Abstract
Hydroxyurea (HU) is a cytostatic drug which has been used as an anti-HIV agent due mainly to its synergistic activity when combined with certain anti-retrovirals. In addition, HU might have a beneficial effect on parameters involved in the pathogenesis of HIV infection, such as immune activation. To test this hypothesis, the effect of HU on T cell proliferation and T cell activation, as well as the potential association between these two phenomena, were examined in an in vitro model. HU exerted a dose-dependent anti-proliferative effect on T cells, and modulated the expression of different activation markers. In cells exposed to HU, expression of CD25 and CD38 diminished in a dose-dependent manner, whereas expression of CD69 increased. However, when the expression of these markers was examined separately on proliferating and non-proliferating lymphocytes, HU did not exert any significant effect. Thus, the effect of HU on T cell activation is not direct and seems to be mediated through its effect on T cell proliferation.
Keywords: HIV, hydroxyurea, T cell activation, T cell proliferation
Introduction
Hydroxyurea (HU) is a cytostatic drug which has been used widely for the treatment of certain neoplasms [1]. It inhibits the cellular enzyme ribonucleotide reductase, which is involved in the synthesis of deoxynucleoside triphosphates (dNTPs). As a consequence, DNA synthesis is blocked and the cell cycle is arrested in the early S phase [2]. The use of HU as an anti-HIV agent has been explored, given its ability to inhibit HIV-1 replication indirectly [3] and because it exerts a synergistic anti-viral activity with some nucleoside reverse transcriptase inhibitors (NRTI), especially didanosine (ddI) [4, 5].
An immune-modulatory effect of HU derived from its cytostatic action has also been postulated. Hypothetically, the use of a cytostatic drug would help T lymphocytes to remain quiescent, becoming refractory to productive HIV infection. This phenomenon has been named as the ‘predator–prey’ hypothesis [6], in which the lower the number of cells available for HIV to replicate, the easier the viral load to be controlled [7]. Several studies have confirmed this hypothesis in the clinical setting, as the association of HU plus ddI is generally able to provide long-term control of virus replication [8, 9].
Finally, HU may have a beneficial effect on parameters involved in the pathogenesis of HIV infection, such as immune activation [9]. In a recent study, we have shown that patients treated with the combination of HU plus ddI had lower levels of T cell activation than patients treated with a standard triple anti-retroviral drug regimen [10]. Moreover, in a protocol of repeated structured treatment interruptions, we noted that T cell activation tended to wane over time, even though episodes of viral rebound were of similar magnitude in each period of therapy in only a group of patients exposed to HU plus ddI [11]. Taken together, these results suggest that HU may exert an effect on T cell activation independent of its anti-viral effect. Herein, we examine in vitro the effect of HU on T cell proliferation and T cell activation, as well as any interaction between these two phenomena.
Materials and methods
Cell samples and viral culture conditions
Peripheral blood mononuclear cells (PBMC) collected from healthy blood donors were isolated from buffy coats by density gradient centrifugation using Ficoll-Hypaque (Sigma Chemical Co., St Louis, MO, USA) and frozen in fetal calf serum (FCS) plus 10% dimethylsulphoxide (DMSO). The viability of thawed PBMC was always greater than 85%. All results were generated from at least three different experiments using samples from different donors. Additionally, PBMCs from six different HIV-infected patients, who were being treated with HU and belonging to a previously reported anti-retroviral therapy simplification protocol [11], were also included in the experiments. Of the six patients, two patients had CD4 counts below 500 cells/µl and the rest above 500 cells/µl. Plasma viral load was detectable in three of them and undetectable in the remainder. Written informed consent was obtained from both healthy donors and HIV patients, and the experimental protocol was approved by the hospital's ethical advisory board.
Cells were cultured in RPMI-1640 medium containing 10% FCS, l-glutamine and penicillin–streptomycin (R10) and stimulated with phytohaemagglutinin (PHA) at a concentration of 1 µg/ml. Cells were cultured for 3–10 days, depending on the type of experiment.
T cell proliferation assay
To evaluate the effect of HU on the ability of T cells to proliferate, 2 × 105 PBMC from blood donors were cultured in 200 µl of R10 in 96-well culture plates, which were stimulated with PHA at 1 µg/ml for 3 days in the absence or presence of HU at three different concentrations: 10, 100 and 1000 µM. This range of HU concentrations was chosen based on the plasma concentrations generated after the oral administration of the drug at doses used typically in HIV infection (500 mg twice daily). Two different methods were employed in parallel to measure the proliferative capacity of T cells in response to PHA; first, using dilution of the carboxyfluorescein succinimidyl ester (CFSE) content, and secondly using the standard [3H]-thymidine incorporation assay.
Dilution of the CFSE content
PBMC were CFSE-labelled by incubation in phosphate-buffered saline (PBS) at a density of 107 cells/ml with 1 µM CFSE for 20 min at 37°C. Thereafter, cells were washed five times in R10, counted and resuspended in R10. CFSE labelling was confirmed by flow cytometry at the moment of initiating the cell culture. At the end of culture (3 days), cells were harvested from the plate, washed in PBS and resuspended in 100 µl for surface staining with CD3 monoclonal antibody. After staining, cells were analysed by multi-parameter flow cytometry using an EPICS XL flow cytometer (Coulter, Madrid, Spain). The percentage of T cells (CD3+) under each division peak (as estimated by CFSE intensity) as well as the absolute number of CD3+ cells recovered were recorded. From these values two different parameters were calculated for data analysis using a previously reported method [12]: the precursor frequency and the mean number of mitosis.
To estimate the precursor frequency, the absolute number of T cells in each division peak was first calculated (daughter cells). The absolute number of original or precursor cells required to originate these daughters was then extrapolated by dividing the number of daughters at n divisions by a factor of 2n. The proportion of the original T cell input (at the beginning of culture) that was able to divide one or more times (precursor frequency) was then determined as the ratio between the number of original T cells able to divide at least once and the total number of cells (those dividing and those not dividing). The mean number of mitosis was calculated for the population of precursor cells that divided at least once using the equation:
>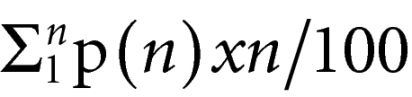
|
, where p(n) is the proportion of precursor cells that divided n times (n ≥ 1).
[3H]-thymidine incorporation assay
On the second day of viral culture, plates were pulsed with 1 µCi/well of tritiated thymidine for 18 h, harvested and radioactivity counted in a beta-counter. The stimulation index was calculated by dividing thymidine incorporation in the presence of stimulus × incorporation in the absence of stimulus. The percentage of proliferation was calculated setting the stimulation index in the absence of HU as 100%.
T cell activation markers
The expression of activation markers CD69, CD25 and CD38 was analysed at different time-points after stimulation with PHA. In a first series of experiments (short-term culture experiments), the expression of CD69 and CD25 by CD3+ cells was measured at 24, 48 and 72 h after stimulation in the absence or presence of HU at 10, 100 and 1000 µM. In order to accommodate the later expression activation marker CD38, a second set of experiments (long-term culture experiments) was performed, and the expression of CD69, CD25 and CD38 was measured at 3, 5, 7 and 10 days using the same experimental conditions. In all these experiments HU was added at the beginning of culture. Finally, the effect of adding HU at different time-points on the expression of activation markers was analysed. For this purpose, the expression of CD69, CD25 and CD38 was measured at day 3 in the absence or presence of HU 10, 100 and 1000 µM addedat days 0, 1 or 2. Moreover, the effect on activation markers of adding HU at day 3 was checked at day 7 of culture.
In all these experiments, cells were harvested from the plates, washed with PBS, resuspended in 100 µl and stained with the next panel of monoclonal antibodies: CD3-PE-Texas Red tandem (ECD), CD25-fluorescein isothiocyanate (FITC), CD69-phycoerythrin (PE) and CD38-PECy5. Samples were analysed with a Coulter EPICS XL flow cytometer.
Relationship between T cell proliferation and T cell activation
In order to investigate the interdependence of T cell proliferation and T cell activation, as well as the effect of HU on these two phenomena, PBMC were CFSE-stained, stimulated with PHA and after 3 days of culture, harvested and stained with the next panel of monoclonal antibodies: CD3-ECD, CD69-PE, CD38-PECy5, and CD3-ECD, CD25-PE and CD38-PECy5. The level of expression of these activation markers was evaluated in both small and large (lymphoblasts) lymphocytes, as well as in cells undergoing at least one mitosis and in quiescent cells which did not undergo mitosis.
Results
Effect of hydroxyurea on T cell proliferation
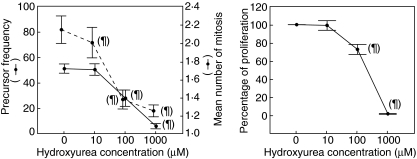
As shown in Fig. 1, HU exerted a dose-dependent anti-proliferative effect on T lymphocytes from normal blood donors, with similar results found in HIV-infected patients. This was reflected in a decrease of both the precursor frequency and the mean number of mitosis per dividing cell. The effect on precursor frequency was significant only at 100 and 1000 µM. However, the mean number of mitosis was already diminished slightly but significantly with HU 10 µM. Data obtained with CFSE staining paralleled what was obtained using the more traditional approach of [3H]-thymidine uptake (Fig. 1). Using this technique, however, the effect was manifest only at 100 µM, with a reduction in the stimulation index to 70% compared to baseline (in the absence of HU).
Fig. 1.
Effect of hydroxyurea (HU) upon the proliferative ability of lymphocytes in response to phytohaemagglutinin stimulation, as evaluated by carboxyfluorescein succinimidyl ester staining (left graph) and by [3H]-thymidine uptake (right graph). Each point on the graphs represents the mean value of at least three different experiments. ¶P < 0·05 for the difference with respect to HU 0.
Effect of hydroxyurea on T cell activation
The effect of different concentrations of HU on the expression of activation markers was analysed in detail. For this purpose, one early (CD69) and two late (CD25 and CD38) activation markers were selected. The expression of CD69 and CD25 was monitored every 24 h during a short-term (3 days) culture. Additionally, the expression of CD25, CD69 and CD38 was monitored at different time-points during a 10-day culture. Figures of results obtained using normal donors PBMCs are shown, with similar results obtained when using PBMCs from HIV patients.
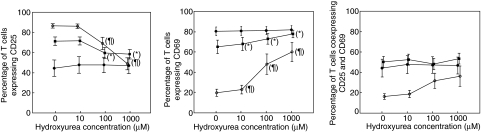
Figure 2 shows the results obtained in the short-term (3 days) culture. In the absence of HU, expression of CD25 increased over time and was maximal at day 3, whereas expression of CD69 was maximal at day 1 and decreased thereafter. Co-expression of both markers was similar at days 1 and 2, and decreased at day 3. HU significantly diminished the expression of CD25 at days 2 and 3 only at high concentrations (100 and 1000 µM). In contrast, HU significantly increased CD69 expression in a dose-dependent manner at day 3, the effect being already manifest with 10 µM of HU. The co-expression of both markers did not changesignificantly with HU, due most probably to the divergent effect exerted on them separately.
Fig. 2.
Effect of hydroxyurea (HU) on the expression of activation markers CD25 and CD69 by T cells at different time-points after phytohaemagglutinin stimulation: day 1 (•), day 2 (▪) and day 3 (○). Each point on the graphs represents the mean value of at least three different experiments. *P < 0·05 for the difference with respect to HU 0 at day 2. ¶P < 0·05 for the difference with respect to HU 0 at day 3.
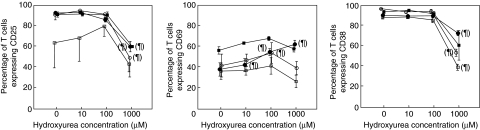
Figure 3 shows the results of the long-term (10 days) culture. In the absence of HU, CD25 remained elevated during the first 7 days and decreased at day 10. Levels of CD69 and CD38 were similar throughout the whole culture period, CD38 being the marker with the highest expression level. HU induced a significant decrease of CD25 expression at days 3, 5 and 7, but only at the highest concentration. Expression of CD69 increased in a dose-dependent manner at day 3, the effect at days 5, 7 and 10 not so clear-cut. On the other hand, CD38 expression decreased significantly at days 3, 7 and 10 only with the highest HU concentration.
Fig. 3.
Effect of hydroxyurea (HU) on the expression of activation markers CD25, CD69 and CD38 by T cells at different time-points after phytohaemagglutinin stimulation: day 3 (•), day 5 (▪), day 7 (○) and day 10 (□). Each point on the graphs represents the mean value of at least three different experiments. ¶P < 0·05 for the difference with respect to HU 0.
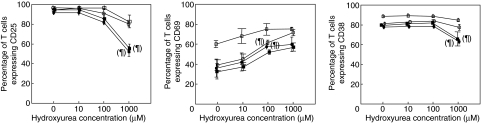
In all these experiments, HU was added at the initiation of culture. The effect of adding HU at different time-points after starting the culture was also investigated (Fig. 4). HU was added at days 0, 1 or 2, and activation markers measured at day 3. In addition, HU was added at day 3 and markers examined at day 7. Expression of CD25 significantly decreased only with the highest HU dose, when the drug was added at days 0 or 1. A similar effect was seen for CD38. In contrast, CD69 expression increased in a dose-dependent manner when HU was added at days 0, 1 or 2, although the increase was significant only with HU 100 µM added at days 0 or 2.
Fig. 4.
Effect of hydroxyurea (HU) on the expression of activation markers CD25, CD69 and CD38 by T cells at different time-points after phytohaemagglutinin stimulation. HU was added to the culture at day 0 (•), day 1 (▪) or day 2 (○) and the expression of activation markers measured at day 3. Additionally HU was added at day 3 (□) and the analysis was carried out at day 7. ¶P < 0·05 for the difference with respect to HU 0.
Relationship between T cell proliferation and T cell activation
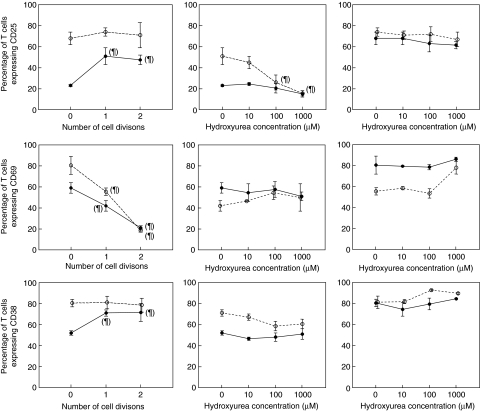
A series of experiments was conducted in order to assess to what extent the effect of HU on T cell activation was a consequence of its effect on T cell proliferation. PBMC were CFSE-stained and stimulated with PHA for 3 days. Cells were harvested and surface-stained with CD3, CD25, CD69 and CD38. Expression of activation markers was evaluated in small and large (blast) cells, as well as in dividing and non-dividing cells (Fig. 5). In the absence of HU, expression of CD25 and CD38 in large cells was maintained at high levels regardless of the division history of cells, whereas in small cells both markers up-regulated expression significantly only in cells that had divided at least once. CD69 expression decreased significantly after cell division in both small and large cells, and continued to decrease with successive divisions.
Fig. 5.
Relationship between T cell proliferation and activation. Left column: effect of cell division of small (•) and large (○) T cells on the expression of activation markers CD25, CD69 and CD38 after 3 days of phytohaemaglutinin (PHA) stimulation. Middle and right columns: effect of hydroxyurea (HU) on the expression of activation markers CD25, CD69 and CD38 by T cells after PHA stimulation in small (middle) and large (right) cells, non-dividing (•) or dividing (○) once. ¶P < 0·05 for the difference with respect to 0 divisions in the left column or with respect to HU 0 in the middle and right columns.
In small non-dividing cells, CD25 was the marker with the lowest expression, with CD69 and CD38 being expressed at similar levels. HU did not have any effect on their level of expression in this cell population. A similar effect was seen in small dividing cells, except for a slight decrease of CD25 expression at high HU concentrations (Fig. 5, middle column). In large cells, expression of CD25 and CD38 was high in both non-dividing and dividing cells, and HU did not have any effect on their expression. However, CD69 expression was down-modulated in large cells after cell division and, again, HU had no effect on its expression (Fig. 5, right column). Similar results were obtained when using PBMCs from HIV-infected patients.
Discussion
In this study we have analysed the effects of HU, a cytostatic agent, on T cell proliferation and activation, as well as the relationship between these two phenomena. Although HU was used for the treatment of HIV infection for a while, based mainly on its synergystic effect when associated with certain anti-retrovirals such as didanosine [4, 5], an additional beneficial effect has therefore been proposed based on its cytostatic action as well as its potential effect on T cell activation [13]. In our study, a dose-dependent effect on T cell proliferation was noticed, being already manifested as a decrease in the mean number of mitosis at a concentration of 10 µM. As this is a concentration that is presumably achieved in vivo in patients treated with the drug [14], our data suggest that a decrease in the proliferative potential of T cells is most probably operating in patients treated with HU. This is supported by the fact that one of the most common side effects of HU therapy in HIV-infected patients is a diminished ability to reconstitute CD4+ T cells compared to individuals treated with HU-sparing regimens [5, 15, 16]. Although this anti-proliferative effect on cells of the immune system could theoretically have a negative impact by diminishing the ability to mount an effective immune response against newly encountered pathogens and/or abolish pre-existing responses, several studies have shown that this is not the case [17, 18].
In this study, a significant effect of HU on the level of expression of three different activation markers was recognized both in short- and long-term cultures. HU down-regulated the expression of CD25 and CD38, although with different intensity. CD25 expression was already decreased at 100 µM, and this effect increased with the highest dose, especially at day 3 of culture. Expression of CD38, however, decreased only with the highest HU dose (1000 µM). At least for CD25, these results may have in vivo implications, as 100 µM is a dose attainable in patients treated with HU 500 mg twice daily [14]. Interestingly, the expression of the early activation marker CD69 was not diminished but increased in cells exposed to HU, and this effect was already manifest with the lowest HU dose. In a recent study, Lova et al., using a similar in vitro system, did not observe any significant effect of HU on the expression of CD25, human leucocyte antigen D-related (HLA-DR) or CD69 [18]. Most probably, these seemingly contradictory results might be explained by the fact that expression of activation markers in that study was measured at a single time-point (day 2) after PHA stimulation and using a single HU dose (100 µM). In our study we found a small (although significant) increase in the expression of CD69 and decrease in the expression of CD25 at that same time-point. In fact, in the study by Lova et al., there was a small increase in CD69 and an almost negligible effect in CD25 expression on CD4+ T cells 2 days after PHA stimulation, although differences were not significant. Curiously, the trend for CD69 markers on CD8+ cells was opposite to that seen on CD4+ cells. However, we did not check separately the effects on CD4+ and CD8+ T cells.
The in vivo implications of these findings are relevant, considering that T cell activation is one of the main mechanisms involved in the pathogenesis of CD4+ depletion in HIV-infected individuals. The level of activation is correlated positively with plasma viral load and decreases when viral replication is controlled with anti-retroviral therapy [19]. Moreover, it is associated with the level of T cell apoptosis [20, 21] and is predictive of subsequent CD4+ T cell loss [22], independently of plasma viral load [23]. In some studies, only T cell activation and not plasma viral load was associated significantly with CD4+ T cell loss [24]. Furthermore, we and others have shown that in patients on highly active anti-retroviral therapy (HAART), the level of activation influences the ability to reconstitute CD4+ T cells [25–27]. Assuming that in vitro results may be extrapolated to the in vivo situation, our results support that HU could have a significant effect on T cell activation beyond its anti-viral activity when used in combination with anti-retrovirals. It has to be pointed out, however, that the in vitro effect on CD38 was significant only with the highest HU dose, which is far beyond HU plasma levels in patients treated with the drug [14]. Moreover, the effect on CD69 was opposite to that observed on CD25 and CD38. Thus, from our in vitro results it is difficult to envisage the net in vivo effect of HU on T cell activation.
Studies in patients treated with the combination of HU plus anti-retrovirals have shown that different immune parameters (including T cell activation) are normalized [9, 28], although this is most probably a consequence of suppressing viral replication, and therefore an independent effect of HU on T cell activation is difficult to prove. In a more recent study conducted in patients treated with a standard HAART regimen, the effect of adding HU to anti-retrovirals was examined. T cell activation was normalized equally in both the HU and the placebo arms [17], suggesting that in the setting of complete viral suppression, HU do not provide any additional benefit on T cell activation. However, this may not be the case in situations where viral replication is not suppressed completely. In these situations, HU may have a beneficial effect on immune parameters, as we have shown recently during a protocol of structured treatment interruptions in patients receiving ddI plus HU [11].
To what extent the effect of HU on T cell activation is driven by its cytostatic action? To our knowledge this has not yet been explored. In the present study, a clear association between the cytostatic activity of HU and its effect on T cell activation was demonstrated, and no effect of HU on activation independently of its cytostatic action was noticed. As Fig. 5 shows clearly, cell activation can be modulated by cell division, and HU had no effect (even at the highest dose) on cell activation when immune markers were analysed separately on dividing and non-dividing cells. Moreover, this interdependence between cell activation and cell division explains the opposite effect of HU on CD25 or CD38 versus CD69. Whereas CD25 and CD38 tend to increase with cell division, CD69 is down-regulated after mitosis. Thus HU, by inhibiting cell division, decreases expression of CD25 and CD38 indirectly, while it increases CD69 expression.
In summary, we have demonstrated that HU, at doses that may be achieved in vivo, has an anti-proliferative effect on T cells and influences the expression of different activation markers in multiple ways, depending on each marker. This latest action, however, is not direct and is mediated through its inhibitory effect on cell division.
Acknowledgments
This work was supported in part by grants from FIS (ISCIII-RETIC RD06/006), FIPSE, AIES and CAM.
References
- 1.Donehower R. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19:11–9. [PubMed] [Google Scholar]
- 2.Yarbro J. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 3.Lori F, Malykh A, Cara A, et al. Hydroxyurea as inhibitor of HIV type 1 replication. Science. 1994;266:801–5. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- 4.Gao W, Cara A, Gallo R, Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit HIV type 1 replication. Proc Natl Acad Sci USA. 1993;90:8925–8. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lori F, Malykh A, Foli A, et al. Combination of a drug targeting the cell with a drug targeting the virus controls HIV type 1 resistance. AIDS Res Hum Retroviruses. 1997;13:1403–9. doi: 10.1089/aid.1997.13.1403. [DOI] [PubMed] [Google Scholar]
- 6.Lori F. Hydroxyurea and HIV: 5 years later − from anti-viral to immune-modulating effects. AIDS. 1999;13:1433–42. doi: 10.1097/00002030-199908200-00001. [DOI] [PubMed] [Google Scholar]
- 7.De Boer R, Boucher A, Perelson A. Target cell availability and the successful suppression of HIV by hydroxyurea and didanosine. AIDS. 1998;12:1567–70. doi: 10.1097/00002030-199813000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lori F, Jessen H, Foli A, Lisziewicz J, Matteo PS. Long-term suppression of HIV-1 by hydroxyurea and didanosine. JAMA. 1997;277:1437–8. [PubMed] [Google Scholar]
- 9.Lori F, Jessen H, Lieberman J, Clerici M, Tinelli C, Lisziewicz J. Immune restoration by combination of a cytostatic drug (hydroxyurea) and anti-HIV drugs (didanosine and indinavir) AIDS Res Hum Retroviruses. 1999;15:619–24. doi: 10.1089/088922299310917. [DOI] [PubMed] [Google Scholar]
- 10.López M, Benito JM, Lozano S, et al. Enhanced HIV-specific immune responses in chronically HIV-infected patients receiving didanosine plus hydroxyurea. AIDS. 2004;18:1251–61. doi: 10.1097/00002030-200406180-00003. [DOI] [PubMed] [Google Scholar]
- 11.Benito JM, López M, Ballesteros C, et al. Immunological and virological effects of structured treatment interruptions following exposure to hydroxyurea plus didanosine. AIDS Res Hum Retroviruses. 2006;22:734–43. doi: 10.1089/aid.2006.22.734. [DOI] [PubMed] [Google Scholar]
- 12.Wells A, Gudmundsdottir H, Turka L. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lori F, Foli A, Groff A, et al. Optimal suppression of HIV replication by low-dose hydroxyurea through the combination of anti-viral and cytostatic (‘virostatic’) mechanisms. AIDS. 2005;19:1173–81. doi: 10.1097/01.aids.0000176217.02743.d1. [DOI] [PubMed] [Google Scholar]
- 14.Villani P, Maserati R, Regazzi M, Giacchino R, Lori F. Pharmacokinetics of hydroxyurea in patients infected with HIV type 1. J Clin Pharmacol. 1996;36:117–21. doi: 10.1002/j.1552-4604.1996.tb04176.x. [DOI] [PubMed] [Google Scholar]
- 15.Rutschmann OT, Opravil M, Iten A, et al. A placebo-controlled trial of didanosine plus stavudine, with and without hydroxyurea, for HIV infection. AIDS. 1998;12:F71–7. doi: 10.1097/00002030-199808000-00003. The Swiss HIV cohort study. [DOI] [PubMed] [Google Scholar]
- 16.Barreiro P, de Mendoza C, Camino N, et al. Hydroxyurea plus didanosine as a maintenance therapy for HIV-infected patients on long-term successful highly active anti-retroviral therapy. HIV Clin Trials. 2003;4:361–71. doi: 10.1310/4GMU-AG3T-Q3CC-GE5D. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra U, Bosch R, Wang R, Collier A, McElrath J. Effect of adjunct hydroxyurea on helper T cell immunity in HIV type 1-infected patients with virological suppression. AIDS Res Hum Retroviruses. 2004;20:807–12. doi: 10.1089/0889222041725226. [DOI] [PubMed] [Google Scholar]
- 18.Lova L, Groff A, Ravot E, et al. Hydroxyurea exerts a cytostatic but not immunosuppressive effect on T lymphocytes. AIDS. 2005;19:137–44. doi: 10.1097/00002030-200501280-00005. [DOI] [PubMed] [Google Scholar]
- 19.Benito JM, López M, Lozano S, Martinez P, Gonzalez-Lahoz J, Soriano V. CD38 expression on CD8+ lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving anti-retroviral therapy. AIDS Res Hum Retroviruses. 2004;20:227–33. doi: 10.1089/088922204773004950. [DOI] [PubMed] [Google Scholar]
- 20.Gougeon ML, Lecouer H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 21.Benito JM, Martin J, López M, et al. Differences in cellular activation and apoptosis in HIV-infected patients receiving protease inhibitors or non-nucleoside reverse transcriptase inhibitors. AIDS Res Hum Retroviruses. 2002;18:1379–88. doi: 10.1089/088922202320935456. [DOI] [PubMed] [Google Scholar]
- 22.Bofill M, Mocroft A, Lipman M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10:827–34. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Deeks S, Kitchen C, Liu L, et al. Immune activation set-point during early HIV infection predicts subsequent CD4+ T cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C, Ellenberg J, Douglas S, Moscicki AB, Holland CA. CD8+CD38+ T cells but not type 1 RNA viral load predict CD4+ T cell loss in a predominantly minority female HIV+ adolescent population. AIDS Res Hum Retroviruses. 2004;20:263–9. doi: 10.1089/088922204322996482. Reach Project of the Adolescent Medicine HIV/AIDS Research Network. [DOI] [PubMed] [Google Scholar]
- 25.Vigano A, Saresella M, Villa ML, Ferrante P, Clerici M. CD38+CD8+ T cells as a marker of poor response to therapy in HIV-infected individuals. Chem Immunol. 2000;75:207–12. doi: 10.1159/000058770. [DOI] [PubMed] [Google Scholar]
- 26.Hunt P, Martin J, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in HIV-infected patients with sustained viral suppression during anti-retroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 27.Benito JM, López M, Lozano S, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active anti-retroviral therapy. J Acquir Immune Defic Syndr. 2005;38:373–81. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 28.Lori F, Rosenberg E, Lieberman J, et al. Hydroxyurea and didanosine long-term treatment prevents HIV breakthrough and normalizes immune parameters. AIDS Res Hum Retroviruses. 1999;15:1133–8. doi: 10.1089/088922299310034. [DOI] [PubMed] [Google Scholar]