Abstract
Cytokine-induced inflammation is involved in the pathogenesis of type 2 diabetes mellitus (DM). We investigated plasma concentrations and ex vivo production of cytokines and chemokines, and intracellular signalling molecules, mitogen-activated protein kinases (MAPK) in T helper (Th) cells and monocytes in 94 type 2 diabetic patients with or without nephropathy and 20 healthy controls. Plasma concentrations of inflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-18 and chemokine CCL2 in patients with diabetic nephropathy (DN) were significantly higher than control subjects, while IL-10, CXCL8, CXCL9, CXCL10 and adiponectin concentrations of DN were significantly higher than patients without diabetic nephropathy (NDN) and control subjects (all P < 0·05). Plasma concentrations of TNF-α, IL-6, IL-10, IL-18, CCL2, CXCL8, CXCL9, CXCL10 and adiponectin exhibited significant positive correlation with urine albumin : creatinine ratio in DN patients. The percentage increases of ex vivo production of IL-6, CXCL8, CXCL10, CCL2 and CCL5 upon TNF-α activation were significantly higher in both NDN and DN patients than controls (all P < 0·05). The percentage increases in IL-18-induced phosphorylation of extracellular signal-regulated kinase (ERK) in Th cells of NDN and DN were significantly higher than controls (P< 0·05), while the percentage increase in TNF-α-induced phosphorylation of p38 MAPK in monocytes and IL-18-induced phosphorylation of p38 MAPK in Th cells and monocytes were significantly higher in NDN patients than controls. These results confirmed that the aberrant production of inflammatory cytokines and chemokines and differential activation of MAPK in different leucocytes are the underlying immunopathological mechanisms of type 2 DM patients with DN.
Keywords: chemokines/monokines, cytokines/interleukins, diabetes, kinases/phosphatases, renal immunology/disease
Introduction
Type 2 diabetes mellitus (DM) is an increasingly prevalent metabolic disease in which the amount of insulin produced by the pancreas is inadequate to meet body needs. Type 2 DM has also been postulated as a disease of the innate immune system [1, 2]. There is increasing evidence that an ongoing cytokine-induced inflammatory response is related closely to the pathogenesis of type 2 DM and the associated complications such as dyslipidaemia, cardiovascular disease and renal failure [1, 3, 4]. Inflammation and fibrosis are common disease mechanisms involved in many forms of progressive renal injury [1]. Previous studies have indicated that the enhanced inflammation in type 2 DM is associated with elevated levels of the prototypic inflammatory marker C-reactive protein (CRP) as well as the proinflammatory cytokines tumour necrosis factor (TNF)-α and interleukin (IL)-6 [4, 5]. Furthermore, insulin resistance, an impaired biological response to circulating insulin, is a common pathological mechanism involved in the development and progression of various metabolic disorders in type 2 DM. Insulin resistance is observed frequently in obese subjects and differentiated adipocytes can secrete elevated levels of TNF-α and IL-6 [6, 7]. Diabetic nephropathy (DN) is a chronic kidney disease that develops as a result of the progression of DM.
Adiponectin is an adipocytokine that is secreted exclusively from the adipocytes [8]. It correlates negatively with insulin resistance [9], fasting serum triglycerides, insulin and plasma glucose concentrations [9]. Low plasma adiponectin concentrations have been shown in patients with obesity and type 2 diabetes [10]. The anti-inflammatory and anti-atherosclerotic activity of adiponectin is supported by the reciprocal association between adiponectin and both CRP and IL-6 in patients with coronary atherosclerosis [11]. Adiponectin concentration has been shown to be elevated significantly in patients with renal disease as well as type 1 and type 2 diabetic patients with impaired renal function compared to healthy control subjects [12–14].
Extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) have been found to play important roles in inflammation by inducing the production of inflammatory cytokine IL-6 and chemokines CXCL8/IL-8, CCL2/monocyte chemoattractant protein-1 (MCP-1), CXCL9/monokine induced by interferon (IFN)-γ (MIG), CCL5/regulated upon activation normal T cell expressed and secreted (RANTES) and CXCL10/IFN-γ-inducible protein-10 (IP-10) [15, 16]. It has been shown that diabetic nephropathy is mediated, at least in part, by the intracellular cell signalling mechanism through the p38 MAPK pathway of macrophages in kidney tissue [3]. Basal p38 MAPK phosphorylation is increased in skeletal muscle from type 2 diabetic patients [17]. However, the detailed dysregulation of various intracellular signal transduction molecules of lymphocytes in type 2 DM is not well defined.
Based on our previous studies of intracellular signal transduction mechanisms in inflammation, our hypothesis is that intracellular signalling molecules ERK, p38 MAPK and c-Jun N-terminal protein kinase (JNK), together with proinflammatory cytokines, chemokines and adiponectin, form a network in orchestrating inflammation in DN. In an attempt to elucidate further the immunopathogenesis of inflammation in type 2 DM, we investigated the above intracellular signalling molecules in Th cells and monocytes, as well as the release of cytokines, chemokines and adiponectin in type 2 DM patients with or without renal disease.
Materials and methods
DM patients, control subjects and blood samples
Ninety-four Chinese adult patients with type 2 DM were recruited from the Diabetes Mellitus and Endocrine Centre of the Prince of Wales Hospital, Hong Kong. DM was diagnosed using oral glucose tolerance test according to the 1985 World Health Organization (WHO) criteria: a fasting plasma glucose concentration ≥7·0 mmol/l or a 2-h glucose concentration ≥11·1 mmol/l [18]. All subjects were non-smokers and free from infection for 4 weeks preceding the study. The DM patients were divided further into two groups: (1) patients with normoalbuminuria and plasma creatinine < 80 (female) or 105 (male) µmol/l [patients without diabetic nephropathy (NDN), n = 28] and (2) patients with albuminuria [fasting urine albumin : creatinine ratio (UACR) > 3·5 mg/mmol in two urine samples] and plasma creatinine ≥80 (female)/≥ 105 (male) µmol/l [patients with diabetic nephropathy (DN), n = 66]. Body weight, body height, waist and hip circumferences were measured for the determination of waist : hip ratio (WHR) and body mass index (BMI). Twenty sex- and age-matched healthy Chinese volunteers were recruited as control subjects (CTL). Twelve millilitres of venous peripheral ethylenediamine tetraacetic acid (EDTA) blood was collected from each participant. Aliquots of whole blood were processed immediately for ex vivo study and fractionation of peripheral blood mononuclear cells (PBMC). Plasma were separated from blood cells by centrifugation (2000 g for 10 min) at 4°C and stored in 300 µl aliquots at −70°C until analysis. The age, sex, fasting plasma glucose (FPG) and creatinine concentrations, UACR, BMI and WHR of the studied subjects are summarized in Table 1. The plasma creatinine and urine ACR were elevated significantly in the DN group rather than the NDN group, indicating the presence of nephropathy in DN patients. The above protocol was approved by the clinical research ethics committee of the Chinese University of Hong Kong–New Territories East Cluster Hospitals, and informed consent was obtainedfrom all participants according to the Declaration of Helsinki.
Table 1.
Demographic and clinical information of type 2 diabetes mellitus (DM) patients and control subjects.
| CTL | NDN | DN | |
|---|---|---|---|
| Number | 20 | 28 | 66 |
| Sex (male/female) | 12/8 | 18/10 | 32/34 |
| Age (years) | 46·5 (41·0–51·0) | 56·5 (53·0–59·0) | 57·0 (54·5–60·5) |
| FPG (mmol/l) | 5·1 (4·9–5·4) | 7·5 (5·8–8·4)b | 7·7 (6·2–9·0)b |
| Plasma Cr (μmol/l) | 81·0 (71·5–88·0) | 88·0 (77·5–100·0) | 114·5 (96·0–129·0)b, c |
| UACR (mg/mmol) | < 3·50 | 2·35 (0·95–3·50) | 34·0 (3·7–107·9)c |
| BMI | 23·0 (21·2–24·5) | 25·4 (23·0–29·1)a | 25·8 (24·3–28·1)b |
| WHR | 0·84 (0·81–0·86) | 0·94 (0·88–0·97)b | 0·93 (0·89–0·98)b |
CTL, control subjects; NDN, patients without diabetic nephropathy; DN, patients with diabetic nephropathy; FPG, fasting plasma glucose; Cr, creatinine; UACR, urine albumin: creatinine ratio; BMI, body mass index; WHR, waist to hip ratio. Data are expressed as median (interquartile range).
P < 0·01
P < 0·001 versus CTL
P < 0·001 versus NDN.
Measurement for chemokines, cytokines and adiponectin in plasma
The plasma concentrations of inflammatory cytokines TNF-α, IL-1β, IL-6, IL-10 and IL-12p70 and chemokines CXCL8, CCL5, CCL2, CXCL10 and CXCL9 were measured using the inflammatory cytokine and chemokine cytometric bead array (CBA) reagent kits from BD Pharmingen (San Diego, CA, USA), respectively. Samples were analysed on a multi-fluorescence BD fluorescence activated cell sorter (FACSCaliburTM) flow cytometer using BD CellQuestTM software and BDTM CBA Software. The assay sensitivities of these five cytokines and five chemokines were 7·2, 2·5, 3·3, 3·7, 1·9, 0·2, 1·0, 2·7, 2·8 and 2·5 ng/l, respectively. The coefficients of variation for all cytokine and chemokine assays were less than 10%. Plasma IL-18 and adiponectin concentrations were measured by enzyme-linked immunosorbent assay (ELISA) from BioSource Corp (Camarillo, CA, USA) and R&D Systems, Inc. (Minneapolis, MN, USA), respectively.
Ex vivo production of cytokines and chemokines
The method of Viallard and co-workers (1999) was adopted [19]. Whole blood was diluted 1 : 1 with culture medium RPMI-1640 (Gibco laboratories, NY, USA), and 1 ml aliquots were dispensed in each well of a 24-well plate (Nalge Nunc International, Rochester, NY, USA). The blood culture was then incubated with or without TNF-α (Peprotech Corp, London, UK) or IL-18 (R&D Systems) at 150 ng/ml for 24 h at 37°C in a 5% CO2 atmosphere. The cell-free supernatant was harvested and stored at −70°C for subsequent assays.
Flow cytometric analysis of intracellular activated MAPK
Activated MAPK in T helper (Th) cells (CD4+) and monocytes in PBMC from patients and controls were assessed by flow cytometric analysis of intracellular phospho-ERK, phospho-p38 MAPK and phospho-JNK. Briefly, PBMC were prepared by centrifuging EDTA venous blood using a Ficoll-Paque density gradient (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden). The viability of PBMC was more than 95%, as determined by the trypan blue exclusion method. PBMC was then fixed by BD CytofixTM Buffer (BD Biosciences) at 37°C for 10 min. Cells were then permeabilized with BD PhosFlow Perm Buffer III for 30 min on ice, washed twice with BD PharmingenTM Stain Buffer (BD) and resuspended in BD PharmingenTM stain buffer at 1 × 107 cells/ml. Fluorochrome-conjugated anti-human phospho-ERK, phospho-p38 MAPK, phospho-JNK antibody or mouse IgG isotypic antibody (BD Pharmingen) was added to each tube and incubated at room temperature for 30 min in the dark. Cells were then washed and resuspended for flow cytometric analysis using CD4+ and forward-scatter (FSC) together with side-scatter (SSC) gating for Th cells and monocytes, respectively (BD FACSCalibur flow cytometer). Results were expressed as mean fluorescence intensity (MFI) for intracellular phospho-p38 MAPK, phospho-ERK and phospho-JNK of 10 000 cells [20, 21].
Statistical analysis
All analyses were performed using statistical software (spss for Windows, version 14·0, SPSS Inc., Chicago, IL, USA). The Kruskal–Wallis test was followed, when significance arose, by Dunn's test for pairwise comparison of data between groups. Spearman's rank correlation test was used to assess the correlations of different parameters. Comparison of basal and ex vivo culture supernatant concentrations was made with the Mann–Whitney U-test. Probability (P) values of less than 0·05 were considered significant. Unless specified otherwise, results are expressed as the median (interquartile range: IQR).
Results
Plasma cytokines, chemokines and adiponectin
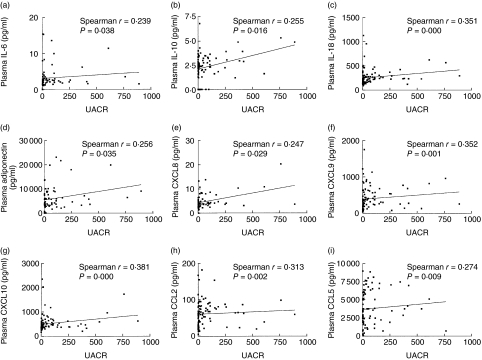
As shown in Table 2, plasma concentrations of inflammatory cytokines TNF-α, IL-6 and IL-18 and chemokine CCL2 of DN patients were significantly higher compared to control subjects (all P < 0·05). Adiponectin, IL-10, CXCL8, CXCL9 and CXCL10 concentrations in DN were significantly higher compared to the NDN group and control subjects (all P < 0·05). Plasma concentrations of TNF-α, IL-6, IL-10, IL-18, CCL2, CXCL8, CXCL9, CXCL10 and adiponectin exhibited a significantly positive correlation with UACR in DN patients but not in NDN patients (all P < 0·05, Fig. 1, Table 3). Plasma concentrations of IL-18 (r = 0·361, P = 0·000), CXCL9 (r = 0·352, P = 0·001) and CXCL10 (r = 0·381, P = 0·000) exhibited the most significant correlation with UACR (Fig. 1, Table 3).
Table 2.
Plasma concentrations of chemokines, cytokines and adiponectin in type 2 diabetic patients and normal control subjects.
| Cytokine/ chemokine | Group | Concentration (IQR) (pg/ml) |
|---|---|---|
| TNF–α | CTL | UD |
| NDN | UD | |
| DN | 1·62 (0·10–2·19)* | |
| IL−1β | CTL | UD |
| NDN | UD | |
| DN | UD | |
| IL−6 | CTL | UD |
| NDN | 2·25 (1·25–3·25) | |
| DN | 2·50 (1·81–3·03)** | |
| IL−10 | CTL | UD |
| NDN | 1·81 (0·61–2·69) | |
| DN | 2·58 (2·03–3·41)***† | |
| IL−12p70 | CTL | UD |
| NDN | UD | |
| DN | UD | |
| IL−18 | CTL | 191 (132–276) |
| NDN | 199 (154–269) | |
| DN | 247 (171–303)* | |
| CXCL8 | CTL | 2·29 (1·72–2·88) |
| NDN | 3·24 (2·48–4·68)* | |
| DN | 4·08 (3·68–6·57)***† | |
| CXCL9 | CTL | 189 (153–273) |
| NDN | 244 (201–283) | |
| DN | 425 (314–653)***†† | |
| CXCL10 | CTL | 345 (264–480) |
| NDN | 331 (282–488) | |
| DN | 482 (389–606)***††† | |
| CCL2 | CTL | 57·4 (37·5–83·5) |
| NDN | 58·7 (54·4–81·8) | |
| DN | 71·0 (61·6–88·3)* | |
| CCL5 | CTL | 2590 (1360–5840) |
| NDN | 2230 (345–5620) | |
| DN | 5040 (2030–6880) | |
| Adiponectin | CTL | 3200 (2200–4940) |
| NDN | 2230 (1500–4290) | |
| DN | 5930 (3720–8970)**††† |
Results were expressed as median (interquartile range). Kruskal–Wallis test was used to assess the differences among groups.
P < 0·05
P < 0·01
P < 0·001 versus CTL
P < 0·05
P < 0·01
P <0·001 versus NDN; UD: below detection limit. TNF: tumour necrosis factor; IL: interleukin; IQR: interquartile range.
Fig. 1.
Correlations between plasma cytokine and chemokine concentrations with urine albumin : creatinine ratio (UACR) in all type 2 diabetes mellitus patients [non-diabetic nephropathy (NDN) + diabetic nephropathy (DN)].
Table 3.
Correlations between plasma cytokine and chemokine concentrations with urine albumin : creatinine ratio in type 2 diabetes mellitus (DM) patients.
| Cytokine | Group | Spearman's r | P-value |
|---|---|---|---|
| TNF-α | NDN | n.a. | n.a. |
| DN | 0·296 | 0·046* | |
| NDN + DN | n.a. | n.a. | |
| IL-6 | NDN | 0·301 | 0·163 |
| DN | 0·280 | 0·038* | |
| NDN + DN | 0·239 | 0·038* | |
| IL-10 | NDN | 0·291 | 0·149 |
| DN | 0·276 | 0·040* | |
| NDN + DN | 0·255 | 0·016* | |
| IL-18 | NDN | −0·074 | 0·786 |
| DN | 0·273 | 0·027* | |
| NDN + DN | 0·361 | 0·000*** | |
| Adiponectin | NDN | −0·333 | 0·385 |
| DN | 0·287 | 0·048* | |
| NDN + DN | 0·256 | 0·035* | |
| CXCL8 | NDN | −0·170 | 0·473 |
| DN | 0·278 | 0·042* | |
| NDN + DN | 0·247 | 0·029* | |
| CXCL9 | NDN | 0·012 | 0·9504 |
| DN | 0·245 | 0·049* | |
| NDN + DN | 0·352 | 0·001*** | |
| CXCL10 | NDN | 0·274 | 0·159 |
| DN | 0·318 | 0·009** | |
| NDN + DN | 0·381 | 0·000*** | |
| CCL2 | NDN | −0·176 | 0·370 |
| DN | 0·271 | 0·031* | |
| NDN + DN | 0·313 | 0·002** | |
| CCL5 | NDN | 0·192 | 0·328 |
| DN | 0·125 | 0·328 | |
| NDN + DN | 0·274 | 0·009** |
Results are expressed as correlation coefficient r
P < 0·05
P < 0·01
P < 0·001. n.a.: Not calculated due to below detection limit of plasma tumour necrosis factor (TNF)- α concentrations. IL: interleukin; NDN: diabetic nephropathy; DN: diabetic nephropathy.
Ex vivo production of chemokines and cytokines
The above findings prompted us to investigate the immunocompetence of PBMC in the ex vivo production of inflammatory cytokines and chemokines from PBMC in the patient cohorts. As shown in Table 4, TNF-α and IL-18 significantly induced the release of IL-6, IL-10 and chemokines CXCL8 and CCL2 in NDN and DN groups compared to the spontaneous production of chemokines/cytokines under basal conditions (all P < 0·05). IL-18 induced CXCL9 release and TNF-α induced CXCL10 release significantly in the DN group (all P < 0·05). IL-18 induced CCL5 release and TNF-α induced IL-18 release significantly in all groups (all P < 0·05).
Table 4.
Ex vivo production of cytokines and chemokines from tumour necrosis factor (TNF)-α- or interleukin (IL)-18-activated peripheral blood mononuclear cells (PBMC) of control subjects (CTL), non-diabetic nephropathy (NDN) and diabetic nephropathy (DN) groups.
| Cytokine/chemokine | Group | Medium control (pg/ml) | Post-TNF-α activation (pg/ml) | Change post-TNF-α activation (%) | Post-IL-18 activation (pg/ml) | Change post-IL-18 activation (%) |
|---|---|---|---|---|---|---|
| TNF-α | CTL | 21·7 (18·0–25·4) | n.d. | n.d. | 20·4 (16·6–22·8) | −11·2 [−22·9–(−1·79)] |
| NDN | 19·0 (13·6–24·0) | n.d. | n.d. | 25·1 (22·2–33·2) | 49·9 (19·8–97·5)†† | |
| DN | 22·0 (17·0–28·6) | n.d. | n.d. | 22·8 (17·7–26·2) | 1·17 (−21·9–46·6)‡ | |
| IL-6 | CTL | 15·6 (12·0–22·8) | 18·2 (12·3–37·5) | 9·52 (−19·6–42·0) | 17·4 (12·1–25·9) | −1·74 (−24·5–69·2) |
| NDN | 16·2 (7·20–22·6) | 35·9 (15·9–80·0)* | 159 (13·9–467)† | 36·0 (21·9–44·1)** | 73·1 (−2·04–216) | |
| DN | 20·0 (11·7–29·8) | 43·8 (28·1–82·3)*** | 95·6 (28·8–264)†† | 27·3 (18·9–53·4)*** | 22·6 (−16·0–134) | |
| IL-10 | CTL | 17·8 (13·2–20·9) | 20·5 (17·9–27·9) | 14·4 (−11·3–61·7) | 21·8 (15·6–26·2) | 15·7 (−3·16–66·1) |
| NDN | 14·0 (11·2–21·5) | 19·9 (15·5–30·1)* | 31·6 (−30·8–117) | 22·8 (19·9–27·4)** | 41·0 (8·10–130) | |
| DN | 19·6 (15·8–25·0) | 28·0 (19·0–38·2)*** | 46·3 (3·75–106) | 22·2 (17·2–27·2)* | 19·9 (−20·1–43·3) | |
| IL-18 | CTL | 76·3 (11·4–128) | 275 (108–348)** | 174 (34·2–279) | n.d. | n.d. |
| NDN | 100 (85·5–221) | 300 (200–390)* | 161 (68·5–204) | n.d. | n.d. | |
| DN | 109 (44·5–226) | 298 (181–570)*** | 164 (77·8–343) | n.d. | n.d. | |
| CXCL8 | CTL | 27·8 (22·1–33·0) | 175 (142–241)*** | 675 (474–820) | 26·7 (21·3–43·7) | 4·20 (−33·8–76·1) |
| NDN | 26·8 (20·4–32·8) | 291 (198–371)*** | 926 (711–1250)† | 47·2 (30·1–76·5)** | 139 (19·3–235)†† | |
| DN | 26·2 (22·6–37·0) | 300 (235–420)*** | 913 (626–1350)† | 36·0 (22·6–51·8)* | 26·3 (−13·0–67·9)‡ | |
| CXCL9 | CTL | 27·7 (19·9–37·4) | 20·0 (15·1–32·8) | −24·8 [−38·0–(−16·3)] | 20·3 (13·6–40·9) | −35·0 [−47·6–(−21·4)] |
| NDN | 36·4 (31·5–43·7) | 41·5 (33·6–56·8) | 20·9 (11·8–35·7)††† | 27·0 (23·1–38·4) | −24·3 [−29·5–(−13·8)] | |
| DN | 62·9 (42·6–112) | 64·9 (40·0–98·7) | 2·00 (−39·6–30·0)‡ | 46·2 (28·0–90·7)* | −29·5 [−45·9–(−6·78)] | |
| CXCL10 | CTL | 12·3 (8·15–30·0) | 9·75 (6·55–19·1) | −34·2 (−60·3–19·9) | 12·1 (6·80–22·1) | 33·8 (−52·3–236) |
| NDN | 9·80 (7·45–16·10) | 12·5 (8·70–39·9) | 49·3 (3·92–185)† | 10·9 (4·95–22·0) | 3·95 (−50·7–83·6) | |
| DN | 16·5 (11·9–28·0) | 25·3 (15·0–51·6)* | 35·1 (−34·8–148)† | 18·6 (9·00–48·0) | −30·7 (−59·0–284) | |
| CCL2 | CTL | 9·00 (7·70–12·2) | 7·85 (6·30–10·2) | −14·6 [−32·7–(−2·13)] | 5·40 (4·80–6·30)*** | −41·0 [−53·7–(−25·7)] |
| NDN | 10·8 (8·60–14·55) | 15·0 (13·5–21·1)* | 48·5 (31·7–58·6)††† | 5·10 (4·65–6·45)*** | −50·4 [−57·0–(−42·9)] | |
| DN | 10·8 (8·50–13·0) | 13·0 (10·8–16·5)*** | 20·9 (0·00–61·9)††† | 4·95 (4·50–5·80)*** | −50·2 [−58·1–(−42·3)]† | |
| CCL5 | CTL | 149 (72·6–278) | 75·8 (39·9–138) | −48·5 [−63·1–(−40·3)] | 50·3 (34·3–154)* | −55·8 [−71·4–(−46·5)] |
| NDN | 91·8 (66·0–165) | 132 (85·9–276) | 22·1 (11·2–46·4)††† | 48·9 (29·8–104)* | −50·3 [−58·8–(−8·21)] | |
| DN | 174 (84·0–292) | 216 (95·2–363) | 5·07 (−37·3–39·9)††† | 118 (36·5–248)* | −43·1 [−65·0–(−33·1)] |
The culture supernatant was derived from whole blood cultured with medium in the absence or presence of TNF-α (150 ng/ml) or IL-18 (150 ng/ml) for 24 hours. Results are expressed as median (IQR). The Kruskal–Wallis test was used to access the differences of production between TNF-α-treated or IL-18-treated and medium control groups
(P < 0·05
P < 0·01
P < 0·001), percentage change between CTL and patient groups
P < 0·05
P < 0·01
P < 0·001) and percentage change between NDN and DN groups
P < 0·05). n.d.: Not done.
In the presence of external stimuli, the percentage increases of ex vivo production of IL-6, CXCL8, CXCL10, CCL2 and CCL5 after activation by TNF-α were significantly higher in both DN and NDN patients than controls (all P < 0·05, Table 4). The percentage increases of IL-18-induced TNF-α and CXCL8 were increased significantly in NDN patients compared to controls (all P < 0·01). Significantly smaller percentage increases of ex vivo release of TNF-α-induced CXCL9, IL-18-induced TNF-α and CXCL8 were found in DN compared to NDN patients (all P < 0·05).
Phosphorylation of intracellular ERK and p38 MAPK in Th cells and monocytes
To characterize the intracellular activation mechanism for the release of cytokines and chemokines from PBMC, we investigated the phosphorylation of intracellular ERK and p38 MAPK in Th cells and monocytes. As shown in Tables 5 and 6, TNF-α and IL-18 could significantly induce the phosphorylation of ERK and p38 MAPK in monocytes of CTL in all DM patients and Th cells in all DM patients, respectively (P< 0·05). Table 5 shows that the percentage increase in IL-18-induced phosphorylation of ERK in Th cells of NDN and DN patients were significantly higher compared to controls (P< 0·05). The percentage increase in TNF-α-induced phosphorylation of p38 MAPK in monocytes and IL-18-induced phosphorylation of p38 MAPK in Th cells and monocytes were significantly higher in NDN patients than CTL (all P < 0·05, Table 6). However, the above percentage changes of phosphorylation of p38 MAPK were significantly lower in DN patients compared to NDN patients (P< 0·05). The TNF-α- and IL-18-induced phosphorylations of JNK in Th cells and monocytes were found to be similar to that of p38 MAPK (data not shown).
Table 5.
Basal and ex vivo expression of phospho-extracellular signal-regulated kinase (ERK) in tumour necrosis factor (TNF)-α and interleukin (IL)-18-activated CD3+/CD4+ T helper cells and monocytes in various groups.
| Cell type | Group | Medium control (MFI/104 leucocytes) | TNF-α activation (MFI/104 leucocytes) | % change TNF-α activation | IL-18 activation (MFI/104 leucocytes) | IL-18 % change activation |
|---|---|---|---|---|---|---|
| Th cells | CTL | 35·1 (25·2–48·4) | 32·4 (28·2–39·5) | 1·11 (−14·7–11·1) | 31·2 (25·5–40·3) | −11·2 (−15·5–4·54) |
| NDN | 36·2 (27·4–51·3) | 39·2 (31·6–49·7) | 8·14 (−3·8–35·3) | 57·5 (37·8–88·9)* | 13·7 (3·11–57·3)††† | |
| DN | 37·6 (23·7–53·6) | 36·5 (25·6–54·0) | 0·82 (−8·1–17·6) | 46·1 (32·7–62·1)* | 2·96 (−5·3–18·3)†‡‡ | |
| Monocytes | CTL | 64·8 (44·9–99·7) | 132 (81·8–188)** | 83·0 (19·8–225) | 63·6 (41·0–90·2) | −6·84 (−27·0–26·5) |
| NDN | 88·2 (65·3–121) | 171 (121–245)*** | 62·4 (24·8–181) | 125 (88·4–159) | −2·93 (−12·5–84·4) | |
| DN | 94·3 (68·2–160) | 198 (140–296)*** | 72·7 (29·4–168) | 97·2 (72·3–164) | 2·10 (−10·0–27·7) |
PBMC were incubated with PBS, TNF-α (20 ng/ml) or IL-18 (20 ng/ml) for 15 min. Results of the intracellular phospho-ERK were expressed as median (IQR). Kruskal–Wallis test was used to access the differences of expression between TNF-α treated or IL-18-treated and medium control groups
P < 0·05
P < 0·01
P < 0·001), percentage change between control (CTL) and patient groups
P < 0·05
P < 0·001) and percentage change between NDN and DN groups
P < 0·01).
Table 6.
Basal and ex vivo expression of phospho-p38 mitogen-activated protein kinases (MAPK) in tumour necrosis factor (TNF)-α and interleukin (IL)-18-activated CD3+/CD4+ T helper cells and monocytes in various groups.
| Cell type | Group | Medium control (MFI/104 leucocytes) | TNF-α activation (MFI/104 leucocytes) | % change TNF-α activation | IL-18 activation (MFI/104 leucocytes) | IL-18 % change activation |
|---|---|---|---|---|---|---|
| Th cells | CTL | 106 (80·4–130) | 124 (83·7–143) | 6·9 (1·04–21·5) | 112 (74·9–130) | −0·36 (−9·69–14·0) |
| NDN | 47·5 (31·1–87·0)†† | 56·8 (42·4–98·1) | 13·7 (−3·33–43·0) | 147 (83·8–176)* | 22·4 (21·8–38·8)††† | |
| DN | 69·0 (34·2–115) | 72·4 (36·0–142) | 12·3 (−3·90–20·8) | 99·3 (44·8–146)** | 5·67 (−3·15–18·2)‡‡‡ | |
| CTL | 198 (129–273) | 324 (199–408)* | 46·3 (17·0–67·5) | 230 (101–290) | 0·42 (−11·2–10·6) | |
| Monocytes | NDN | 161 (122–235) | 327 (150–442)** | 93·4 (34·6–198)† | 281 (218–364)* | 15·4 (0·11–33·3)† |
| DN | 219 (133–324) | 373 (236–428)*** | 41·6 (20·8–80·3)‡‡ | 245 (156–343) | 2·01 (−20·6–17·6)‡‡‡ |
Peripheral blood mononuclear cells were incubated with phosphate-buffered saline, TNF-α (20 ng/ml) or IL-18 (20 ng/ml) for 15 min. Results of phospho-p38 MAPK were expressed as median (interquartile range). The Kruskal–Wallis test was used to access the differences of basal expression among groups
P < 0·01), expression between TNF-α treated or IL-18-treated and medium control groups
P < 0·05
P < 0·01
P < 0·001), percentage change between control (CTL) and patient groups
(P < 0·05
P < 0·001) and percentage change between NDN and DN groups
P < 0·01
P < 0·001).
Discussion
Inflammation, deranged glucose and lipid metabolism, and overactivated adipocytes have been implicated in the pathogenesis of type 2 DM [1]. Hypercytokinaemia and activated innate immunity may be the common antecedent of both type 2 diabetes and atherosclerosis [1]. We have reported previously that elevation of pro-inflammatory cytokines could play an important immunopathological role in the chronic inflammation of chronic renal failure patients [22]. In the present study, we showed first that plasma concentrations of inflammatory cytokines TNF-α, IL-6, IL-18 and chemokine CCL2 in DN patients but not NDN patients were significantly higher compared to control subjects. Adiponectin, IL-10, CXCL8, CXCL9 and CXCL10 concentrations in DN were significantly higher compared to the NDN group and control subjects. Previous studies have also shown the elevation of inflammatory cytokines and chemokines in type 2 diabetes [1, 23, 24]. Hyperglycaemia can increase circulating cytokine concentrations by oxidative mechanisms, and this effect is more pronounced in subjects with impaired glucose tolerance [25, 26]. Adiponectin has been postulated to have pathological implications in DN [27]. Elevation of plasma concentration of adiponectin in DN in the present study may be due to renal insufficiency and/or increase in synthesis [14, 27]. In our present investigation, the striking findings are the significant and positive correlations for plasma concentrations of TNF-α, IL-6, IL-10, IL-18, CCL2, CXCL8, CXCL9, CXCL10 and adiponectin with severity of nephropathy in DN group. Moreover, IL-18, CXCL9 and CXCL10 exhibited the most significant correlation with severity of nephropathy. Results therefore confirmed that the above-studied panel of inflammatory cytokines, chemokines andadiponectin but not IL-1β, IL-12 and CCL5 are involved inthe nephropathy-related inflammation in type 2 DM patients, and Th1-related cytokine IL-18 and Th1-related chemokines CXCL9 and CXCL10 may play a crucial role for diabetic nephropathy. We observed that the elevated adiponectin exhibited significant and positive correlation with plasma concentrations of TNF-α, IL-6, CXCL10 and CCL5 in DN patients but not in NDN patients (data not shown). It suggested that the elevated adiponectin in DN patients may also be due to an increase in de novo synthesis.
Elevated circulating TNF-α and IL-18, the upstream cytokines for Th1 immunity, have been shown to be associated with DN in previous reports [28–30]. In the present study, IL-18 has been shown to have strong correlation with severity of DN. Moreover, TNF-α is also a modulator of glucose metabolism by the direct induction of insulin resistance and down-regulation of insulin receptor signalling [31]. In order to mimic the local Th1-mediated inflammatory reaction and the responsiveness of PBMC upon activation in type 2 DM patients, we studied the ex vivo production of cytokines and chemokines from TNF-α- or IL-18-activated PBMC. In the presence of external stimuli, the percentage increases of ex vivo production of TNF-α-induced IL-6, CXCL8, CXCL10, CCL2 and CCL5 were significantly higher in both DN and NDN patients compared to controls (all P < 0·05). The percentage increases of IL-18 induced TNF-α and CXCL8 were increased significantly in NDN patients compared to controls (P< 0·01). However, significantly less percentage increases of TNF-α-induced CXCL9 and IL-18-induced TNF-α and CXCL8 were found in DN compared to NDN patients (all P < 0·05). A previous study has also shown that type 1 DM patients did not express higher lipopolysaccharide-induced TNF-α, IL-1β and IL-6 levels than controls using whole blood assay [32]. The elevated TNF-α-induced IL-6, CXCL8, CXCL10, CCL2 and CCL5 from PBMC indicated further the aberrant production of the above inflammatory cytokine, chemokines for neutrophils (CXCL8), Th1 cells (CXCL10), macrophages (CCL2) and activated T cells (CCL5) in type 2 DM patients. In fact, it has been shown that CXCL8 increased in the early stage of DN, and CCL2 increased in the advanced stage of type 2 DN [33].
To elucidate further the abnormalities of the activation of leucocyte subsets in type 2 DM patients, we have investigated the activation of intracellular signalling molecules in TNF-α- and IL-18-treated lymphocytes and monocytes. Results indicated that the percentage increases in IL-18-induced phosphorylation of ERK in Th cells of NDN and DN patients were significantly higher compared to controls. As ERK is responsible for cell proliferation, transformation, differentiation and cytokine production [15, 16, 34], the increased activation of ERK in Th cells in type 2 DM patients implies hyperactivation of Th cell-mediated inflammation in type 2 DM. Moreover, the percentage increases in TNF-induced phosphorylation of p38 MAPK in monocytes and IL-18-induced phosphorylation of p38 MAPK in Th cells and monocytes were significantly higher in NDN patients compared to CTL. JNK also showed similar activation patterns upon TNF-α and IL-18 stimulation. p38 MAPK and JNK are both responsible for the regulation of stress response and inflammation of the pathology of chronic inflammation, heart disease, stroke, the debilitating effects of diabetes mellitus and the side effects of cancer therapy by the up-regulation of inflammatory cytokines and regulation of apoptosis of different leucocytes, especially macrophages [35, 36]. It has been shown that glucose could regulate CXCL8 production in aortic endothelial cells through activation of p38 MAPK pathway in diabetes [37]. Therefore, our results of the hyperactivation of p38 MAPK in monocytes and Th cells further implied that activated monocytes and Th cells mediated inflammation in type 2 DM patients. Our study may therefore provide a biochemical basis for treatment strategy of type 2 DM targeting intracellular signal transduction [38].
In conclusion, our results revealed that elevated concentrations of cytokines and chemokines were correlated with disease severity of DN and illustrated the potential roles of TNF-α and IL-18 in the exacerbation of inflammatory reactions. Although the plasma cytokines and chemokines show relatively low correlations with UACR, the concentrations of cytokines and chemokines and their correlations with UACR at local inflammatory sites should be much higher than that of the circulation. Therefore, further study is required for investigating the expression of inflammatory cytokines and chemokines and their correlation with disease severity at the local inflammatory kidney tissue in DN. Nevertheless, the above immunological mechanisms probably involved the abnormal activation of p38 MAPK, JNK and ERK in activated lymphocytes and monocytes. The results of this clinical study may therefore provide a further biochemical basis for the elucidation of the pathological mechanisms of diabetic nephropathy and the development of a novel therapeutic approach (e.g. using inhibitors of signalling molecules) in the treatment of type 2 DM and its associated inflammation.
Acknowledgments
The study was supported by a Direct Grant for Research, The Chinese University of Hong Kong (2041203).
References
- 1.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 2.Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36:1331–6. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- 3.Adhikary L, Chow F, Nikolic-Paterson DJ, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–22. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- 4.Madonna R, Pandolfi A, Massaro M, Consoli A, De Caterina R. Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated protein-kinase. Diabetologia. 2004;47:532–6. doi: 10.1007/s00125-004-1330-x. [DOI] [PubMed] [Google Scholar]
- 5.Devaraj S, Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790–2. doi: 10.1016/s0891-5849(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 6.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi K, Kanazawa A, Tsukada S, Maeda S. PKCepsilon induces interleukin-6 expression through the MAPK pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:707–12. doi: 10.1016/j.bbrc.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 8.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002;103:137–42. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C, Mallamaci F, Panuccio V, et al. Adiponectin is markedly increased in patients with nephrotic syndrome and is related to metabolic risk factors. Kidney Int Suppl. 2003;84:S98–102. doi: 10.1046/j.1523-1755.63.s84.49.x. [DOI] [PubMed] [Google Scholar]
- 13.Saraheimo M, Forsblom C, Fagerudd J, et al. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005;28:1410–14. doi: 10.2337/diacare.28.6.1410. [DOI] [PubMed] [Google Scholar]
- 14.Komaba H, Igaki N, Goto S, et al. Increased serum high-molecular-weight complex of adiponectin in type 2 diabetic patients with impaired renal function. Am J Nephrol. 2006;26:476–82. doi: 10.1159/000096870. [DOI] [PubMed] [Google Scholar]
- 15.Wong CK, Wang CB, Ip WK, Tian YP, Lam CW. Role of p38 MAPK and NF-kB for chemokine release in coculture of human eosinophils and bronchial epithelial cells. Clin Exp Immunol. 2005;139:90–100. doi: 10.1111/j.1365-2249.2005.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CK, Cheung PF, Ip WK, Lam CW. IL-25 induced chemokines and IL-6 release from eosinophils is mediated by p38 MAPK, JNK and NF-{kappa}B. Am J Respir Cell Mol Biol. 2005;33:186–94. doi: 10.1165/rcmb.2005-0034OC. [DOI] [PubMed] [Google Scholar]
- 17.Koistinen HA, Chibalin AV, Zierath JR. Aberrant p38 mitogen-activated protein kinase signalling in skeletal muscle from Type 2 diabetic patients. Diabetologia. 2003;46:1324–8. doi: 10.1007/s00125-003-1196-3. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Diabetes mellitus. Geneva: WHO; 1985. Technical report series 727. [Google Scholar]
- 19.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN-gamma) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–95. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, Chen RF, Liu JW, et al. Altered p38 mitogen-activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J Immunol. 2004;172:7841–7. doi: 10.4049/jimmunol.172.12.7841. [DOI] [PubMed] [Google Scholar]
- 21.Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004;110:206–21. doi: 10.1016/j.clim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Wong CK, Szeto CC, Chan MH, Leung CB, Li PK, Lam CW. Elevation of pro-inflammatory cytokines, C-reactive protein and cardiac troponin T in chronic renal failure patients on dialysis. Immunol Invest. 2007;36:47–57. doi: 10.1080/08820130600745505. [DOI] [PubMed] [Google Scholar]
- 23.Esposito K, Nappo F, Giugliano F, et al. Cytokine milieu tends toward inflammation in type 2 diabetes. Diabetes Care. 2003;26:1647. doi: 10.2337/diacare.26.5.1647. [DOI] [PubMed] [Google Scholar]
- 24.Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437–43. doi: 10.1046/j.1365-2249.2000.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 26.Morohoshi M, Fujisawa K, Uchimura I, Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45:954–9. doi: 10.2337/diab.45.7.954. [DOI] [PubMed] [Google Scholar]
- 27.Koshimura J, Fujita H, Narita T, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun. 2004;316:165–9. doi: 10.1016/j.bbrc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Ng MC, So WY, et al. Association between tumour necrosis factor-alpha G-308A polymorphism and risk of nephropathy in obese Chinese type 2 diabetic patients. Nephrol Dial Transplant. 2005;20:2733–8. doi: 10.1093/ndt/gfi101. [DOI] [PubMed] [Google Scholar]
- 29.Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol. 2005;117:152–60. doi: 10.1016/j.clim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud RA, el-Ezz SA, Hegazy AS. Increased serum levels of interleukin-18 in patients with diabetic nephropathy. Ital J Biochem. 2004;53:73–81. [PubMed] [Google Scholar]
- 31.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276:E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 32.Araya AV, Pavez V, Perez C, et al. Ex vivo lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2 secretion in whole blood from Type 1 diabetes mellitus patients with or without aggressive periodontitis. Eur Cytokine Netw. 2003;14:128–33. [PubMed] [Google Scholar]
- 33.Tashiro K, Koyanagi I, Saitoh A, et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochimica et Biophysica Acta – Molecular Cell Research. 2006 doi: 10.1016/j.bbamcr.2006.11.010. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 36.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan S, Bolick DT, Hatley ME, et al. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogen-activated protein kinase pathway in diabetes. J Biol Chem. 2004;279:31930–6. doi: 10.1074/jbc.M400753200. [DOI] [PubMed] [Google Scholar]
- 38.Kaneto H, Nakatani Y, Miyatsuka T, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–32. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]