Abstract
Alefacept, a recombinant leucocyte function-associated antigen-3 (LFA-3)/IgG1 fusion protein approved for the treatment of psoriasis, is reported to reduce selectively the numbers of circulating CD4+ CD45RO+ and CD8+ CD45RO+ T cells, while sparing the naive cells. The purpose of the present study was to elucidate further the effect of alefacept on various circulating lymphocyte subsets. Sixteen patients, 12 with chronic plaque psoriasis and four with pustular psoriasis, received alefacept 7·5 mg once weekly for 12 weeks. Blood samples collected at study entry and after 12 weeks of treatment were analysed by four-colour flow cytometry. There were statistically significant reductions in the total number of conventional memory (CD45RA– CD27+) and effector (CD45RA– CD27– or CD45RA+ CD27–) T cells, including CD4+ and CD8+ T cells expressing CD161 and CD8+ T cells expressing cutaneous lymphocyte-associated antigen (CLA). Natural killer (NK) T cells were also reduced significantly, while no statistically significant changes were seen in NK cells and CD4+ CD25high cells. The affected subpopulations were all characterized by a high expression of CD2. However, CD4+ CD25low, and CD4+ CLA+ cells, which also expressed relative high levels of CD2, were not reduced significantly. Our results suggest a heterogeneous effect of alefacept on the circulating memory T cell population, indicating that high expression of CD2 may not, by itself, be sufficient to explain the reduction in cell count for a specific subpopulation.
Keywords: alefacept, CLA, NK T, psoriasis, T cells
Introduction
Alefacept is a recombinant leucocyte function-associated antigen-3 (LFA-3)/IgG1 fusion protein (Amevive, Biogen, Cambridge, UK) designed to bind to CD2 molecules on T cells via the LFA-3 part and to FcγRIII on accessory cells via the Fc part, thereby mediating T cell apoptosis [1]. In large clinical trials, alefacept has been shown to produce significant clinical improvements in patients with chronic plaque psoriasis and to reduce circulating levels of CD4+ and CD8+ memory T cells, while sparing the naive populations [2–6]. Adding to the growing body of evidence that T cells with a memory or activated phenotype are pathogenic in psoriasis, the selective reduction in memory cells found in these studies, which has been explained by an up-regulation of CD2 compared to naive cells [7–10], was well correlated with clinical efficacy. However, as memory/activated T cells make up a large and heterogeneous population, the detailed phenotype and functional nature of the true pathogenic immunocyte in psoriasis still remains to be established. Among several possible candidates, special interest has been concentrating on T cells expressing the cutaneous lymphocyte-associated antigen (CLA) or other homing or chemokine receptors that may guide these cells to the psoriatic lesions [11, 12]. Other studies have focused on the role of regulatory T cells and T cells expressing natural killer (NK) cell markers [13–15]. The latter includes (1) NK T cells, which are CD1d-restricted T cells that express certain NK cell markers variably, and (2) non-CD1d-restricted T cells expressing natural killer cell receptors such as CD94, CD158 and CD161, sometimes referred to as NK T-like cells [16].
In the present study, we wished to characterize further the effect of alefacept on various subpopulations of memory T lymphocytes, and to clarify whether certain other subsets, inparticular skin-homing lymphocytes, NK T, NK T-like cells and CD4+ CD25+ cells, were affected. Also, we were interested to analyse the correlation between the surface expression of CD2, assessed by median fluorescent intensity (MFI), and the reduction in cell count for the relevant lymphocyte subsets.
Materials and methods
Patients and ethics
Sixteen patients (age 33–74 years, mean 53), 12 with chronic plaque psoriasis and four with pustular psoriasis, were enrolled into the study. Nine of the patients had not been receiving systemic therapy for at least 4 weeks prior to entry in the study. In the plaque psoriasis group, where five patients also had psoriasis arthritis, three patients received 15–17·5 mg methotrexate (MTX) weekly at study entry and continued this treatment, while two other patients continued treatment with 3–4 mg/kg cyclosporin daily. In the pustular psoriasis group, one patient continued treatment with MTX, while another patient continued treatment with both MTX and cyclosporin. All patients received 7·5 mg alefacept (Amevive) intravenously once weekly for 12 weeks. In the plaque psoriasis group, psoriasis area and severity index (PASI) evaluations were performed at baseline and once weekly during treatment, and patients were classified as responders (PASI score reduction ≥50%) or non-responders (PASI score reduction < 50%), respectively. The study protocol was approved by the Scientific Ethical Committee for Copenhagen County (KF 01–162) and conducted in accordance with the Declaration of Helsinki V. All subjects gave written informed consent.
Peripheral blood samples
Blood samples were obtained from each subject just prior to the first dose of alefacept (baseline) and after 12 weeks of treatment. Venous blood was collected into heparin and ethylemediamine tetraacetic acid (EDTA) anti-coagulant vacutainers for isolation of peripheral blood mononuclear cells (PBMC) and analysis of haematology parameters, respectively. PMBC were isolated by density gradient centrifugation over Lymphoprep (Axis-Shield, Oslo, Norway) and cryopreserved in medium containing RPMI-1640 (50%), serum (30%), and dimethylsulphoxide (DMSO, 20%). The average viability of PBMC after thawing was approximately 95%.
Antibodies
The following monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), allophycocyanin (APC) or Alexa Fluor 647 (AF647) were used: anti-CD2-APC, anti-CD3-PE, anti-CD3-PerCP, anti-CD4-FITC, anti-CD4-PerCP, anti-CD4-APC, anti-CD8-PE, anti-CD8-PerCP, anti-CD16-FITC, anti-CD19-FITC, anti-CD25-PE, anti-CD27-PE, anti-CD56-PE, anti-CD161-FITC, anti-CD45RA-FITC, anti-CD45RO-PE, anti-CD62L-PE, anti-CCR7-AF647, anti-CLA-FITC, anti-Vα24-PE, anti-Vβ11-FITC and 6B11-PE. Anti-Vα24-PE and anti-Vβ11-FITC were purchased from Immunotech (Marseille, France). All other antibodies were purchased from BD Biosciences Pharmingen (San Jose, CA, USA).
Flow cytometry analysis
Thawed cryopreserved aliquots were washed twice in phosphate buffered saline (PBS) and stained with fluorochrome-conjugated monoclonal antibodies following the manufacturer's protocol. After incubation at 4°C in the dark for 30 min, cells were washed with PBS and analysed by four-colour flow cytometry on a fluorescence activated cell sorter (FACSAria) apparatus (BD Biosciences) using Facs Diva software. Cells isolated from pretreatment samples and from samples obtained after 12 weeks of treatment from each individual patient were analysed in the same run.
For detection of major lymphocyte subsets a minimum of 10 000 events were collected for each sample, while at least 250 000 events were collected to ensure possible detection of NK T cells. Samples were gated on lymphocytes using forward and side light-scatter parameters and a CD45/CD14 antibody combination (Leucogate) was used to establish this gate (i.e. lymphocytes identified as CD45bright CD14negative cells). The lymphocyte purity within the gate was checked by the lymphosum (sum of CD3+ cells, CD19+ cells and CD3–CD16+ CD56+ cells), and samples with < 85% purity and/or < 90% recovery were excluded from analysis. Absolute counts of the analysed lymphocyte subsets were calculated by multiplying the specific subset percentage by the absolute lymphocyte count determined from the EDTA-containing samples analysed on a Sysmex XE2100 (Sysmex Corporation, Kobe, Japan).
Statistical analysis
Data are presented as mean ± standard error of the mean (s.e.m.). The Wilcoxon matched-pairs signed-rank sum test was used to analyse differences in paired data. Spearman's correlation test was used to access the relationship between variables, where appropriate. Results with P-values < 0·05 were considered significant.
Results
Identification of lymphocyte subsets by flow cytometry
We used the combination of anti-CD45RA and anti-CD27 to distinguish between naive (CD45RA+ CD27+), conventional memory (CD45RA– CD27+), effector (CD45RA– CD27–) and terminally differentiated effector (CD45RA+ CD27–) T cells. In agreement with previous reports, the latter subpopulation of terminally differentiated T cells was found exclusively among CD8+ cells. Naive and memory T cells, as well as terminally differentiated CD8+ cells and effector CD4+ cells, all separated into distinct populations. In contrast, effector CD8+ cells, which were generally few in number or even missing, appeared as a less well-defined population and were excluded from further analysis.
We also attempted to characterize naive and memory subpopulations with combinations of anti-CD45RA with anti-CCR7 and anti-CD62L. However, anti-CCR7 generally showed very poor staining, while staining with anti-CD62L did not provide fully reproducible results, as a fraction of CD45RA positive cells in some samples apparently lost their expression of CD62L during the freezing/thawing procedure, a problem that has been reported by others [17]. As expected, CLA, CD161 and CD25, like CD45RA and CD27, displayed a continuum of expression that did not resolve into distinct positive and negative populations. Thus, for these markers, demarcation of positive cells was based only on comparison with the isotype control. Among CD4+ cells, all three markers were found exclusively on CD45RO+ T cells, while within CD8+ cells a minor proportion of the CLA+ and CD161+ cells were also found among CD45RO– cells, probably TD cells, but further characterization of these subsets with other memory/effector markers was not performed. In the case of CD25, we used the gating strategy described by Lundgren and co-workers, which is based on comparison with the expression of CD25 on CD8+ T cells, to discriminate CD4+CD25high from CD4+ CD25low T cells [18]. The CLA+ and CD161+ T cells represented predominantly different cell populations, as the vast majority of CLA+ cells were negative for CD161+ and vice versa (i.e. less than 0·5% were CLA+ CD161+).
Type 1 NK T cells were identified by combinations of anti-CD3 with anti-Vβ11 and anti-Vα24. However, distinct populations (> 10 events) of type 1 NK T cells could be demonstrated clearly in only seven of 16 patients, where they comprised about 0·07% (range 0·01–0·16%) of the total peripheral lymphocytes at study entry. This lack of detectable NK T cells in nine patients was not due to poor staining with the antibodies used, as CD3+ Vα24+ cells and CD3+ Vβ11+ cells were seen in all patients, both constituting around 0·5% of the total lymphocyte population. In addition, we obtained similar results using a combination of anti-CD3 and monoclonal antibody (MoAb) 6B11, the latter of which recognizes the conserved region of Vα24–Jα18 T cell receptor (TCR).
Expression of CD2 on lymphocyte subsets
The level of CD2 expression at baseline for each examined lymphocyte subpopulation, as assessed by the MFI, is shown in Table 1.
Table 1.
Pretreatment expression of CD2 and post-treatment changes in total cell counts for specific lymphocyte subpopulations.
| Phenotype | MFI ratio | Reduction (%) | P |
|---|---|---|---|
| Total lymphocytes | 115·9 ± 8·1 | 23·1 ± 4·2 | 0·001 |
| (112·0 ± 11·0) | (22·9 ± 5·2) | (0·0078) | |
| B cells | 0·6 ± 0·1 | 19·5 ± 6·2 | 0·004 |
| (0·6 ± 1·1) | (13·7 ± 7·6) | (0·0547) | |
| NK cells | 73·9 ± 11·1 | 10·7 ± 5·2 | 0·0813 |
| (69·3 ± 12·3) | (6·3 ± 6·7) | (0·4961) | |
| Total T cells | 149·8 ± 12·5 | 28·4 ± 5·1 | 0·0012 |
| (145·6 ± 19·2) | (27·7 ± 6·9) | (0·0117) | |
| CD4+ | 137·3 ± 12·2 | 22·4 ± 5·7 | 0·0029 |
| (135·1 ± 18·8) | (20·8 ± 6·0) | (0·0117) | |
| CD8+ | 170·1 ± 14·8 | 32·0 ± 6·1 | 0·004 |
| (161·5 ± 20·6) | (30·9 ± 9·3) | (0·0391) | |
| Naive CD4+ | 120·2 ± 12·0 | 4·8 ± 6·8 | 0·3074 |
| (118·3 ± 18·3) | (6·0 ± 14·1) | (0·8203) | |
| Memory CD4+ | 157·0 ± 14·9 | 32·7 ± 6·0 | 0·001 |
| (157·3 ± 23·6) | (33·9 ± 8·2) | (0·0117) | |
| Effector CD4+ | 191·4 ± 17·6 | 71·4 ± 4·8 | 0·0005 |
| (194·3 ± 29·6) | (74·1 ± 7·1) | (0·0039) | |
| Naive CD8+ | 154·6 ± 14·4 | 19·6 ± 7·3 | 0·0354 |
| (148·3 ± 19·4) | (16·8 ± 10·6) | (0·1289) | |
| Memory CD8+ | 182·5 ± 16·6 | 49·8 ± 7·9 | 0·0008 |
| (174·8 ± 26·6) | (56·4 ± 10·1) | (0·0078) | |
| TD CD8+ | 185·9 ± 17·2 | 36·4 ± 6·3 | 0·0024 |
| (179·9 ± 23·9) | (42·1 ± 8·9) | (0·0039) | |
| CD4+ CD45RA+ RO– | 118·4 ± 11·6 | 4·1 ± 7·4 | 0·237 |
| (117·6 ± 18·2) | (4·1 ± 5·3) | (0·4258) | |
| CD4+ CD45RA+ RO+ | 133·1 ± 12·2 | 27·6 ± 6·9 | 0·0024 |
| (135·0 ± 19·5) | (26·5 ± 7·0) | (0·0195) | |
| CD4+ CD45RA– RO+ | 143·4 ± 14·4 | 42·9 ± 6·2 | 0·0002 |
| (163·4 ± 22·6) | (44·5 ± 8·6) | (0·0039) | |
| CD8+ CD45RA+ RO– | 168·6 ± 18·8 | 20·2 ± 8·5 | 0·0681 |
| (156·9 ± 17·5) | (20·8 ± 10·3) | (0·0742) | |
| CD8+ CD45RA+ RO+ | 209·8 ± 25·7 | 59·2 ± 6·3 | 0·0002 |
| (195·6 ± 28·1) | (63·1 ± 8·5) | (0·0039) | |
| CD8+ CD45RA– RO+ | 169·5 ± 15·7 | 57·5 ± 7·4 | 0·0002 |
| (188·5 ± 23·0) | (66·3 ± 8·0) | (0·0039) | |
| CD4+ CD161+ | 209·3 ± 22·2 | 51·0 ± 5·5 | 0·0008 |
| (208·8 ± 35·8) | (49·8 ± 8·8) | (0·0078) | |
| CD8+ CD161+ | 198·6 ± 19·7 | 47·5 ± 7·6 | 0·0005 |
| (203·3 ± 33·1) | (53·3 ± 8·4) | (0·0039) | |
| CD4+ CLA+ | 180·3 ± 18·5 | 18·4 ± 10·6 | 0·0907 |
| (186·7 ± 30·1) | (22·8 ± 11·2) | (0·1641) | |
| CD8+ CLA+ | 181·9 ± 18·7 | 39·7 ± 6·2 | 0·0005 |
| (179·6 ± 31·5) | (40·6 ± 7·8) | (0·0039) | |
| CD4+ CD25high | 138·6 ± 13·0 | 11·2 ± 11·4 | 0·0727 |
| (142·7 ± 20·8) | (20·6 ± 12·0) | (0·2500) | |
| CD4+ CD25low | 153·8 ± 13·7 | 18·6 ± 13·0 | 0·0512 |
| (149·9 ± 19·6) | (13·9 ± 7·7) | (0·3008) | |
| NK T (Vα24+Vβ11+) | 157·8 ± 22·6 | 51·1 ± 6·7 | 0·0156 |
The relative expression of CD2 for each subpopulation was assessed as the median fluorescent intensity (MFI) ratio (MFI of cells staining positive for allophycocyanin-conjugated anti-CD2/MFI of isotype). The reduction in total cell count was calculated as: [total cell count at baseline − total count after 12 weeks of treatment with alefacept)/total cell count at baseline × (100)]. Positive values denote reduction in cell count, negative values denote an increase. All data are expressed as mean ± standard error of the mean (s.e.m.). Values for all included patients (n = 16, except for natural killer (NK) T cells where n = 7) in the study protocol are shown in bold type. Values for patients treated with alefacept only (n = 9) are shown in brackets. The Wilcoxon signed-rank test P-values for the reduction in cell count are specified.
CD2 expression was significantly higher on memory T cells compared to naive cells, within both CD4+ (in 16 of 16 patients; P < 0·0001) and CD8+ (in 15 of 16 patients; P = 0·0017). However, while effector CD4+ cells had even higher CD2 expression compared to memory CD4+ cells (P < 0·0005), there was no difference between memory cells, effector cells and TD cells within the CD8+ population.
As circulating T cells expressing CD25, CLA or CD161 probably belong exclusively to the memory/effector cell population, it was not surprising that the expression of CD2 on subsets expressing one of these markers was closer to that of memory/effector cells than to that of naive cells (P < 0·0001). The expression of CD2 on CD4+ CD25low cells, being similar to that of CD4+ memory cells, was on average slightly higher than on CD4+ CD25high cells (P = 0·0155).
On average, approximately 70% of NK cells expressed CD2, but with considerable variability between subjects (range 30–100%), and the level of CD2 expression on these cells was only about half the value of that for the average T cells.
For the seven patients in whom type 1 NK T cells could be detected, the CD2 expression on these cells was not different from that of memory CD4+ cells.
Following alefacept treatment all examined lymphocyte subsets, except for B cells, showed on average a significant decrease in CD2 signal of 25·2 ± 1·1% (P < 0·0001). There was some variation in this decrease between the different subsets (range 15–32%) and a substantial variation between individuals (range −25% to 60%), but it was not correlated with changes in cell count for any of the examined subsets. One possible explanation for this change and for the difference between subjects could be that variations in alefacept concentration in the blood at the time of blood sample drawing causes a more or less effective binding of alefacept that could hinder sterically the binding of fluorochrome-labelled anti-CD2.
Changes in major lymphocyte subsets
Only minor changes were seen in the percentages of total T cells, CD4+ cells, CD8+ cells, B cells and NK cells following treatment with alefacept (data not shown). In 14 of 16 patients, total lymphocyte counts were reduced significantly, which was accounted for primarily by a significant decrease in T cells affecting both CD4+ and CD8+ cells (Table 1). The reduction in CD8+ cells was correlated with the reduction in CD4+ cells (r = 0·6794, P = 0·038), with CD8+ being slightly more affected (P = 0·0273). No statistically significant changes were observed for NK cells. In contrast, B cells showed an unexpected decrease which was, however, not statistically significant when patients receiving MTX in addition to alefacept were excluded from analysis.
Changes in naive and memory T lymphocyte subsets
Following treatment with alefacept there was a significant enrichment in the proportion of naive cells (8–10%) within both CD4+ and CD8+, with a corresponding decrease in the proportion of memory/effector cells. However, there was a moderate reduction in the absolute number of naive CD8+ cells that did reach statistical significance, whereas no significant change for CD4+ cells in the absolute number of naive cells was observed (Table 1). Another difference between CD4+ and CD8+ cells was a more pronounced decrease in the absolute number for effector CD4+ cells than for memory CD4+ cells (P = 0·0005), whereas the reductions in memory cells and TD cells were more similar for CD8+ cells. Similarly, in experiments where effective staining with anti-CD62L was obtained, CD4+ with an ‘effector memory’ phenotype (CD45RA– CD62L–) were more affected than cells with a ‘central memory’ (CD45RA– CD62L+) phenotype while there was no difference between CD45RA- CD62L- and CD45RA- CD62L+ CD8 cells (data not shown).
Changes in CD4+ CD25+, CD161+ and CLA+ T cells
Although T cells expressing CD25low, CD161 or CLA all belong to the memory/effector pool, subsets expressing one of these markers were not affected equally. Hence, neither CD4+ CD25low nor CD4+ CLA+ cells showed statistically significant changes in percentages (data not shown) or absolute counts following treatment (Table 1). By contrast, CD4+ and CD8+ T cells expressing CD161 and CD8+ CLA+ decreased significantly in absolute numbers.
Changes in CD4+ CD25high and NK T cells
As shown in Table 1, there was a significant reduction in the absolute number of type 1 NK T cells, whereas the smaller reduction in absolute numbers of CD4+ CD25high did not reach statistically significance.
Correlation between CD2 expression and reductions in cell counts
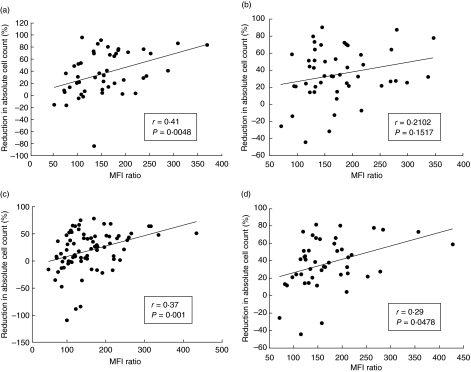
From the data presented in Table 1 it appears that for a specific lymphocyte subset there was a good correlation between the average CD2 expression and the average reduction in absolute cell count, except for CD4+ CD25low and CD4+ CLA+ T cells, which were not reduced significantly despite a relative high CD2 expression. However, the correlation was not strong when analysed for pools of naive, memory and effector subsets within CD4+ or CD8+ cells, respectively (Fig. 1), and did not reach statistical significance when analysed within each single subset (data not shown), except for the CD8+ CLA+ cells (P = 0·0142).
Fig. 1.
Correlation between mean pretreatment expressions of CD2 and post-treatment changes in total cell counts. (a) Individual values for naive, memory and effector cells within the CD4+ population (n = 16, n = 48). (b) Individual values for naive, memory and terminally differentiated effector cells within the CD8+ population (n = 16, n = 48). (c) Individual values for naive, CD25high, CD25low, CLA+ and CD161+ cells within the CD4+ population (n = 16, n = 80). (d) Individual values for naive, CLA+ and CD161+ cells within the CD8+ population (n = 16, n = 48). r = Spearman's correlation coefficient. Positive values denote reduction in cell count, negative values denote an increase. The relative expression of CD2 for each subpopulation was assessed as the median fluorescent intensity (MFI) ratio (see Table 1).
Influence of methotrexate and cyclosporin
Overall, the changes in the various lymphocyte subsets showed rather similar patterns when patients receiving no other systemic therapy than alefacept were compared with patients continuing treatment with MTX or cyclosporin in addition to alefacept (Table 1). An apparent difference was seen for B cells and for naive CD4+ and CD8+ cells, as these subsets had lower pretreatment values and seemed to be more affected in the group of patients who received MTX (data not shown). Of the two patients who received cyclosporin in addition to alefacept, one had generally higher reductions, whereas the other had generally lower reductions compared to the means for the respective subsets of lymphocytes in patients receiving alefacept only. However, patient numbers in these treatment groups were too small to perform statistical analysis.
Clinical observations
Alefacept was generally well tolerated and only minor adverse effects were observed. In the plaque psoriasis group reductions in PASI score during the 12 weeks of treatment ranged from 0 to 93% (mean 46 ± 10%), with six of 12 being responders (PASI score reduction > 50%). The PASI score showed no statistically significant correlation with the reductions in any of the investigated lymphocyte subpopulations (data not shown). In the group of patients with pustular psoriasis, only one patient showed clinical improvements during the treatment course. None of the five patients with psoriasis arthritis experienced improvement in joint symptoms.
Discussion
Consistent with previous reports, our data show that memory populations of CD4+ and CD8+ T cells are reduced significantly in response to alefacept therapy, whereas naive cells are affected only slightly (CD8+ cells) or practically unchanged (CD4+ cells). The aim of the present study was to characterize further the effect of alefacept on the memory/effector T cell compartment in peripheral blood. To this end, CD45RA and CD27 were used as the primary combination to identify naive, conventional memory, effector and terminally differentiated effector cells. Using this combination, the main findings were that within CD4+ cells, the effector subpopulation (CD45RA– CD27–) was even more affected than conventional memory cells (CD45RA– CD27+), whereas within CD8+ cells, terminally differentiated effector cells (TD; CD45RA+ CD27–) and memory cells were affected equally. Although the use of cryopreserved cells impeded reliable staining with CD62L, some successful experiments nevertheless showed the same tendency; that is, CD45RA– 62L– cells (‘effector memory’) were more affected than CD45RA- CD62L+ cells (‘central memory’) within CD4+ cells, while there was no difference between CD45RA+ CD62L– and CD45RA– CD62L+ cells within CD8+ cells. However, a direct comparison between CD4+ and CD8+ cells on this issue is difficult, as many reported data have emphasized the complexity of memory T cells and the difficulties in finding useful markers to distinguish memory and effector functions (reviewed in [19]). Hence, the impact of the obvious difference with the markers used here, the occurrence of CD45RA+ CD27– (equivalent to CD45RA+ CD62L–) cells almost exclusively within CD8+ cells, is not understood fully and may reflect differences between CD4+ and CD8+ cells in the differentiation pathway from naive to memory/effector cells. Nevertheless, TD CD8+ cells are thought to be antigen-primed, so their existence is a main reason why discrimination between naive and memory cells based solely on the CD45 isoform expression has been proved to be unreliable. TD CD8+ cells are present in large numbers in some individuals, in health and disease, and are of interest because ‘effector’ T cells, in particular within CD8+ cells, have been suggested to play a key role in psoriasis. Meanwhile, the T cells which have been found to dominate the lymphocyte infiltration in psoriasis plaques are often referred to as ‘effector memory’ cells although, to our knowledge, the use of that term has so far been based only on the demonstration of T cells expressing CD45RO+ and/or markers of activation such as CD25, and not on further characterization by other effector or memory phenotypic markers.
Next, we were interested to see if we could differentiate further the effect of alefacept on separate subpopulations within the memory/effector T cell compartment and to see if other lymphocyte phenotypes that might play a role in the pathogenesis of psoriasis, specifically NK, NK T and CD4+ CD25+ cells, were affected. Although T cells expressing the activation marker CD25low or the cutaneous homing-receptor CLA all belong to the memory/effector pool and have been found in increased numbers in psoriatic lesions [10–12], they were not affected equally by alefacept. Thus, whereas CD8+ CLA+ cells decreased significantly in absolute numbers, neither CD4+ CD25low nor CD4+ CLA+ cells showed statistically significant changes in percentages or absolute counts following treatment. It is noteworthy that one study has demonstrated a correlation between disease severity in psoriasis patients and the frequency of CD8+ CLA+, but not CD4+ CLA+ cells [20].
NK cells and T cells expressing NK cell markers have also been suggested to play a role in the pathogenesis of psoriasis. As some NK cells express CD2 they might be targets for alefacept, although the death of target cells caused by alefacept is, paradoxically, in itself NK cell-mediated [21]. However, we observed no significant changes in the NK cell population, in agreement with previously reported data [3]. In contrast, within both CD4+ and CD8+ subsets, we found that T cells expressing the NK marker CD161 were reduced in parallel to memory and/or effector T cells. In concordance with this these cells expressed CD45RO, indicating that they were part of the memory/effector population, as has been suggested previously by others [22]. Of note, T cells expressing NK markers such as CD161, some in combination with Vβ11 but not Vα24, have been found in psoriasis plaques [14], but it has become clear in recent years that only a very small fraction of T cells expressing NK cell markers are true so-called type 1 or type 2 NK T cells [16]. We were able to demonstrate cells only with the CD3+ Vα24+ Vβ11+ phenotype, generally accepted to be type 1 NK T cells, in approximately half the patients; that is, in the other half of patients they comprised less than 0·01% of the T cell population. Regulatory T cells [CD4+ CD25high CTLA+ forkhead box P3 (FoxP3)high] represent another lymphocyte population that has been hypothesized to play a role in the pathogenesis of psoriasis [15], but in contrast to type 1 NK T cells, CD4+ CD25high cells were unaffected by alefacept in our study.
It is obvious to assume that the differential effect on the various lymphocyte subsets found herein can be explained by differences in the cell surface expression of CD2. Indeed, our results, using MFI as a relative measure of CD2 surface expression, confirm that CD2 is up-regulated on conventional memory cells compared with naive cells [8, 10] and in addition demonstrates that, within CD4+ cells, CD2 expression is even higher on effector cells than on memory cells. We actually observed a weak but statistically significant correlation between the reductions in absolute count and the pretreatment expression of CD2 when the analysis was performed for pools of naive, memory and effector subsets within CD4+ and CD8+ cells, respectively. Moreover, CD8+ cells, which on average had a higher level of CD2 expression compared to CD4+ cells, were also more affected by alefacept whereas, for example, NK cells, which had a level of CD2 expression that was only about half that for the average T cells, were not significantly reduced. Hence, alefacept might simply target cells with a CD2 expression over a certain threshold. However, some observations in our study did not comply with this assumption. First, CD4+ cells expressing CLA or CD25 were not reduced significantly despite a CD2 expression similar to that of other memory CD4+ cells. Secondly, for each identified subset, the correlation between the sometimes highly variable levels of CD2 expression and the corresponding change in cell count for each subject did not reach statistical significance. Thirdly, the differences in CD2 expression between cell subsets that decreased and subsets that were unaffected after alefacept treatment seemed, in some cases, to be small. For instance, CD2 expression was ‘only’ 30% higher on memory CD4+ cells compared to naive cells. These observations indicate that factors other than high CD2 expression might also be important for the susceptibility to alefacept, such as the differential expression of Fas antigen on naive/memory T cell subpopulations, as suggested by others [21].
The limitations of our study include the relative small number of patients, the inclusion of patients with different forms of psoriasis and the inclusion of seven patients in whom maintenance therapy with MTX and/or cyclosporin were continued. However, it was beyond the scope of this study to look specifically at the clinical response of alefacept in psoriasis and the possible correlation of this response with changes in specific lymphocyte subpopulations. Although such correlations could provide important clues to the pathophysiology of psoriasis, they would certainly have to be combined with data from the skin to complete the picture, as changes in the peripheral circulation may not necessarily mirror those in the psoriatic lesions [23]. In fact, the local action of alefacept in the affected skin may be complicated by a variety of immunoregulatory factors such as possible expression of FCγRIII on keratinocytes induced by interferon-γ [24].
The treatment with MTX and/or cyclosporin may have affected the action of alefacept. Thus, MTX have been found to reduce the number of CD4+ and CD8+ cells expressing CLA in psoriasis patients [25] and the number of B cells in patients with lupus erythematosus [26], whereas cyclosporin has been found to reduce the number of CD4+ CLA+ cells, but not CD8+ CLA+ cells, in children with atopic dermatitis [27]. However, except for the pretreatment values and the affection of B cells and naive T cells in the group of patients who were treated with MTX, we found similar patterns in the response to alefacept regardless of whether or not the patients were treated with MTX and/or cyclosporin. This justified pooling the data from all the included patients, although we cannot exclude the possibility that for certain subsets these drugs may have affected susceptibility to alefacept.
In summary, this study has evaluated the effect of alefacept on a variety of circulating lymphocyte subsets hypothesized to play a role in psoriasis and/or other diseases with an autoimmune pathogenesis and has demonstrated a differentiated effect on specific subsets within the memory/effector compartment of T cells. The results suggest that the effect of alefacept may be more complex than simply a matter of reducing the number of CD45RO+ cells with a high level of CD2 expression, and thus further studies are warranted to clarify the mechanism of action of alefacept in vivo.
Acknowledgments
We thank study coordinator Ms Britt Kaae for the help with the patients. We are grateful to Mrs Ingelise Petersen for her technical help with lymphocyte purification and freezing. Biogen-Idec AS is acknowledged for providing Amevive for the present study.
References
- 1.da Silva AJ, Brickelmaier M, Majeau GR, et al. Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cells. J Immunol. 2002;168:4462–71. doi: 10.4049/jimmunol.168.9.4462. [DOI] [PubMed] [Google Scholar]
- 2.Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 3.Gordon KB, Vaishnaw AK, O'Gorman J, Haney J, Menter A. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563–70. doi: 10.1001/archderm.139.12.1563. [DOI] [PubMed] [Google Scholar]
- 4.Liu CM, McKenna JK, Krueger GG. Alefacept: a novel biologic in the treatment of psoriasis. Drugs Today (Barcelona) 2004;40:961–74. doi: 10.1358/dot.2004.40.12.872572. [DOI] [PubMed] [Google Scholar]
- 5.Ortonne JP, Lebwohl M, Em GC. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117–23. [PubMed] [Google Scholar]
- 6.Vaishnaw AK, TenHoor CN. Pharmacokinetics, biologic activity, and tolerability of alefacept by intravenous and intramuscular administration. J Pharmacokinet Pharmacodyn. 2002;29:415–26. doi: 10.1023/a:1022995602257. [DOI] [PubMed] [Google Scholar]
- 7.Prince HE, York J, Jensen ER. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992;145:254–62. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- 8.Sanders ME, Makgoba MW, Sharrow SO, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–7. [PubMed] [Google Scholar]
- 9.Vissers WH, Arndtz CH, Muys L, Van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–9. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- 10.Wallace DL, Beverley PC. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990;69:460–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res. 2000;292:519–21. doi: 10.1007/s004030000167. [DOI] [PubMed] [Google Scholar]
- 12.Teraki Y, Miyake A, Takebayashi R, Shiohara T. Homing receptor and chemokine receptor on intraepidermal T cells in psoriasis vulgaris. Clin Exp Dermatol. 2004;29:658–63. doi: 10.1111/j.1365-2230.2004.01638.x. [DOI] [PubMed] [Google Scholar]
- 13.Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076–85. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- 14.Nickoloff BJ, Bonish B, Huang BB, Porcelli SA. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212–25. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 17.Reimann KA, Chernoff M, Wilkening CL, Nickerson CE, Landay AL. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. Clin Diagn Lab Immunol. 2000;7:352–9. doi: 10.1128/cdli.7.3.352-359.2000. The ACTG Immunology Advanced Technology Laboratories. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren A, Stromberg E, Sjoling A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–31. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 20.Sigmundsdottir H, Gudjonsson JE, Jonsdottir I, Ludviksson BR, Valdimarsson H. The frequency of CLA+ CD8+ T cells in the blood of psoriasis patients correlates closely with the severity of their disease. Clin Exp Immunol. 2001;126:365–9. doi: 10.1046/j.1365-2249.2001.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper JC, Morgan G, Harding S, et al. Alefacept selectively promotes NK cell-mediated deletion of CD45R0+ human T cells. Eur J Immunol. 2003;33:666–75. doi: 10.1002/eji.200323586. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–6. doi: 10.4049/jimmunol.176.1.211. [DOI] [PubMed] [Google Scholar]
- 23.Chamian F, Lowes MA, Lin SL, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci USA. 2005;102:2075–80. doi: 10.1073/pnas.0409569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauza K, Grassauer A, Hinterhuber G, et al. FcgammaRIII expression on cultured human keratinocytes and upregulation by interferon-gamma. J Invest Dermatol. 2002;119:1074–9. doi: 10.1046/j.1523-1747.2002.19527.x. [DOI] [PubMed] [Google Scholar]
- 25.Rentenaar RJ, Heydendael VM, van Diepen FN, de Rie MA, ten Berge IJ. Systemic treatment with either cyclosporin A or methotrexate does not influence the T helper 1/T helper 2 balance in psoriatic patients. J Clin Immunol. 2004;24:361–9. doi: 10.1023/B:JOCI.0000029107.47085.1b. [DOI] [PubMed] [Google Scholar]
- 26.Bohm I. Decrease of B-cells and autoantibodies after low-dose methotrexate. Biomed Pharmacother. 2003;57:278–81. doi: 10.1016/s0753-3322(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Shim JY, Kim JH, et al. Cyclosporine treatment decreases the percentage of cutaneous lymphocyte antigen (CLA)(+) CD4(+) T cells in children with severe atopic dermatitis. Allergy. 2004;59:1129–30. doi: 10.1111/j.1398-9995.2004.00639.x. [DOI] [PubMed] [Google Scholar]