Abstract
Toll-like receptor 4 (TLR4) is a member of the Toll-like receptor family, which can bridge innate and adaptive immune responses. Activation of the TLR4 signalling pathway may induce the release of proinflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-12, which was considered to play an important role in pathogenesis of immune-mediated diseases. Ankylosing spondylitis (AS) is an immune-mediated disease whose aetiology remains unknown. The aim of the study was to investigate the expression of TLR4 and serum TNF-α, IL-12 and soluble tumour necrosis factor-related apoptosis-inducing ligand (sTRAIL) level in AS patients. The results indicated that TLR4 protein and mRNA levels were significantly higher in AS patients than in healthy controls; however, there was no significant difference between human leucocyte antigen (HLA)-B27-positive and -negative AS patients, as well as serum levels of TNF-α, IL-12 and sTRAIL. In addition, in HLA-B27-positive AS patients, TLR4 level showed close associations with the cytokines and laboratory parameters of disease activity [erythrocyte sedimentation rate (ESR) and plasma C-reactive protein (CRP)], respectively. Similarly, the strong associations between the cytokines or between IL-12 and ESR or CRP were observed in HLA-B27-positive AS patients. Interestingly, in HLA-B27-positive AS patients, TNF-α correlated significantly with ESR, but did not with CRP. In contrast, sTRAIL correlated with CRP, but did not with ESR. Among HLA-B27-negative patients, no close correlation was found. In our study, it was suggested that the abnormal activation of TLR4 signalling and serum TNF-α, IL-12 and sTRAIL may play a key role in the development and progression of AS, which may be dependent on the status of HLA-B27 antigen.
Keywords: ankylosing spondylitis, IL-12, TNF-α, Toll-like receptor 4, TRAIL
Introduction
Ankylosing spondylitis (AS) is a chronic rheumatic disease characterized by axial skeletal ankylosing, inflammation at the insertions of tendons (enthesitis) and, occasionally, peripheral arthritis. Over time, the spinal and peripheral joint involvement of AS may cause severe disability and functional limitations. Its aetiology and pathogenesis are not yet understood fully, and the diagnosis is still difficult. As a result, the management and treatment of AS has always been unsatisfactory. Although AS shows a strong association with the major histocompatibility complex class I molecule human leucocyte antigen (HLA)-B27 hereditary, environmental and other factors, detailed associations remain unclear. Previous studies have shown that bacteria were thought to play a crucial role in the pathogenesis of AS. In animal models such as B27 transgenic rats, in ∼50% of the cases the presence of bacterial flora was obligatory for the development of inflammatory gut lesions and peripheral and axial inflammatory joint lesions, similar to AS. When raised in a germfree environment, inflammatory intestinal or joint disease does not develop until the normal bacterial flora are restored [1]. Similar to reactive arthritis, there is an indication that T cells play an important role in AS, and T cell responses to bacteria-derived antigens such as Klebsiella have been demonstrated in AS [2, 3].
Toll-like receptors (TLRs) are a family of such pattern-recognition receptors which discriminate pathogens from self and activate suitable defence mechanisms [4, 5]. TLRs on antigen-presenting cells also initiate and modulate adaptive immunity during infection [6]. TLR4 is a member of this family, which recognizes lipopolysaccharide (LPS), a major integral component of the outer membrane of Gram-negative bacteria. Activation of the TLR4 signalling pathway following binding ligand may induce the release of an array of proinflammatory cytokines and increase production of interleukin (IL)-12, a critical factor for generation of T helper 1 (Th1)-type responses. TLR4 has also been shown to be important for the pathogenesis of immune-mediated diseases, such as rheumatoid arthritis (RA) [7, 8], multiple sclerosis [9] and inflammatory bowel disease [10]. In addition, it has been reported that TLR4 may mediate the production of perforin by CD28null T helper cells in ankylosing spondylitis [11], while another report indicated that there was no evidence for involvement of the TLR4 A896G polymorphisms in susceptibility to AS [12]. However, previous studies demonstrated differences only between AS patients (n = 8) and healthy individuals at the level of TLR4 protein on peripheral-blood leucocytes in a minimum-sized sample, almost failing to include the level of TLR4 mRNA as well as the associations of TLR4 expression with serum proinflammatory cytokines in AS patients [13].
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a transmembrane (type II) glycoprotein, including membrane-binding TRAIL (mTRAIL) and soluble TRAIL (sTRAIL), which triggers apoptosis through interaction with the death receptors (DR) 4 and DR5 [14, 15]. It is known that TRAIL shows a close association with many autoimmune diseases, such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), RA and so on. However, it is still unclear whether TRAIL might play a role in pathogenesis of AS. Recently, although it has been reported that TLR4 agonists, Mycobacterium bovis bacillus Calmette–Guerin (BCG), could induce the release of soluble TRAIL from neutrophils [16], previous studies have failed to demonstrate the association of TLR4 with sTRAIL in AS.
Therefore, the present study was designed to determine the expression levels of TLR4 protein and mRNA in leucocytes and the serum levels of TNF-α, IL-12 and sTRAIL in 60 AS patients to determine whether the abnormal levels contribute to the development of AS.
Materials and methods
Subjects
The study was approved by the research ethics committee of our institution. After informed consent, the subjects were recruited from the in-patient and out-patient clinics of the Shanghai Changzheng Hospital. The study group consisted of 60 patients [38 HLA-B27-positive: 28 men and 10 women; mean ± standard deviation (s.d.), 30 ± 11 years; 22 HLA-B27-negative: 15 men and seven women; mean ± s.d., 36 ± 13 years] who fulfilled the modified 1984 New York criteria for a diagnosis of AS [17], and were excluded if they had a spondylitis other than AS, clinical or radiographic evidence of complete spinal ankylosis, a history of recurrent infections or cancer or a serious liver, renal, haematological or neurological disorder. The disease control consisted of 20 patients with RA (11 men and nine women; mean ± s.d., age 48 ± 15 years) who fulfilled the American College of Rheumatology criteria for RA [18]. Healthy volunteers (21 men and nine women; mean ± s.d., age 33 ± 8 years), with no AS history, AS family history or other diseases served as controls.
Analysis of expression of TLR4 protein
Flow cytometry (FCM) was performed using phycoerythrin (PE)-Cy5-labelled monoclonal antibody for human TLR4 (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. In brief, 100 μl whole blood was incubated with 20 μl PE-conjugated mouse anti-human TLR4 antibody for 30 min at room temperature in the dark. After incubation with the red blood cell (RBC) lysis buffer, the mixture was washed in staining buffer containing bovine serum albumin and sodium azide. Cells were resuspended in 500 μl staining buffer and analysed by flow cytometer (Beckman Coulter, Fullerton, CA, USA) as follows. First, gating was employed effectively in the use of forward and side light-scatter signals at different intensities for the selection of lymphocytes, monocytes and neutrophils within a mixed population of cells from peripheral blood, according to their heterogeneous characteristics. Secondly, the percentages of TLR4-positive cells were analysed in every gate. Negative control was prepared by incubating with an isotype-matched control antibody (IgG2a).
Peripheral blood mononuclear cell (PBMC) preparation and RNA extraction
PBMCs were isolated from peripheral venous blood of the subjects by Ficoll-Hypaque density gradient centrifugation. Total cellular RNA was extracted using the Trizol RNA extraction kit in accordance with the manufacturer's instructions. RNA yield and purity were determined spectrophotometrically at 260/280 nm.
Standard plasmid construction
For real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR), primers and Taqman probes were designed as described elsewhere [19] and synthesized by Shanghai Genecore Biotechnologies (Shanghai, China). The sequences are shown in Table 1. cDNA was synthesized by RT–PCR reactions using a SuperScript™ III Platinum® two-step quantitative RT–PCR kit (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. PCR products were purified through gel extraction. Ligation of the pGM-T plasmid vector and purified PCR fragment was performed with T4 DNA ligase (Promega, Madison, WI, USA). Plasmids were purified on columns with the Qiagen kit and quantified by A260 measurement. To control the validity of our primers, the amplified products were sequenced. Recombinant plasmid pGM-T-TLR4 was used as positive control and pGM-T-β-actin was used as endogenous control.
Table 1.
Primer and probe sequences of Toll-like receptor 4 (TLR4) and β-actin.
| Sequence | Amplicon size (base pairs) | |
|---|---|---|
| TLR4 | ||
| Sense-primer | 5′-TGGAAGTTGAACGAATGGAATGTG-3′ | 148 |
| Anti-sense-primer | 5′-ACCAGAACTGCTACAACAGATACT-3′ | |
| Probe | 5′-FAM-AGCACACTGAGGACCGACACACCAA-TAMRA-3′ | |
| β-actin | ||
| Sense-primer | 5′-AGATCAAGATCATTGCTCCTCCTG-3′ | 145 |
| Anti-sense-primer | 5′-CATTTGCGGTGGACGATGGA-3′ | |
| Probe | 5′-FAM-CGGACTCGTCATACTCCTGCTTGCTG-TAMRA-3′ |
Real-time quantitative RT–PCR
To prepare a standard curve, the recombinant plasmids were gradient diluted with sterilized water to 1 × 109, 108, 107, 106, 105, 104 and 103 copies μl. We synthesized cDNA from the extracted total RNA using the SuperScript™ III Platinum® two-step quantitative RT–PCR kit. Real-time quantitative PCR reactions were performed in an ABI-Prism 7000 sequence detector under the following cycling conditions: 50°C for 2 min, 95°C for 2 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Each reaction mixture (50 μl total volume) contained 4 μl cDNA template, 25 μl Platinum® Quantitative PCR SuperMix-UDG, 1 μl ROX, 1 μl sense primer, 1 μl anti-sense primer, 1 μl Taqman probe and 17 μl sterilized water. For each sample PCR reactions were performed in triplicate. The results were analysed by Sequence Detection Software (Applied Biosystems, Foster City, CA, USA). The level of gene expression was determined by interpolation with a standard curve (reference for the detail).
Determination of TNF-α, IL-12 and sTRAIL in serum
Serum samples were prepared immediately by centrifugation of peripheral venous blood. All cytokine levels were determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol: TNF-α and IL-12 kits were from Bender (Burlingane, CA, USA) and sTRAIL kit was from Diaclone (Besancon Cedex, France). Sensitivities of the various assays were as follows: TNF-α, 8 pg/ml; IL-12, 3 pg/ml and sTRAIL less than 64 pg/ml. Cytokine concentrations were determined by the optical densities obtained and the standard curves.
Determination of the erythrocyte sedimentation rate (ESR) and plasma C-reactive protein (CRP)
The ESR was determined by Auto ESR Analyser (Electa Laboratory, Forli, Italy) and the level of serum CRP was obtained by Specific Protein Analyser (Dade Behring, Deerfield, IL, USA).
Statistical analysis
Results were expressed as mean ± s.d. Student's t-test was made to test for differences in means between groups. The correlation of two different parameters within one group was calculated with the Spearman's rank correlation coefficient. P-values less than 0·05 were considered significant.
Results
Increased expression levels of TLR4 protein and mRNA in peripheral blood cells in AS patients
Leucocytes are well suited to flow analysis because the normal suspension of blood leucocytes contains a mixture of cell types that give off forward and side light-scatter signals of different intensities, thereby allowing them to be distinguished from each other by flow cytometric light scatter parameters. TLR4 protein expression on peripheral blood leucocytes was determined by FCM in this study. The results indicated that TLR4 was expressed mainly in human monocytes, and the positive rates of TLR4 on monocytes, lymphocytes and neutrophils were significantly higher in HLA B27-positive AS, HLA B27-negative AS or RA group than controls (each P < 0·001). When we compared the positive rates of TLR4 in the three types of cells between any two disease groups of the patients with HLA-B27-positive, -B27-negative AS and RA, no significant difference was found (Fig. 1).
Fig. 1.
The positive rates of Toll-like receptor 4 (TLR4) protein on peripheral blood cells. L = lymphocytes; M = monocytes; N = neutrophils. *P < 0·01 versus healthy controls.
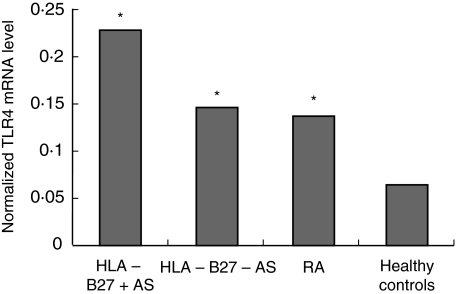
For each sample, copy numbers of TLR4 mRNA were divided by those of β-actin mRNA to normalize for TLR4 mRNA expression and thus avoid sample-to-sampledifferences in RNA quantity. The normalized TLR4 expression levels in patients with HLA B27-positive (0·23 ± 0·20; P < 0·001) and B27-negative AS (0·15 ± 0·13; P < 0·001) and RA (0·14 ± 0·12; P < 0·001) were all increased significantly compared with controls, but there was no significant difference between the two AS groups. (shown in Fig. 2)
Fig. 2.
The normalized Toll-like receptor 4 (TLR4) mRNA level in peripheral blood mononuclear cells. P < 0·01 versus healthy controls.
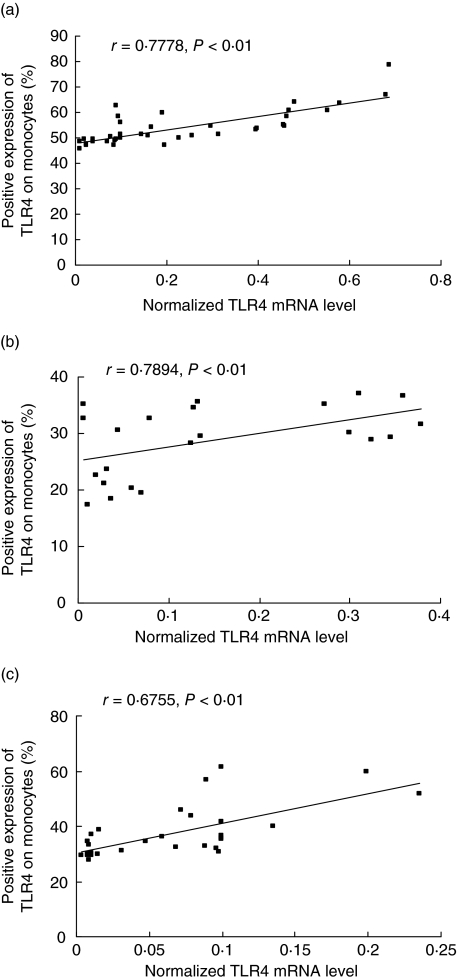
The normalized TLR4 mRNA expression showed a close association with TLR4 protein expression on monocytes in both AS groups and controls (each P < 0·01) (Fig. 3).
Fig. 3.
The association of Toll-like receptor 4 (TLR4) mRNA with the expression of TLR4 protein on monocytes from various populations. [a, b and c for human leucocyte antigen (HLA)-B27+, HLA-B27– ankylosing spondylitis (AS) patients and healthy controls, respectively].
Increased ESR and levels of serum cytokines and CRP in AS patients
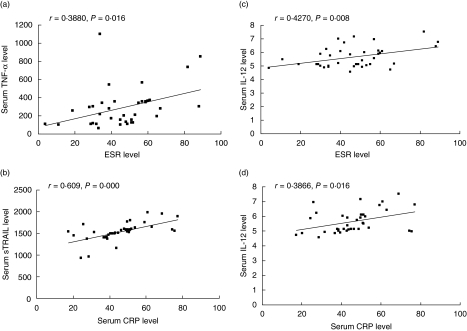
The serum concentrations of TNF-α, IL-12 and sTRAIL were elevated significantly in AS patients compared to healthy controls (231·242 ± 193·531 versus 15·247 ±2·646 pg/ml, P < 0·001; 5·573 ± 0·714 versus 4·385 ±0·778 pg/ml, P < 0·001 and 1514·375 ± 261·836 versus 936·992 ± 239·53 pg/ml, P < 0·001, respectively, for TNF-α, IL-12 and sTRAIL), while in RA patients only the serum concentration of TNF-α was significantly up-regulated (147·79 ± 70·93 pg/ml) and no significant differences for IL-12 (4·50 ± 1·54 pg/ml) and sTRAIL (1067·01 ±280·93 pg/ml) were observed, compared with healthy controls. In AS patients, HLA-B27-positive patients showed higher TNF-α serum concentrations than HLA-B27-negative patients (290·624 ± 223·292 versus 129·296 ±50·549 pg/ml, P < 0·001). However, there was no significant difference for IL-12 and sTRAIL between HLA-B27-positive and -negative patients (Table 2). Among HLA-B27-positive patients, there were significant correlations between TNF-α and IL-12 and sTRAIL, respectively. However, with the exception of IL-12 and sTRAIL (r = 0·483, P = 0·023), the above correlations observed in HLA-B27-positive patients were not observed in HLA-B27-negative patients. HLA-B27-positive patients showed significantly increased ESR compared to the HLA-B27-negative patients (46·47 ± 18·53 versus 30·82 ± 18·16, P = 0·003), and both were significantly higher than healthy controls. There were elevated levels of serum CRP in HLA-B27-positive patients compared to the HLA-B27-negative patients and controls. However, no significant difference was observed between the last two groups (Table 2). As disease control, RA patients showed higher levels of ESR and CRP than healthy controls. When we studied the correlations between cytokines and ESR or CRP, IL-12 was correlated strongly with ESR (r = 0·427, P = 0·008) and CRP (r = 0·3866, P = 0·016) in HLA-B27-positive patients. Surprisingly, HLA-B27-positive patients showed a close association of TNF-α with ESR (r = 0·388, P = 0·016) but not with CRP; and in contrast, of sTRAIL with CRP (r = 0·609, P = 0·000) but not with ESR (Fig. 4). Among HLA-B27-negative patients, no strong correlation was seen between various cytokines and ESR or CRP.
Table 2.
The level of erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP) and cytokines in populations.
| Healthy controls (n = 30) | Disease controls (n = 20) | HLA-B27-negative patients (n = 22) | HLA-B27-positive patients (n = 38) | |
|---|---|---|---|---|
| TNF-α (pg/ml) | 15·25 ± 2·65 | 147·79 ± 70·93** | 129·30 ± 50·55** | 290·62 ± 223·29** |
| IL-12 (pg/ml) | 4·39 ± 0·78 | 4·50 ± 1·54 | 5·43 ± 0·60** | 5·67 ± 0·78** |
| sTRAIL (pg/ml) | 936·99 ± 239·53 | 1067·01 ± 280·93 | 1474·16 ± 329·40** | 1537·66 ± 214·99** |
| ESR | 7·25 ± 3·68 | 41·90 ± 19·10** | 30·82 ± 18·16 | 46·47 ± 18·53 |
| CRP | 5·38 ± 2·99 | 37·04 ± 25·74** | 6·18 ± 3·27 | 46·01 ± 14·45** |
P < 0·01 versus healthy controls. HLA: human leucocyte antigen; TNF: tumour necrosis factor; IL: interleukin; sTRAIL: soluble tumour necrosis factor-related apoptosis-inducing ligand.
Fig. 4.
Relationship between serum cytokines and laboratory parameters of disease activity in human leucocyte antigen (HLA)-B27-positive patients with ankylosing spondylitis (AS).
Correlation studies between expression of TLR4 protein on monocytes or mRNA and serum cytokines or laboratory parameters of disease activity in AS
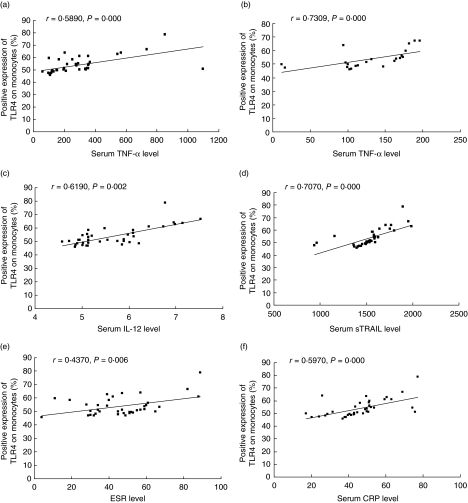
When correlation studies were performed, close correlations of expression of TLR4 protein on monocytes and TLR4 mRNA with serum TNF-α were observed in either HLA-B27-positive (r = 0·589, P = 0·000 and r = 0·555, P = 0·001, respectively, for TLR4 protein and mRNA) or HLA-B27-negative (r = 0·619, P = 0·002 and r = 0·601, P = 0·003, respectively, for TLR4 protein and mRNA) AS patients. Close correlations between TLR4 mRNA or protein and IL-12 (r = 0·552, P = 0·001 or r = 0·7309, P = 0·000), sTRAIL (r = 0·508, P = 0·001 or r = 0·707, P = 0·000), ESR (r = 0·471, P = 0·003 or r = 0·437, P = 0·006) or CRP (r = 0·569, P = 0·000 or r = 0·597, P = 0·000) were found in HLA-B27-positive AS patients, but no correlation was found in the HLA-B27-negative patients (Fig. 5)
Fig. 5.
The correlations of expression of Toll-like receptor 4 (TLR4) protein on monocytes with erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP) and cytokines [B for human leucocyte antigen (HLA)-B27– ankylosing spondylitis (AS) patients, others for HLA-B27+ AS patients].
Discussion
The present data demonstrate that the expression of TLR4 on lymphocytes, monocytes and neutrophils is all increased significantly in either patients with AS (HLA-B27-positive and-negative) or RA patients, compared with healthy individuals, although it is expressed mainly on monocytes. This result extends a previous study, which showed increased expression of TLR4 on total PBMCs from only eight patients with AS [13]. Furthermore, in our study the level of TLR4 mRNA in PBMCs was also determined by real-time PCR, and a significant increase was found in both HLA-B27-positive and -negative patients with AS, compared with healthy individuals. Although AS shows a strong association with HLA-B27, its value as a diagnostic tool for early AS has been a subject of long-standing debate that has not yet been clarified, and in several studies the advantages and disadvantages of HLA-B27 as a diagnostic test for AS have been discussed [20].The current study indicates that increased expression of TLR4 in PBMC may play a role in the pathogenesis of AS, independent of HLA-B27. However, the close correlations between the level of TLR mRNA or protein and ESR or CRP are clearly influenced by HLA-B27, as HLA-B27-positive AS patients showed a close correlation but the HLA-B27-negative patients did not.
It has been known that upon stimulation by LPS, macrophages secrete proinflammatory cytokines, including IL-12 and TNF-α by the activated TLR4 signalling pathway [21]. TNF-α is a potent proinflammatory immunomodulatory cytokine produced by monocytes/macrophages, which plays a central role in the initiation and regulation of the immune response, exerting both beneficial and deleterious effects. A finding showed significantly higher TNF-α serum levels in patients with AS compared with healthy individuals and patients with non-inflammatory back pain [22, 23]. Recent trials have shown a positive benefit of anti-TNF treatment in both PsA [24] and AS [25]. IL-12 is an inducer of the Th1 subset that is of major importance in RA. IL-12 is a key factor in the induction of T cell-dependent and -independent activation of macrophages, generation of Th1 and cytotoxic T cells and induction of organ-specific autoimmunity [26]. However, to date, the association of IL-12 with AS and its role in the pathogenesis of AS are still unknown. In our study, we found that TNF-α and IL-12 are elevated in either HLA-B27-positive or -negative AS patients, and in HLA-B27-positive patients there is a close correlation between these two cytokines. In HLA-B27-positive patients the expression of TLR4 mRNA or protein shows strong associations, respectively, with TNF-α and IL-12. Furthermore, the HLA-B27-negative AS patients also show a clear association between TLR4 and TNF-α. However, in RA patients only TNF-α shows a significant difference as described in previous studies and IL-12 does not, when compared with healthy controls.
There are several reasons why TRAIL could be involved in the pathophysiology of autoimmune diseases. In patients with RA, sTRAIL are elevated from synovial fluids [27]. In patients with multiple sclerosis, FasL and TRAIL are up-regulated in peripheral blood mononuclear cells [28]. In patients with Sjögren's syndrome, DR4 and DR5 are expressed strongly on the ductal epithelial cells in the salivary glands [29]. Recently it has been reported that increased expression of TRAIL and FasL found on activated T cells contributes to increased monocyte apoptosis in patients with SLE. Furthermore, increased TRAIL mRNA in peripheral blood mononuclear cells and soluble TRAIL concentrations in sera were found in SLE patients [30, 31]. In this study, we explored the association between serum sTRAIL and AS. The results indicated that the serum sTRAIL level is up-regulated in AS patients and there are clear associations with CRP, TNF-α, IL-12 and TLR4 mRNA or protein in HLA-B27-positive AS patients. However, the serum sTRAIL level is not up-regulated significantly in RA patients, and we propose that sTRAIL may enter mainly into synovial fluid, involved in apoptosis of synovial fibroblasts. These results have demonstrated that as far as sTRAIL and IL-12 are concerned, there might be a difference between the mechanisms of AS and RA.
Taken together, our study has three important implications. First, the abnormal activation of TLR4 signalling, including increased levels of TLR4 mRNA and protein, serum TNF-α and IL-12 may correlate significantly with the development and progression of AS. Secondly, in AS patients there is a close association between TLR4 and serum sTRAIL, both of which may play a role in the pathogenesis of AS. Thirdly, associations of TLR4 signalling, TNF-α, IL-12 and sTRAIL with AS may be dependent upon HLA-B27 antigen status. However, the molecular mechanism driving TLR4 overexpression and detailed associations of AS with the TLR4 signalling pathway, TRAIL and HLA-B27 antigen remain to be clarified.
Acknowledgments
This study was supported by a grant from the China National Natural Science Foundation Council (30471616).
References
- 1.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollow M, Fischer T, Reisshauer H, et al. Quantitative analysis of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroilitis-cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–40. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermann E, Sucke B, Droste U, et al. Klebsiella pneumoniae- reactive T cells in blood and synovial fluid of patients with ankylosing spondylitis. Comparison with HLA-B27+ healthy control subjects in a limiting dilution study and determination of the specificity of synovial fluid T cell clones. Arthritis Rheum. 1995;38:1277–82. doi: 10.1002/art.1780380916. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya SA, Cheng G. Toll-like receptors and innate antiviral responses. Curr Opin Immunol. 2003;15:402–7. doi: 10.1016/s0952-7915(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 6.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 7.Roelofs MF, Boelens WC, Joosten LA, et al. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176:7021–7. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 8.Radstake TR, Roelofs MF, Jenniskens YM, et al. Expression of Toll-like receptor 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–65. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 9.Kerfoot SM, Long EM, Hickey MJ, et al. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7. doi: 10.4049/jimmunol.173.11.7070. [DOI] [PubMed] [Google Scholar]
- 10.Shi D, Das J, Das G. Inflammatory bowel disease requires the interplay between innate and adaptive immune signals. Cell Res. 2006;16:70–4. doi: 10.1038/sj.cr.7310009. [DOI] [PubMed] [Google Scholar]
- 11.Raffeiner B, Dejaco C, Duftner C, et al. Between adaptive and innate immunity: TLR4-mediated perforin production by CD28null T-helper cells in ankylosing spondylitis. Arthritis Res Ther. 2005;7:1412–20. doi: 10.1186/ar1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Paardt M, Crusius JB, de Koning MH, et al. No evidence for involvement of the Toll-like receptor 4 (TLR4) A896G and CD14-C260T polymorphisms in susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2005;64:235–8. doi: 10.1136/ard.2004.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rycke LD, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor-α blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondyloarthropathy. Arthritis Rheum. 2005;52:2146–58. doi: 10.1002/art.21155. [DOI] [PubMed] [Google Scholar]
- 14.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc HN, Ashkenazi A. Apo 2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 16.Kemp TJ, Ludwig AT, Earel JK, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette–Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–82. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987: revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Geng HL, Lu HQ, Zhang LZ, et al. Increased expression of Toll-like receptor 4 on peripheral-blood mononuclear cells in patients with coronary arteriosclerosis disease. Clin Exp Immunol. 2006;143:269–73. doi: 10.1111/j.1365-2249.2005.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Sun R, Wei HM, Tian ZG. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of Toll-like receptor 4 expression on macrophages. Proc Natl Acad Sci USA. 2005;102:17077–82. doi: 10.1073/pnas.0504570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gratacos J, Collado A, Filella X, et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994;33:927–31. doi: 10.1093/rheumatology/33.10.927. [DOI] [PubMed] [Google Scholar]
- 23.Imrich R, Rovensky J, Zlnay M, et al. Hypothalamic–pituitary–adrenal axis function in ankylosing spondylitis. Ann Rheum Dis. 2004;63:671–4. doi: 10.1136/ard.2003.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 25.Brandt J, Haibel H, Cornely D, et al. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum. 2000;43:1346–52. doi: 10.1002/1529-0131(200006)43:6<1346::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 27.Jungel A, Baresova V, Ospelt C, et al. Trichostatin A sensitizes rheumatoid arthritis synovial fibroblasts for TRAIL-induced apoptosis. Ann Rheum Dis. 2006;65:910–12. doi: 10.1136/ard.2005.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang WX, Huang MP, Gomes MA, Hillert J. Apoptosis mediators FasL and TRAIL are upregulated in peripheral blood mononuclear cells in MS. Neurology. 2000;55:928–34. doi: 10.1212/wnl.55.7.928. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura R, Umemiya K, Kagami M, et al. Expression of TNF-related apoptosis inducing ligand (TRAIL) on infiltrating cells and of TRAIL receptors on salivary glands in patients with Sjögren's syndrome. Clin Exp Rheumatol. 2002;20:791–8. [PubMed] [Google Scholar]
- 30.Kaplan MJ, Lewis EE, Shelden EA, et al. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–9. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 31.Lub-de Hooge MN, de Vries EGE, de Jong S, Bijl M. Soluble TRAIL concentrations are raised in patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64:854–8. doi: 10.1136/ard.2004.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]