antigens can induce tolerance [8]. Indeed, Koelman et al. reported that oral sex and swallowing sperm is associated with a low incidence of pre-eclampsia [6].
These findings support the hypothesis that the immune maladaptation is present in pre-eclampsia. Indeed, the fetus is detected by the maternal immune system, but it is well known that maternal T cells acquire a transient state of tolerance of specific paternal alloantigens by deletion of cells expressing receptors with fetal antigens [9, 10] and the suppression of reactive cells by regulatory T (Treg) cells [11]. Because of these observations, it has been proposed recently that CD4+ CD25+ Treg cells play a critical role in maternal tolerance in mice and human [11–13].
In the setting of pre-eclampsia, little is known about the dynamics of CD4+ CD25+ Treg cells. Recent data show that CD4+ CD25+ Treg cells express Toll-like receptors (TLR)-4, -5, -7 and -8 [14], that the Toll pathway blocks the suppressive effect of CD4+ CD25+ Treg cells by interleukin (IL)-6 production [15] and persistent TLR-4 and -8 signals are required to reverse CD4+ CD25+ Treg-mediated tolerance in cancer and other diseases [16, 17].
Interestingly, an excessive maternal inflammatory response in pre-eclampsia has been reported, and Redman et al. proposed that systemic inflammatory responses during pregnancy might be a major cause of pre-eclampsia [18]. Many supportive data have been reported [19, 20], and inflammation could activate the TLR systems. Indeed, Kim et al. reported that TLR-4 expression on interstitial extravillous trophoblast (EVT) was enhanced in pre-eclampsia [21]. This chronic inflammation might impair the immunosuppressive effect of CD4+ CD25+ Treg cells through the TLR pathway in pre-eclampsia.
In humans, CD4+ CD25bright Treg cells in CD4+ CD25+ cells are the only cells that exhibit a regulatory function [22]. Here, we examined the population of peripheral blood CD4+ CD25bright Treg cells using flow cytometry in Japanese and Polish pre-eclamptic cases. Forkhead box P3 (FoxP3) is the most reliable marker to detect CD4+ CD25+ Treg cells [23], and we could not distinguish CD25bright cells from CD25dim cells by immunohistochemical staining; therefore, we examined the localization of FoxP3+ Treg cells at the placental bed using immunohistochemical staining.
Materials and methods
Subjects
This study was approved by the University of Toyama Institutional Review Board.
The 38 women with pre-eclampsia for study had gestational onset of hypertension (diastolic pressure exceeding 90 mmHg or systolic pressure 140 mmHg) together with new proteinuria > 300 mg/24 h. Seventeen pre-eclamptic cases were Japanese and 21 were Polish. None of these patients was complicated by clinical chorioamnionitis or any infectious disorder. These patients were matched individually with 40 normal pregnant women (20 Japanese; 20 Polish) of similar age, gestational age and parity (Table 1). Twenty-one age-matched non-pregnant healthy women were selected as controls. Informed consent was obtained from all subjects and patients.
Table 1.
Characteristics of subjects.
| Pre-eclampsia | Normal pregnant | Non-pregnant | ||||
|---|---|---|---|---|---|---|
| JPN (n = 17) | POL (n = 21) | JPN (n = 18) | POL (n = 20) | JPN (n = 10) | POL (n = 11) | |
| Age (years) | 32 (28–34) | 28 (21–40) | 31 (22–41) | 27 (24–32) | 32 (25–37) | 28 (24–36) |
| Gestational age (weeks) | 31 (27–34) | 33 (27–35) | 30 (23–38) | 33·5 (29–38) | – | |
| Para | ||||||
| Primipara | 15/17 (88%) | 14/21 (67%) | 13/18 (72%) | 13/20 (65%) | – | |
| Multipara | 2/17 (12%) | 7/21 (33%) | 5/18 (28%) | 7/20 (35%) | – | |
| Blood pressure (mmHg) | ||||||
| Systolic | 170 (160–184) | 170 (160–190) | 99 (96–110) | 120 (110–125) | 102 (88–130) | 115 (110–120) |
| Diastolic | 108 (95–110) | 110 (100–120) | 64 (50–72) | 75 (70–85) | 70 (51–80) | 75 (70–80) |
Data were shown as median (range).
Flow cytometric analysis
Heparinized peripheral venous blood was obtained from normal pregnant women, pre-eclamptic patients and healthy control women. Peripheral blood mononuclear cells (PBMCs) were obtained using a standard Ficoll-Hypaque method. Cells were stained with fluorescein isothiocyanate (FITC)-labelled anti-CD4 monoclonal antibody (MoAb) (Becton Dickinson, San Jose, CA, USA) and phycoerythrin (PE)-labelled anti-CD25 MoAb (Becton Dickinson). In some cases, the cells were stained with FITC-labelled anti-FoxP3 MoAb (e Bioscience, San Diego, CA, USA), allophycocyanin (APC)-labelled anti-CD25 MoAb (e Bioscience) and PE-labelled anti CD4 MoAb using a FITC anti-human FoxP3 staining set (e Bioscience). Flow cytometry was performed on a fluorescence activated cell sorter (FACScan) instrument (Becton Dickinson) as described previously [12].
Immunohistochemistry
Placental bed biopsies were obtained from women undergoing elective caesarean section. After delivery, the position of the placenta was determined and two or three placental bed biopsies were taken under direct vision using biopsy forceps. Placental bed biopsies were included in this study if they contained decidua with interstitial EVT.
Five-micron sections from formalin-fixed, paraffin-enbedded placental bed biopsy samples (nine Japanese samples; pre-eclampsia, 12 Japanese samples; normal pregnancy subjects) were deparaffinized in xylene and rehydrated in graded alcohols, followed by antigen retrieval by boiling in citrate buffer at 121°C for 15 min in an autoclave. After the quenching of endogenous peroxidase activity by treating with 3% H2O2 for 5 min, these sections were rinsed with phosphate-buffered saline and incubated with 5% bovine serum albumin for 5 min. Goat polyclonal anti-FoxP3 antibody (10 µg/ml; Abcam Ltd, Cambridge, UK), mouse monoclonal anti-human CD3 antibody (5 µg/ml; Novocastra, Newcastle upon Tyne, UK) or mouse monoclonal anti-human CD8 antibody (5 µg/ml; Dako, Tokyo, Japan) were applied to the specimens in a plastic moist chamber for 15 min under intermittent microwave irradiation (M1-77, Azumaya, Tokyo, Japan; 250 W, 4 s on/3 s off) followed by incubation at 4°C overnight. After washing with Tris-buffered saline (TBS) for 5 min, peroxidase-conjugated immune polymer reagents for goat polyclonal antibody or mouse monoclonal antibody (Simple Fine Stain for goat or mouse, Nichirei Co., Japan) were hybridized as the second antibody for 1 h at room temperature. After washing with TBS, the sections were developed with 3-3′ diaminobenzidine (Nichirei Co.), and counterstained with haemotoxylin. We counted the numbers of CD3+ cells, CD8+ cells and FoxP3+ cells in five high-power fields (HPFs). Data were showed as average per HPF. CD4+ cell numbers were determined as numbers of CD3+ cells minus numbers of CD8+ cells, because we could not obtain commercially available antibody to detect CD4 in formalin-fixed sections.
Statistical analysis
Data were presented as the median and range. Differences between pre-eclamptic patients, healthy pregnant women and non-pregnant subjects were analysed with the Mann–Whitney U-test. A value of P < 0·05 was considered to indicate statistical significance.
Results
Proportion of CD4+ CD25bright T cells in normal pregnant subjects, pre-eclamptic cases and non-pregnant subjects
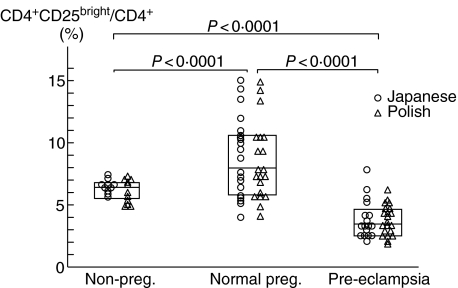
CD4+ T cells can be classified as CD4+ CD25bright T cells, CD4+ CD25dim T cells and CD4+ CD25– T cells by the expression pattern of CD25. A subset within the CD4+ CD25bright T cells in humans exhibit a strong regulatory function, demonstrating that CD4+ CD25bright T cells are Treg cells [22, 24]. As shown in Fig. 1 (left panel), we classified CD4+ T cells into CD4+ CD25bright T cells, CD4+ CD25dim T cells and CD4+ CD25– T cells. There were no significant differences in populations of CD4+ CD25bright/CD4+ T cells between non-pregnant Japanese women and non-pregnant Polish women, normal pregnant Japanese women and normal pregnant Polish women, and pre-eclamptic Japanese cases and pre-eclamptic Polish cases (Fig. 2). The median levels of CD4+ CD25bright T cells in CD4+ T cells in the Japanese and Polish normal pregnancy group (median 8·0%, range 4·4–15·0%) was significantly higher (P < 0·0001) compared to that in the Japanese and Polish non-pregnancy groups (median 6·5%, range 4·6–7·4%). On the other hand, the population of CD25bright cells in CD4+ T cells in the Japanese and Polish pre-eclamptic cases (median 3·1%, range 1·9–7·9%) was significantly lower compared to that in the Japanese and Polish normal pregnant subjects (P < 0·0001) and in the Japanese and Polish non-pregnant subjects (P < 0·0001) (Fig. 2). These findings suggest that peripheral blood Treg cells increased in normal pregnancy subjects, but decreased in pre-eclamptic cases.
Fig. 1.
Expression of forkhead box P3 (FoxP3) in CD4+ CD25bright T cells, CD4+ CD25dim T cells and CD4+ CD25– T cell subsets in pre-eclampsia (a) and normal pregnancy (b). Lymphocytes were stained with allophycocyanin (APC)-labelled anti-CD25 monoclonal antibody (MoAb), phycoerythrin (PE)-labelled anti-CD4 MoAb and fluorescein isothiocyanate (FITC)-labelled anti-human FoxP3 MoAb. CD4+ lymphocytes were classified into CD4+ CD25bright cells, CD4+ CD25dim cells and CD4+ CD25– cells (left panels). The expression of FoxP3 and CD25 in CD4+ cells is shown in the middle panels. The percentages of FoxP3-expressing cells in CD4+ CD25bright, CD4+ CD25dim and CD4+ CD25– cells are shown in the middle panels. Most CD4+ CD25bright cells expressed FoxP3 and least CD4+ CD25dim cells and CD4+ CD25– cells expressed FoxP3.
Fig. 2.
Population of CD4+ CD25bright/CD4+ T cells in non-pregnant subjects, normal pregnant subjects and pre-eclamptic cases in the Japanese (○) and Polish (▵) subjects. The percentage of CD4+ CD25bright cells to CD4+ cells was comparable between Japanese non-pregnant subjects and Polish non-pregnant subjects, Japanese pre-eclamptic cases and Polish pre-eclamptic cases. The box means median ± 75% quartiles of the total of Japanese and Polish in each group. The percentage of CD4+ CD25bright cells to CD4+ cells in pregnant women was significantly higher (P < 0·0001) than that in non-pregnant women. This percentage in pre-eclamptic cases was significantly lower than that in normal pregnant women (P < 0·0001) and non-pregnant women (P < 0·0001).
It has been reported that FoxP3 is the most reliable marker for Treg cells [23]. We further evaluated that the expression of FoxP3 in CD4+ CD25bright T cell, CD4+ CD25dim T cell and CD4+ CD25– T cell subsets of three Japanese pre-eclampsia cases and five Japanese normal pregnancy subjects and five Japanese non-pregnant women using flow cytometry (Fig. 1). The major population of FoxP3-expressing cells was CD4+ CD25bright cells in pre-eclamptic cases, normal pregnancy subjects and non-pregnant women. The median percentage of FoxP3+ CD4+ CD25bright/CD4+ CD25bright cells of pre-eclamptic cases, normal pregnancy subjects and non-pregnant women was 67·9%, 65·9% and 70·6%, respectively. The median percentage of FoxP3+ CD4+CD25dim/CD4+ CD25dim cells of pre-eclamptic cases, normal pregnant subjects and non-pregnant women was 6·6%, 6·1% and 6·3%, respectively, and the percentages of FoxP3+ CD4+ CD25–/CD4+ CD25– cells of pre-eclamptic cases, normal pregnant subjects and non-pregnant women was 0·3%, 0·3% and 0·2%, respectively.
These data suggest that the majority of CD4+ CD25bright T cells in pre-eclamptic cases, normal pregnancy subjects and non-pregnant women are FoxP3+ Treg cells.
Localization of FoxP3+ cells at the placental bed in pre-eclampsia
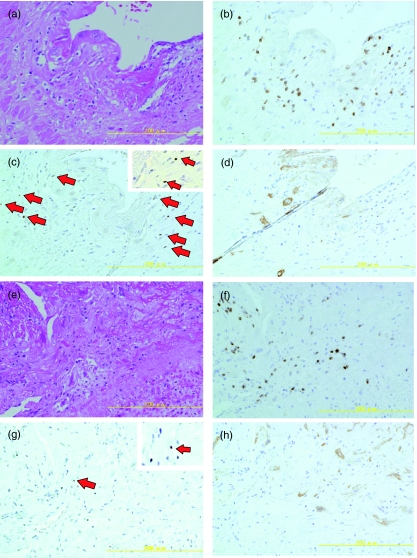
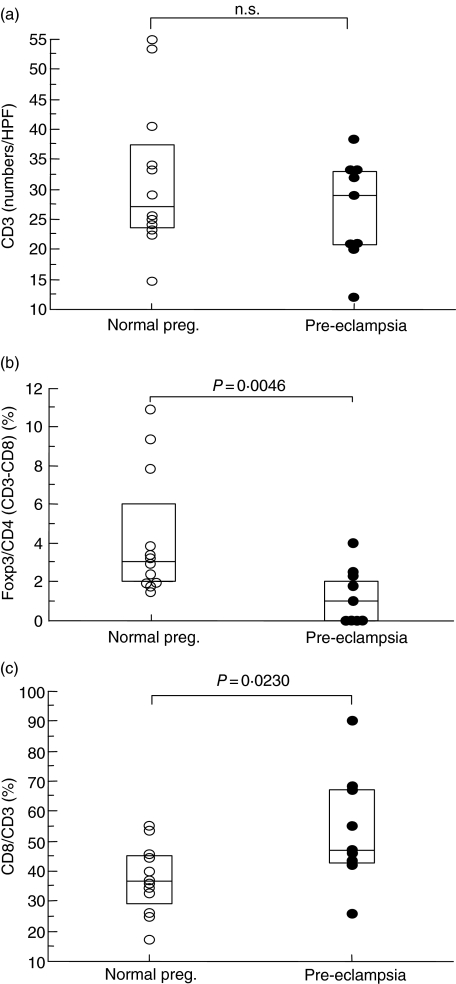
The numbers of CD3+ T cells at placental bed biopsy samples were similar between pre-eclamptic cases and normal pregnancy subjects (Fig. 3b,f), suggesting that the total T cell numbers did not change in pre-eclampsia at the placental bed (Fig. 4a). On the other hand, the ratio of CD8+ T/CD3+ T cells in pre-eclampsia was significantly higher (P = 0·023) than those in normal pregnancy subjects (Fig. 4c), suggesting that cytotoxic T cells increased at the decidua basalis in pre-eclampsia. We examined the immunostaining for FoxP3 to detect CD4+ CD25bright Treg cells, because FoxP3 is the most reliable marker for CD4+ CD25+ Treg cells [23]. Indeed, our flow cytometric analysis showed that 68·5 ± 5·1% [mean ± standard deviation (s.d.), n = 13] of FoxP3+ cells were CD4+ CD25bright cells, suggesting that the majority of FoxP3+ cells are CD4+ CD25bright T cells. We have reported previously that the majority of FoxP3+ cells are CD3+ CD8–CD25bright T cells using two-colour immunofluorescent staining [24]. FoxP3+ cells in the placental bed of pre-eclamptic cases (Fig. 3g) were fewer compared to those in normal pregnancy subjects (Fig. 3c). Interestingly, the median level of FoxP3+/CD4+ T (number of CD3+ T minus number of CD8+ T) cells in pre-eclampsia (0·9%) was significantly lower (P = 0·0046) compared to that in normal pregnancy subjects (3·0%), suggesting that Treg cells decreased at the placental bed in pre-eclampsia (Fig. 4b).
Fig. 3.
Haematoxylin and eosin staining of placental bed biopsy samples in a normal pregnancy subject (a) and a pre-eclamptic case (e). Immunohistochemical staining of CD3 (b,f), forkhead box P3 (FoxP3) (c,g) and cytokeratin (d,h) on formalin-fixed paraffin-embedded placental biopsy samples in a normal pregnancy subject (b,c,d) and a pre-eclamptic case (f,g,h). Red arrows in (c) and (g) show FoxP3-stained cells, and FoxP3+ cells in the high-power field are shown at the upper right.
Fig. 4.
The numbers of CD3+ T cells (a), the population of forkhead box P3/CD4 (b) and the population of CD8/CD3 (c) at the placental bed biopsy of pre-eclamptic cases (•) and normal pregnant subjects (○). The box shows median ± 75% quartiles. CD4+ cell numbers were determined as numbers of CD3+ cells minus numbers of CD8+ cells.
Discussion
Epidemiological studies suggest that the maladaption of maternal tolerance is present in pre-eclamptic cases [2–6]. We and other groups have reported that T helper 1 (Th1)-type immunity, which induces rejection, is predominant in pre-eclampsia, supporting this hypothesis [25–31]. Recent studies have demonstrated that CD4+ CD25bright T cells play a central role in the induction and maintenance of tolerance [32], and CD4+ CD25+ Treg cells are essential for the maintenance of allogeneic pregnancy in mice [11, 13].
This study showed that the population of peripheral blood CD4+ CD25bright T cells was high in the late pregnancy period of normal pregnancy subjects. Somerset et al. also reported an increase in circulating CD4+ CD25+ Treg cells during early pregnancy, peaking during the second trimester and then declining postpartum [33].
Our interesting finding was that peripheral blood CD4+ CD25bright FoxP3+ Treg cells decreased in pre-eclamptic cases. Furthermore, FoxP3+ cells from placental bed biopsy decreased in pre-eclampsia, suggesting that both peripheral blood and decidual Treg cells decreased in pre-eclampsia.
Very recently, Paeschke et al. reported conflicting data [34]; they could not find a significant difference in the level of the CD4+ CD25bright T cell in pre-eclampsia. They measured the surface antigens CD4 and CD25 in peripheral blood from patients suffering from pre-eclampsia (n = 8) and age-matched patients undergoing normal pregnancies (n = 9) by flow cytometry. They used frozen stocked peripheral blood mononuclear cells and examined the surface markers on these cells.
In this study, we used fresh peripheral blood mononuclear cells and analysed the surface markers on fresh cells. The sample number of pre-eclamptic cases was smaller in Paeschke's report compared to those in our study.
In our study, the median levels of systolic blood pressure and diastolic blood pressure in pre-eclamptic cases were 170 mmHg and 110 mmHg, respectively, suggesting that the majority of these cases were the severe type. We did not know the clinical characteristics in Paeschke's paper because they did not show these data. Mild-type pre-eclamptic cases might have been the major population in their study, and CD4+ CD25bright T cells might not decrease in mild-type pre-eclampsia. Furthermore, Paeschke et al. did not show the proportion of CD4+ CD25bright cells in non-pregnant subjects, so it is unclear whether or not CD4+ CD25bright T cells increased in normal pregnancy subjects. In this study, we confirmed that the majority of CD4+ CD25bright T cells expressed the specific marker for Treg, FoxP3, and that peripheral blood CD4+ CD25bright T cells decreased in both Japanese and Polish pre-eclamptic cases. Furthermore, immunohistochemical staining showed that FoxP3+ Treg cells at the placental bed decreased in pre-eclamptic cases. Our findings suggest that Treg cells decreased systematically in pre-eclampsia. We have not obtained direct proof as to why Treg cells decrease in pre-eclampsia. Matarese et al. reported that leptin production was increased significantly in both the serum and cerebrospinal fluid of multiple sclerosis patients and correlated with the Th1-type cytokine, interferon (IFN)-γ[35]. Interestingly, they reported an inverse correlation between serum leptin and the percentage of circulating Treg cells [35]. It is well known that serum leptin increases in pre-eclampsia [36, 37], so increased leptin might reduce the population of Treg cells. CD4+ CD25+ Treg cells express CD95 (Fas) on their surface, and freshly isolated CD4+ CD25+ Treg cells are highly sensitive towards CD95+-mediated apoptosis [38]. The rapid elimination of CD4+ CD25bright Treg cells by CD95 ligand might be present in pre-eclampsia.
In pre-eclamptic patients, chronic inflammation is reported [18–21] and a persistent TLR signal could reverse the CD4+ CD25bright Treg cell-mediated immunosuppression [16, 17]. We need to study the expression of TLRs on FoxP3+ Treg cells by two-colour immunohistochemical staining to strengthen our data in the near future. Our data showed that the number of CD4+ CD25bright Treg cells was suppressed systemically in pre-eclamsia. Inflammation at the decidua might impair the immunoregulatory function of CD4+ CD25bright Treg cells. Regulatory T cells suppress systemic and mucosal activation to control inflammation [39, 40], therefore decreased CD4+ CD25bright Treg cells might augment the systemic inflammation in pre-eclampsia, so inflammation may be trapped within a vicious circle.
These maternal immunological changes might reverse maternal tolerance, resulting in fetal rejection. The accumulation of CD8+ T cells at the placental bed in pre-eclampsia in our study supports this idea. It should be clarified whether reduced Treg cells are the cause or result in pre-ecalmpsia. Further studies are needed to clarify these points.
Acknowledgments
This research was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan [Grant-in Aid for Scientific Research (B)-17390447 (C)-16591648 (C)-16591647, (C)-18591797 and Grant-in-Aid for Exploratory Research 18659482] and from the 21st Century COE Program.
References
- 1.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–51. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 2.Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, Papiernik E. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J Reprod Immunol. 1993;24:1–12. doi: 10.1016/0165-0378(93)90032-d. [DOI] [PubMed] [Google Scholar]
- 3.Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology. 1996;7:240–4. doi: 10.1097/00001648-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344:973–5. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 5.Salha O, Sharma V, Dada T, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14:2268–73. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- 6.Koelman CA, Coumans ABC, Nijman HW, Doxiadis IIN, Dekker GA, Claas FHJ. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? (Hypothesis) J Reprod Immunol. 2000;46:155–66. doi: 10.1016/s0165-0378(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 7.Zavazava N, Kronke M. sHLA class I molecules induce apoptosis in alloreactive cytotoxic T cells. Nat Med. 1996;2:1005–11. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- 8.Brandzaeg P. History of oral tolerance and mucosal immunity. Ann NY Acad Sci. 1996;13:1–27. doi: 10.1111/j.1749-6632.1996.tb21110.x. [DOI] [PubMed] [Google Scholar]
- 9.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 10.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal ‘allograft’. J Immunol. 1998;160:3086–90. [PubMed] [Google Scholar]
- 11.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 13.Zenclussen AC, Gerlof K, Zenclussen MC, et al. Abnormal T cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caramalho I, Lopes-Carvalho T, Ostler D, Zejenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 17.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 18.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 19.Steinborn A, Sohn C, Sayehli C, Niederhut A, Schmitt E, Kaufmann M. Preeclampsia, a pregnancy-specific disease, is associated with fetal monocyte activation. Clin Immunol. 2001;100:305–13. doi: 10.1006/clim.2001.5081. [DOI] [PubMed] [Google Scholar]
- 20.Meziani F, Tesse A, David E, et al. Shed membrane particles from preeclamptic women generate vascular wall inflammation and blunt vascular contractility. Am J Pathol. 2006;169:1473–83. doi: 10.2353/ajpath.2006.051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between ‘danger signals’, the innate immune system and preeclampsia? Am J Obstet Gynecol. 2005;193:921–7. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 22.Beacher-Allan C, Brown J, Freeman G, Hafler D. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Shima T, Saeki A, et al. The expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Science, in press. [DOI] [PMC free article] [PubMed]
- 25.Saito S, Umekage H, Sakamoto Y, et al. Increased Th1-type immunity and decreased Th2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 26.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1 : Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwajima T, Suzuki S, Yoneyama Y, Sawa R, Asakura H, Araki T. Relation between plasma endothelin 1 levels and T helper 1 : T helper 2 cell immunity in women with preeclampsia. Gynecol Obstet Invest. 2001;52:260–3. doi: 10.1159/000052987. [DOI] [PubMed] [Google Scholar]
- 28.Rein DT, Schondorf T, Gohring UJ, et al. Cytokine expression in peripheral blood lymphocytes indicates a switch to T HELPER cells in patients with preeclampsia. J Reprod Immunol. 2002;54:133–42. doi: 10.1016/s0165-0378(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 29.Wilczynski JR, Tchorzewski H, Glowacka E, et al. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Med Inflamm. 2002;11:105–11. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1 type and T helper 2 type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86:165–70. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 31.Darmochwal-olarz D, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The expressions of intracellular cytokines in the lymphocytes of pre-eclamptic patients. Am J Reprod Immunol. 2002;48:381–6. doi: 10.1034/j.1600-0897.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 33.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD4+CD25+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paeschke S, Chen F, Horn N, et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–9. doi: 10.1111/j.1600-0897.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 35.Matarese G, Carrieri PB, Cava AL, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. PANS. 2005;102:5150–5. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagawa N, Yura S, Itoh H, et al. Role of leptin in pregnancy. Placenta Suppl. 2002;1:S80–6. doi: 10.1053/plac.2002.0814. [DOI] [PubMed] [Google Scholar]
- 37.Poston L. Leptin and preeclampsia. Semin Reprod Med. 2002;20:131–8. doi: 10.1055/s-2002-32504. [DOI] [PubMed] [Google Scholar]
- 38.Fritzsching B, Oberle N, Eberhardt N, et al. In contrast to effector T cells, CD4+CD25+Foxp3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR- mediated cell death. J Immunol. 2005;175:32–6. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 39.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 40.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med. 2005;202:1459–63. doi: 10.1084/jem.20052211. [DOI] [PMC free article] [PubMed] [Google Scholar]