Abstract
Systemic sclerosis (SSc) is a complex and heterogeneous autoimmune disorder with a multi-factorial pathogenesis. Like other autoimmune disorders, the possible role of specific cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphisms in predisposing to SSc has been hypothesized, but it remains controversial. CTLA-4 promoter (−318C/T) and exon 1 (+49 A/G) polymorphisms have been analysed in 43 Italian females with SSc and in 93 unrelated matched healthy controls by a newly designed tetra-primer amplification refractory mutation system–polymerase chain reaction (T-ARMS–PCR) method. No significant association has been found with either polymorphisms. Nevertheless, SSc patients without concomitant Hashimoto's thyroiditis (HT) were carrying both the −318T allele (P = 0·031) and the +49 G allele (P = 0·076) more frequently than SSc patients with HT [defined by positivity for anti-thyroperoxidase (TPO) and anti-thyroglobulin (TGA) autoantibodies] than controls. Haplotype analysis confirms this association (P = 0·028), and suggests the predominant role of the −318T, whereas that of the +49 G, if any, seems weak. Thus, in Italian SSc patients the CTLA-4 −318C/T promoter polymorphism appears to be associated with the susceptibility to develop SSc without thyroid involvement. Larger studies are needed to confirm these findings and to clarify whether the −318C/T polymorphism is the functional responsible or whether it reflects the presence of another linked genetic element in the same chromosomal region.
Keywords: CTLA-4, single nucleotide polymorphisms, systemic sclerosis, T-ARMS–PCR, thyroiditis
Introduction
Systemic sclerosis (SSc, scleroderma) is an autoimmune disease characterized by various clinical features, including progressive induration and thickening of the skin, microvascular abnormalities (e.g. Raynaud's phenomenon) and fibrosis of multiple internal organs. SSc can be classified as limited (lSSc) when it involves only the skin of distal extremities and face, or as diffuse (dSSc) when it affects the whole body surface. SSc is a rare disease with an estimated incidence of 19 individuals per million per year and almost exclusively affects women [1–3].
The pathogenesis of SSc is very complex and its aetiology still unknown, although both environmental and genetic factors play an important role in the development of disease [4]. Among the genetic factors, the possible contribution of certain human leucocyte antigen (HLA) class II alleles has been reported [5], but no single major HLA allele predisposes to SSc in all ethnic groups [2] and some individuals develop SSc in the absence of known predisposing alleles, suggesting that other non-HLA loci might contribute to susceptibility to SSc. Recently, the cytotoxic T lymphocyte antigen-4 (CTLA-4, CD152) gene has been pointed out as a good candidate locus for susceptibility to SSc, as reported widely for other autoimmune disorders [6].
CTLA-4 is a transmembrane homodimer glycoprotein of about 40 kDa expressed transiently on activated CD4+ and CD8+ T cells and constitutively on CD4+ CD25+ T regulatory cells, few non-lymphoid normal cells [7] and a variety of neoplastic cells [8, 9].
CTLA-4 behaves as a negative regulator of activation and effector function of T cells by inhibiting interleukin (IL)-2 and interferon (IFN)-γ production, IL-2 receptor expression and cell cycle progression upon interaction with its ligands CD80/CD86 expressed on antigen-presenting cells [7]. As such ligands are shared with CD28, one possibility for CTLA-4 action mechanism is ligand recruitment and prevention of CD28-mediated positive T cell signalling [10]. However, other mechanisms have been reported, including association with intracellular signalling molecules and direct or indirect interference with T cell receptor (TCR) signalling events [7].
The CTLA-4 gene maps on the 2q33 region and consists of four exons. Polymorphisms of the CTLA-4 gene have been identified in the promoter, exons 1 and 4, and in many different populations found to be associated with several autoimmune diseases [11], including systemic lupus erythematosus [12], Graves' disease [13], insulin-dependent diabetes mellitus [14], rheumatoid arthritis [15], myasthenia gravis [16], multiple sclerosis [17] and coeliac disease [18]. Few studies have been reported on SSc and their results are controversial [19–22].
In the present study, we investigated whether two CTLA-4 single nucleotide polymorphisms (SNPs) found commonly to be associated with autoimmune diseases, located at position −318 (SNP C/T) of the promoter region and at position +49 (SNP A/G) of exon 1, respectively, were associated with SSc in Italian patients. CTLA-4 genotyping was performed by a simple and inexpensive SNP genotyping method, tetra-primer amplification refractory mutation system–polymerase chain reaction (T-ARMS–PCR), that amplifies and discriminates both alleles of each SNP by a single tube PCR reaction.
Materials and methods
Subjects
Forty-three consecutive unrelated Italian women suffering from SSc (mean age 54 years; range 35–77 years) diagnosed at the Department of Internal Medicine (University of Genoa, Italy) between January 2003 and June 2004 and 93 sex- and age-matched healthy blood donor volunteers (Transfusion Service, Galliera Hospital, Genoa, Italy) with neither clinical evidence nor a family history of autoimmune disorders have been enrolled in this study. Informed consent was obtained from both patients and controls according to institutional procedures. All patients were diagnosed on the basis of clinical signs according to the American College of Rheumatology (ACR) criteria [23]. According Le Roy's classification of SSc subsettings [24], 17 patients were affected by diffuse scleroderma and 23 patients by limited scleroderma. Forty patients (three patients were lost to follow-up) were tested for the presence of specific autoantibodies by standard methods: 15 were anti-Scl-70 positive, 18 were anti-centromere positive and 37 were anti-nuclear (ANA) positive. These patients were also tested for the presence of other autoantibodies: anti-mitochondrial (AMA) antibodies have been found in one patient and anti-RO/SSA (anti 60KD ribonuclerprotein also known as ‘Sjögren's syndrome A antigen’) antibodies in four patients. In addition, anti-thyroperoxidase (TPO) and anti-thyroglobulin (TGA) antibodies were tested by an immunoenzymometric assay (Radim Diagnostic, Rome, Italy): for anti-TPO normal and elevated levels were < 40 and > 75 U/ml, respectively; for anti-TGA normal and elevated levels were < 100 and > 120 U/ml. Elevated levels of anti-TPO (> 230 U/ml) and of anti-TGA (> 120 U/ml) antibodies have been found in 20 patients (positive), while in the remaining patients (negative) the levels were below the normal level (anti-TPO < 40 U/ml and anti-TGA < 100 U/ml).
CTLA-4 genotyping by ARMS–PCR
Genomic DNA was extracted from 300 µl of peripheral blood in sodium citrate using Puregene DNA Purification System (Gentra Systems, Minneapolis, MN, USA).
The −318 C/T and +49 A/G SNPs (rs5742909 and rs231775, respectively (available from NCBI at: http://www.ncbi.nlm.nih.gov/SNP/index.html), have been genotyped by the T-ARMS–PCR [25–27] that is able to identify both alleles of a SNP by a single tube PCR reaction. In T-ARMS–PCR a pair of common (outer) primers produces a non-allele-specific control amplicon, and in combination with two allele-specific (inner) primers (designed in opposite orientation) produce allele-specific amplicons. The allele-specific amplicons are of different lengths and can be separated easily by standard gel electrophoresis, because the mutation is located asymmetrically with respect to the common (outer) primers.
The primers for the T-ARMS–PCR for −318 C/T and +49 A/G polymorphisms were designed on the basis of the published CTLA-4 sequence (GenBank accession no. M74363). The primer sequences and the amplicon sizes are reported in Table 1.
Table 1.
Primers and conditions for the tetra-primer amplification refractory mutation system–polymerase chain reaction (T-ARMS–PCR) cytotoxic T lymphocyte antigen-4 (CTLA-4) polymorphisms.
| SNP | Name1 | Primer sequences2 | Conc3 (µM) | Amplicon size (bp)4 | Ta5 |
|---|---|---|---|---|---|
| −318 C/T | 318Fo | 5′-CAATGAAATGAATTGGACTGGATG-3′ | 0·5 | K 296 | 58°C |
| 318Ro | 5′-TGCACACACAGAAGGCTCTTGAATA-3′ | 0·5 | C 201 | ||
| 318Fi(C) | 5′-CTCCACTTAGTTATCCAGATCTTC-3′ | 0·8 | T 141 | ||
| 318Ri(T) | 5′-ACTGAAGCTTCATGTTCACTCTA-3′ | 1·0 | |||
| +49 A/G | 49Fo | 5′-GTGGGTTCAAACACATTTCAAAGCTTCAGG-3′ | 0·25 | K 229 | 62°C |
| 49Ro | 5′-TCCATCTTCATGCTCCAAAAGTCTCACTC-3′ | 0·5 | A 162 | ||
| 49Fi(G) | 5′-GCACAAGGCTCAGCTGAACCTGGATG-3′ | 2·0 | G 120 | ||
| 49Ri(A) | 5′-ACAGGAGAGTGCAGGGCCAGGTCCTAGT-3′ | 1·0 |
F: forward; R: reverse. o: outer (common). i: inner (allele-specific). The nucleotide specificity is indicated in parentheses.
GenBank sequence ID number M74363. Deliberate mismatches (shown in italics) have been introduced to increase the specificity of the allele-specific primers.
The concentration (Conc) of each primer has been determined experimentally to increase specificity and to produce similar amplification of all amplicons.
K size of the control amplicon (shown in italics).
Ta: annealing temperature. Cycling conditions are 10 min at 95°C followed by 35 cycles of 94°C for 30 s, Ta (see above) for 30 s, 72°C for 30 s and a final cycle 72°C for 7 min.
The PCR reaction (total volume 20 µl) contained 100 µM deoxyribonucleoside triphosphate (dNTP), 1 U DNA polymerase AmpliTaq with its buffer (Applera Europe BV, Nieuwerkerk aan den Ijssel, the Netherlands), 100 ng of genomic DNA and the appropriate concentration of each primer (see Table 1). The cycling conditions were 10 min at 95°C followed by 35 cycles of 94°C for 30 s, annealing temperature as in Table 1 for 30 s, 72°C for 30 s and a final cycle 72°C for 7 min. PCR products were separated by standard electrophoresis on 2·5% agarose gel containing ethidium bromide.
The T-ARMS–PCR procedures for both −318 C/T and +49 A/G polymorphisms have been validated by analysis of the same samples by two standard methods such as restriction fragment length polymorphism (RFLP)–PCR [28] and direct DNA sequencing [17].
Statistical analysis
Deviation from Hardy–Weinberg equilibrium (HWE) was tested with Pearson's χ2 test using the Finetti program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Association between polymorphisms and disease status has been tested by using χ2 test or Fisher's exact test, as appropriate, with software statview version 4·51 (Abacus concept for Mac OS 9·2·2). Statistical significance has been accepted for any P < 0·05.
Results
Genotyping of CTLA-4 promoter (−318 C/T) and exon 1 (+49 A/G) by T-ARMS–PCR
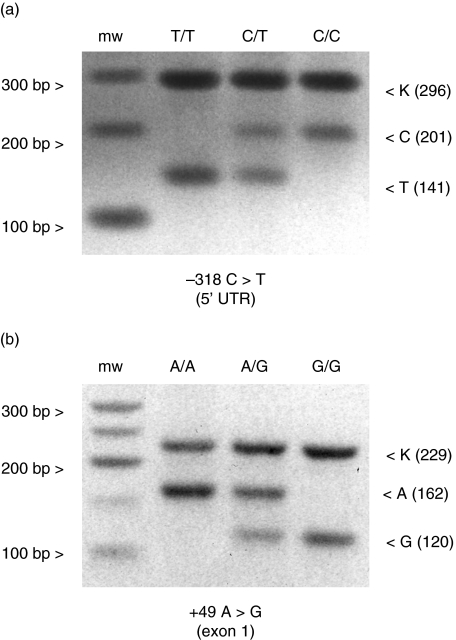
We have analysed two CTLA-4 SNP polymorphisms at position −318 (C/T substitution) of the promoter region and at position +49 (A/G substitution) of exon 1 in 136 subjects (43 female SSc patients and 93 age-matched healthy women) by a newly designed T-ARMS–PCR method (Fig. 1a for −318 C/T and Fig. 1b for +49 A/G). In order to validate the T-ARMS–PCR all samples were genotyped subsequently using two independent and established methods, RFLP–PCR [28] and direct DNA sequencing [17]: the results were identical to that obtained by the T-ARMS–PCR. No single discrepancy was observed.
Fig. 1.
Tetra-primer amplification refractory mutation system–polymerase chain reaction (T-ARMS–PCR) for cytotoxic T lymphocyte antigen-4 (CTLA-4) −318 C/T and +49 A/G. Representative analysis of each one of homozygotes and of one heterozygote are shown for each polymorphism: (a) −318 C/T; (b) +49 A/G. mw: Molecular weight marker. Bands: K: non-allele-specific control band; C: C allele-specific band; T: T allele-specific band; A: A allele-specific band; G: G allele-specific band. The number in parenthesis by each band indicates the amplicon size in base pairs.
Genotype frequencies of CTLA-4 −318 C/T and +49 A/G polymorphisms in SSc patients and controls
The allele and the genotype frequencies of the −318 C/T and the +49 A/G CTLA-4 polymorphisms in SSc patients (n = 43) and in healthy controls (n = 93) are reported in Table 2. No deviation from the Hardy–Weinberg equilibrium was observed for both polymorphisms either in SSc patients or in healthy controls (Table 2). The allele and the genotype frequencies in our healthy controls are similar to that reported previously for healthy Italian population [28].
Table 2.
Frequencies of cytotoxic T lymphocyte antigen-4 (CTLA-4) −318 C/T and +49 A/G polymorphisms in systemic sclerosis (SSc) patients and in healthy controls.
| n (%) | n (frequency) | ||||||
|---|---|---|---|---|---|---|---|
| Genotypes | Patients (n = 43) | Controls (n = 93) | P | Alleles | Patients (2n = 86) | Controls (2n = 186) | P |
| −318 C/T | −318 C/T | ||||||
| C/C | 31 (72·1) | 71 (76·4) | 0·341 | C | 74 (0·86) | 161 (0·87) | 0·992 |
| C/T | 12 (27·9) | 19 (20·4) | T | 12 (0·14) | 25 (0·13) | ||
| T/T | 0 (0) | 3 (3·2) | |||||
| HWE | HWE | ||||||
| P = 0·29 | P = 0·24 | ||||||
| +49 A/G | +49 A/G | ||||||
| A/A | 18 (41·9) | 53 (57) | 0·221 | A | 59 (0·69) | 141 (0·76) | 0·242 |
| A/G | 23 (54·5) | 35 (37·6) | G | 27 (0·31) | 45 (0·24) | ||
| G/G | 2 (4·6) | 5 (5·4) | |||||
| HWE | HWE | ||||||
| P = 0·11 | P = 0·80 | ||||||
| Diplotypes −318 and +49 | Haplotypes −318 and +49 | ||||||
| CA/CA | 11 (25·6) | 36 (38·7) | 0·391 | −318C +49 A: CA | 47 (0·55) | 116 (0·62) | 0·421 |
| CA/CG | 18 (41·9) | 30 (32·3) | −318C +49 G: CG | 27 (0·31) | 45 (0·24) | ||
| CA/TA | 7 (16·3) | 14 (15·1) | − 318T +49 A: TA | 12 (0·14) | 25 (0·14) | ||
| CG/TA | 5 (11·6) | 5 (5·4) | |||||
| CG/CG | 2 (4·7) | 5 (5·4) | |||||
| TA/TA | 0 (0) | 3 (3·2) | |||||
| HWE | HWE | ||||||
| P = 0·33 | P = 0·67 | ||||||
HWE: Hardy–Weinberg equilibrium tested by Pearson's χ2 test (P < 0·05 indicates a lack of HWE).
χ2 test
Fisher's test.
We found that SSc patients and healthy controls have similar frequencies of alleles (Fisher's test: −318 C/T, P = 0·99; +49 A/G, P = 0·24), of genotypes (χ2 test: −318 C/T, P = 0·34; +49 A/G, P = 0·22) and of subjects carrying the less common allele (−318T, 27·9% versus 23·6%, P = 0·34 + 49 G, 59·1% versus 43%, P = 0·22) for both polymorphisms.
Haplotypes have been inferred based upon our study (this and other unpublished series) and on previously reported data [29, 30]. Because of the strict linkage between the two loci, only three of the theoretically four possible haplotypes have been observed (−318C and +49 A: CA; −318C and +49 G: CG; −318T and +49 A: TA). Diplotype frequencies fitted Hardy–Weinberg equilibrium in both patients and controls. The frequencies of haplotypes (χ2 test, P = 0·42) and of their combinations (diplotypes) (χ2 test, P = 0·39) are not statistically different between patients and controls (Table 2).
Genotype frequencies of CTLA-4 −318 C/T and +49 A/G polymorphisms and serological characteristics of SSc patients
SSc is a complex disease with high heterogeneity of clinical manifestations and serological features; thus, we have explored further the possibility that CTLA-4 polymorphisms could be associated with some of these features. In the 40 patients for whom follow-up data were available, we found no association of the two CTLA-4 polymorphisms with positivity for tested autoantibodies (anti-Scl-70, anti-centromere, ANA, AMA, SSA) (data not shown) but positivity for anti-TPO and anti-TGA antibodies. Positivity for anti-TPO and anti-TGA antibodies defines a group of clinically unusual SSc patients with the associate occurrence of Hashimoto's thyroiditis (HT). In our series 20 patients were negative (SSc/HT−) and 20 were positive (SSc/HT+) for the presence of anti-TPO and anti-TGA antibodies. Genotype frequencies according to positivity for anti-TPO/TGA antibodies are reported in Table 3. No statistically significant deviation from the Hardy–Weinberg equilibrium was observed for both polymorphisms either in SSc/HT− or in SSc/HT+ patients (Table 3).
Table 3.
Frequencies of cytotoxic T lymphocyte antigen-4 (CTLA-4) −318 C/T and +49 A/G polymorphisms in systemic sclerosis (SSc) patients according to anti-thyroperoxidase (TPO) and anti-thyroglobulin (TGA) status.
| n (%) | ||||
|---|---|---|---|---|
| CTLA-4 genotypes | HT− (n = 20) | HT+ (n = 20) | Controls (n = 93) | P1 |
| –318 C/T | ||||
| C/C | 11 (55%) | 18 (90%) | 71 (76·4%) | 0·060 |
| C/T | 9 (45%) | 2 (10%) | 19 (20·4%) | |
| T/T | 0 (0%) | 0 (0%) | 3 (3·2%) | |
| HWE | HWE | HWE | ||
| P = 0·19 | P = 0·81 | P = 0·24 | ||
| T-allele | ||||
| T− (C/C) | 11 (55%) | 18 (90%) | 71 (76·3%) | 0·034 |
| T+ (C/T, T/T) | 9 (45%) | 2 (10%) | 22 (23·7%) | |
| +49 A/G | ||||
| A/A | 6 (30%) | 9 (45%) | 53 (57%) | 0·23 |
| A/G | 13 (65%) | 10 (50%) | 35 (37·6%) | |
| G/G | 1 (5%) | 1 (5%) | 5 (5·4%) | |
| HWE | HWE | HWE | ||
| P = 0·08 | P = 0·39 | P = 0·80 | ||
| G-allele | ||||
| G− (A/A) | 6 (30%) | 9 (45%) | 53 (57%) | 0·076 |
| G+ (A/G, G/G) | 14 (70%) | 11 (55%) | 40 (43%) | |
HWE: Hardy–Weinberg equilibrium tested by Pearson's χ2 test (P < 0·05 indicates a lack of HWE).
χ2 test. HT−: SSc patients anti-TPO/TGA). HT+: SSc patients positive for anti-TPO/TGA.
Comparison of these two groups of patients has shown that the frequency of subjects carrying the less common allele (mutant) −318T (genotypes C/T and T/T: T+) is significantly higher in SSc/HT− patients (45%) than in both SSc/HT+ patients (10%) and healthy controls (23%) (χ2 test, P = 0·034). Pairwise comparison of SSc/HT− with SSc/HT+ patients has confirmed this significant difference [Fisher's test, P = 0·031; odds ratio (OR) = 7·36; confidence interval (CI) 95%: 1·34–40·55], and has shown a trend towards a significant difference between SSc/HT− patients and healthy controls (Fisher's test, P = 0·094; OR = 2·64; CI 95%: 0·97–7·19). The frequencies of T+ subjects are similar in SSc/HT+ patients and healthy controls (Fisher's test, P = 0·24).
In addition, 70% of SSc/HT− patients carry the less common allele (mutant) +49 G (genotypes A/G and G/G: G +): this frequency is higher, but not significantly, than that observed in both SSc/HT+ patients and healthy controls (55% and 43%, respectively (χ2 test, P = 0·076). In particular, the frequency of G+ subjects is significantly higher in SSc/HT− patients than healthy controls (Fisher's test, P = 0·047; OR = 3·09; CI 95%: 1·09–8·75), while there are no significant differences between SSc/HT− and SSc/HT+ patients (Fisher's test, P = 0·51) and between SSc/HT+ patients and healthy controls (Fisher's test, P = 0·46)
Haplotype and diplotype frequencies of CTLA-4 −318 C/T and +49 A/G polymorphisms in SSc/HT– and SSc/HT+ patients
The frequencies of haplotypes and of diplotypes have been analysed within the two groups of SSc patients with respect to healthy controls. The frequencies of diplotypes in all three groups fitted the Hardy–Weinberg equilibrium (Table 4). Haplotype frequencies are significantly different in SSc/HT−, SSc/HT+ and healthy control groups (χ2 test: P = 0·043); in fact, the more rare TA haplotype is more frequent in SSc/HT− patients (23%) than in SSc/HT+ patients and in healthy controls (5% and 14%, respectively), the CA haplotype is more frequent in SSc/HT+ patients and in healthy controls (65% and 62%, respectively) than in SSc/HT− patients (40%), while the frequency of the CG haplotype appears to be similar (37% in SSc/HT−; 30% in SSc/HT+; 24% in healthy controls) in all three groups.
Table 4.
Frequencies of haplotypes and diplotypes of cytotoxic T lymphocyte antigen-4 (CTLA-4) −318 C/T and +49 A/G polymorphisms in systemic sclerosis (SSc) patients according to anti-thyroperoxidase (TPO) and anti-thyroglobulin (TGA) status.
| n (frequency) | ||||
|---|---|---|---|---|
| HT− (2n = 40) | HT+ (2n = 40) | Controls (2n = 186) | P | |
| Haplotypes | ||||
| –318C +49 A: CA | 16 (0·40) | 26 (0·65) | 116 (0·62) | 0·0431 |
| –318C +49 G: CG | 15 (0·37) | 12 (0·30) | 45 (0·24) | |
| –318T +49 A: TA | 9 (0·23) | 2 (0·05) | 25 (0·14) | |
| n (%) | ||||
|---|---|---|---|---|
| Diplotype combinations | HT−2 (n = 20) | HT+3 (n = 20) | Controls (n = 93) | P |
| T+G+ | 4 (20) | 1 (5) | 5 (5·4) | 0·0281 |
| CG/TA | 4 (20) | 1 (5) | 5 (5·4) | |
| T+/G− | 5 (25) | 1 (5) | 17 (18·3) | |
| CA/TA | 5 (25) | 1 (5) | 14 (15·1) | |
| TA/TA | 0 (0) | 0 (0) | 3 (3·2) | |
| T−/G+ | 10 (50) | 10 (50) | 35 (37·7) | |
| CA/CG | 9 (45) | 9 (45) | 30 (32·3) | |
| CG/CG | 1 (5) | 1 (5) | 5 (5·4) | |
| T−/− | 1 (5) | 8 (40) | 36 (38·6) | |
| CA/CA | 1 (5) | 8 (40) | 36 (38·6) | |
| HWE | HWE | HWE | ||
| P = 0·11 | P = 0·81 | P = 0·67 | ||
HWE: Hardy–Weinberg equilibrium tested by Pearson's χ2 test (P < 0·05 indicates a lack of HWE). HT −: SSc patients negative for anti-TPO/TGA. HT+: SSc patients positive for anti-TPO/TGA. In plain text are indicated diplotypes. Bold type indicates the combinations of diplotypes according the presence of both the mutant alleles (−318T and +49 G: T+/G+), only the −318T (T+/G−), only the +49 G (T −/G+) and none of them (T −/G–).
χ2 test
χ2 test: HT −versus HT+, P = 0·019 and HT −versus controls, P = 0·011.
χ2 test: HT+versus controls–, P = 0·48.
In addition, analysis of the diplotypes has shown a significant difference in the frequency of individuals carrying both the mutant alleles (−318T and +49 G: T+/G+), only the −318T (T+/G−), only the +49 G (T−/G+) and none of them (T−/G–): χ2 test, P = 0·028 (Table 4). In fact, T+/G+ and T+/G− individuals are much more frequent in SSc/HT− patients (20% and 25%, respectively) than in SSc/HT+ patients (5% and 5%, respectively) and in healthy controls (5·4% and 18·3%, respectively), T−/G− individuals are more frequent in SSc/HT+ patients and in healthy controls (40% and 38·6%, respectively, versus 5% in SSc/HT−), while the frequency of T−/G+ is similar in the thee groups (HT−: 50%; HT+ : 50%; controls: 37·7%). Pairwise comparison has confirmed the significant difference of frequencies of these diplotype combinations of SSc/HT− patients in the respect of SSc/H+ patients and of healthy controls (χ2 test: P = 0·019 and P = 0·011, respectively), while they are not different between SSc/HT+ patients and healthy controls (χ2 test: P = 0·48).
Discussion
CTLA-4 is a negative regulator of the immune response that provides a basal level of immune suppression. The activation state of T cells plays an important role in the complexpathogenesis of systemic sclerosis (SSc). Thus, it is possible that polymorphisms affecting CTLA-4 expression and/or function could affect susceptibility to SSc. Indeed, several studies have reported the association of specific CTLA-4 gene polymorphisms with a variety of autoimmune disorders [6, 11] but only few controversial studies have investigated a possible association with SSc.
The association of SSc with +49 A/G but not with −318 C/T has been reported in African American patients [20] and in Japanese patients with anti-RNP autoantibodies [22], whereas the association with −318 C/T but not with +49 A/G has been reported in Caucasian patients from Iran [19, 21]. We found no significant association of SSc with either −318 C/T or +49 A/G polymorphisms in 43 Italian women suffering from SSc compared to 93 age-matched healthy women.
Various factors could explain these discrepancies: for instance, differences in genetic background. In fact, the allele frequencies of both polymorphisms for healthy controls reported in these studies are quite different: in our controls the frequency of −318T is 13% and of +49G is 24%, compared to 3·6% and 36·4% in Caucasian (Iran) [19, 21], 1·5% and 21·2% in African Americans [20], 5·7% and 37% in Caucasian (USA) [20] and 12·6% and 66·8% in Japanese patients, respectively [22]. Differences in the definition of SSc are possible but unlikely, because all these studies refer to the ACR clinical criteria [23]. However, as SSc is a complex and heterogeneous disease, it is possible that each study includes variable proportions of patients with different biological backgrounds, such as the association with other autoimmune diseases that could be affected differentially by genetic factors. In keeping with this, the association with CTLA-4 +49 A/G polymorphism has been reported only in Japanese SSc patients positive for anti-RNP autoantibodies [22]. Indeed, in our SSc patients the positivity of anti-TPO and anti-TGA autoantibodies (but no other serological characteristics) was associated significantly with CTLA-4 polymorphisms. The positivity for these autoantibodies defines an unusual, and not infrequent, group of SSc patients suffering from concomitant HT [31].
A significant association of the CTLA-4 −318 C/T, in terms of T positive genotypes, was observed in SSc patients without HT (HT−) with respect to both SSc patients with HT (HT+) and healthy controls (P = 0·034). In fact, the frequency of patients carrying the more rare −318T allele is significantly much higher in HT− than HT+ SSc patients (P = 0·031). This observation is supported further by the finding that the haplotype TA (−318T and +49A), the unique haplotype containing the −318T allele, is significantly more frequent in HT− SSc patients than in HT+ SSc patients and in controls (P = 0·043).
With respect to the CTLA-4 +49 A/G, an association was apparently present with the G positive genotypes (HT− versus controls: P = 0·047). However, the frequency of haplotype CG (−318C and +49G), the unique haplotypecontaining the +49G allele, is similar in all three groups (Table 4) suggesting that the weak, if any, association of the +49 G allele with HT− SSc patients is due mainly to individuals carrying both TA and CG haplotypes. In fact, diplotypes containing the haplotype TA (CG/TA and CA/TA) are more frequent in HT− SSc patients, whereas diplotypes containing only the haplotype CG (CA/CG and CG/CG) have similar frequencies in all three groups (Table 4).
Our finding of an association of the −318 C/T polymorphism with SSc patients who are not co-affected with HT may not be surprising, if we consider that the role of CTLA-4 polymorphisms in the pathogenesis of HT is controversial [32] and that +49 A/G polymorphism does not play a role in susceptibility to HT in Italian patients [33]. We cannot rule out that CTLA-4 polymorphism protects from thyroiditis per se. However, it is reasonable to infer that in the Italian population such CTLA-4 polymorphisms may increase the risk of developing SSc and, on the other hand, may protect from the development of a concomitant HT. Indeed, the observation that our SSc patients carrying TA haplotypes are less prone to suffer from HT is in keeping with the recent report that the lowest levels of anti-TPO autoantibodies among patients with HT are present in patients with the TA haplotypes [30].
The functional effects of the CTLA-4 −318 C/T promoter polymorphism have not yet been elucidated completely. However, it is likely that it affects CTLA-4 transcription by altering a putative binding site for the transcription factor LEF1 [13, 34]. In particular, −318T up-regulates CTLA-4 transcription, resulting in an increased expression of both CTLA-4 isoforms: the soluble (sCTLA-4) and membrane-bound (mCTLA-4) isoforms [35]. It has been suggested that the increased serum levels of sCTLA-4, produced by resting T cells, could reduce CTLA-4 inhibitory function by competing with mCTLA-4 for binding to CD80/CD86 ligands on antigen-presenting cells [32]. It is possible that this mechanism underlies the association between such group of SSc patients without HT and CTLA-4 −318 C/T polymorphism. Indeed, high serum levels of sCTLA-4 have been reported in SSc patients [36].
The T-ARMS–PCR methodology, developed for this study, has provided a rapid, reproducible and cost-effective genotyping of both −318 C/T and +49 A/G CTLA-4 polymorphisms. It is noteworthy that this simple PCR technique allows the genotyping of a large number of samples without requiring any special equipment.
In conclusion, in Italian SSc patients the CTLA-4 −318 C/T promoter polymorphism appears to be associated with the susceptibility to develop SSc without thyroid involvement, whereas association with the +49 A/G is weak, if any. Larger epidemiological studies on fully clinical and serological characterized patients are needed to confirm definitively these findings. In addition, this kind of study would clarify whether the −318 C/T polymorphism is the functional responsible for this association or whether it is only a tag of another strictly linked polymorphism present in the same genomic region [29, 37].
Acknowledgments
This work was supported in part by grants from CARIGE (Genova, Italy), CARIVE (Verona, Italy), Ministero della Salute (Italy), CIPE (02/07/2004, CBA project) and Compagnia di San Paolo (Torino, Italy).
References
- 1.Derk CT, Jimenez SA. Systemic sclerosis: current views of its pathogenesis. Autoimmun Rev. 2003;2:181–91. doi: 10.1016/s1568-9972(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. [PubMed] [Google Scholar]
- 3.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 4.Tan FK. Systemic sclerosis: the susceptible host (genetics and environment) Rheum Dis Clin North Am. 2003;29:211–37. doi: 10.1016/s0889-857x(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RW, Tew MB, Arnett FC. The genetics of systemic sclerosis. Curr Rheumatol Rep. 2002;4:99–107. doi: 10.1007/s11926-002-0004-2. [DOI] [PubMed] [Google Scholar]
- 6.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 7.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 8.Contardi E, Palmisano GL, Tazzari PL, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–50. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 9.Pistillo MP, Tazzari PL, Palmisano GL, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–9. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 10.Carreno BM, Bennett F, Chau TA, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352–6. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 11.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–15. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar F, Torres B, Sanchez-Roman J, Nunez-Roldan A, Gonzalez-Escribano MF. CTLA4 polymorphism in Spanish patients with systemic lupus erythematosus. Hum Immunol. 2003;64:936–40. doi: 10.1016/s0198-8859(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 13.Chistiakov DA, Savost'anov KV, Turakulov RI, Efremov IA, Demurov LM. Genetic analysis and functional evaluation of the C/T (-318) and A/G (-1661) polymorphisms of the CTLA-4 gene in patients affected with Graves' disease. Clin Immunol. 2006;118:233–42. doi: 10.1016/j.clim.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Van der Auwera BJ, Vandewalle CL, Schuit FC, et al. CTLA-4 gene polymorphism confers susceptibility to insulin-dependent diabetes mellitus (IDDM) independently from age and from other genetic or immune disease markers. Clin Exp Immunol. 1997;110:98–103. doi: 10.1046/j.1365-2249.1997.5121410.x. The Belgian Diabetes Registry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya B, Pearce SH, Charlton S, et al. An association between the CTLA4 exon 1 polymorphism and early rheumatoid arthritis with autoimmune endocrinopathies. Rheumatology (Oxf) 2002;41:180–3. doi: 10.1093/rheumatology/41.2.180. [DOI] [PubMed] [Google Scholar]
- 16.Wang XB, Kakoulidou M, Qiu Q, et al. CDS1 and promoter single nucleotide polymorphisms of the CTLA-4 gene in human myasthenia gravis. Genes Immun. 2002;3:46–9. doi: 10.1038/sj.gene.6363816. [DOI] [PubMed] [Google Scholar]
- 17.Malferrari G, Stella A, Monferini E, et al. CTLA4 and multiple sclerosis in the Italian population. Exp Mol Pathol. 2005;78:55–7. doi: 10.1016/j.yexmp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Mora B, Bonamico M, Indovina P, et al. CTLA-4 +49 A/G dimorphism in Italian patients with celiac disease. Hum Immunol. 2003;64:297–301. doi: 10.1016/s0198-8859(02)00782-6. [DOI] [PubMed] [Google Scholar]
- 19.Almasi S, Erfani N, Mojtahedi Z, Rajaee A, Ghaderi A. Association of CTLA-4 gene promoter polymorphisms with systemic sclerosis in Iranian population. Genes Immun. 2006;7:401–6. doi: 10.1038/sj.gene.6364313. [DOI] [PubMed] [Google Scholar]
- 20.Hudson LL, Silver RM, Pandey JP. Ethnic differences in cytotoxic T lymphocyte associated antigen 4 genotype associations with systemic sclerosis. J Rheumatol. 2004;31:85–7. [PubMed] [Google Scholar]
- 21.Rajaee A, Ebrahimi A, Ghiam AF, Kalantari T, Ghaderi A. Exon-1 polymorphism of CTLA-4 gene is not associated with systemic sclerosis in Iranian patients. Rheumatol Int. 2006;26:687–92. doi: 10.1007/s00296-005-0047-6. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi F, Kawasugi K, Nabeta H, Mori M, Tanimoto K. Association of CTLA-4 with systemic sclerosis in Japanese patients. Clin Exp Rheumatol. 2002;20:823–8. [PubMed] [Google Scholar]
- 23.American Rheumatism Association. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 24.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 25.Ye S, Humphries S, Green F. Allele specific amplification by tetra-primer PCR. Nucl Acids Res. 1992;20:1152. doi: 10.1093/nar/20.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamajima N, Saito T, Matsuo K, Kozaki K, Takahashi T, Tajima K. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res. 2000;91:865–8. doi: 10.1111/j.1349-7006.2000.tb01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccioli P, Serra M, Gismondi V, et al. Multiplex tetra-primer amplification refractory mutation system PCR to detect 6 common germline mutations of the MUTYH gene associated with polyposis and colorectal cancer. Clin Chem. 2006;52:739–43. doi: 10.1373/clinchem.2005.060137. [DOI] [PubMed] [Google Scholar]
- 28.Svahn J, Capasso M, Lanciotti M, et al. 18C > T in the promoter and 49A > G in exon 1 of CTLA4 and the risk of aplastic anemia in a Caucasian population. Bone Marrow Transplant. 2005;35(Suppl. 1):S89–92. doi: 10.1038/sj.bmt.1704855. The polymorphisms −3. [DOI] [PubMed] [Google Scholar]
- 29.Holopainen PM, Partanen JA. Technical note. linkage disequilibrium and disease-associated CTLA4 gene polymorphisms. J Immunol. 2001;167:2457–8. doi: 10.4049/jimmunol.167.5.2457. [DOI] [PubMed] [Google Scholar]
- 30.Zaletel K, Krhin B, Gaberscek S, Hojker S. Thyroid autoantibody production is influenced by exon 1 and promoter CTLA-4 polymorphisms in patients with Hashimoto's thyroiditis. Int J Immunogenet. 2006;33:87–91. doi: 10.1111/j.1744-313X.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 31.Farzati B, Mazziotti G, Cuomo G, et al. Hashimoto's thyroiditis is associated with peripheral lymphocyte activation in patients with systemic sclerosis. Clin Exp Rheumatol. 2005;23:43–9. [PubMed] [Google Scholar]
- 32.Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003;31:21–36. doi: 10.1677/jme.0.0310021. [DOI] [PubMed] [Google Scholar]
- 33.Petrone A, Giorgi G, Mesturino CA, et al. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto's thyroiditis in an Italian population. Thyroid. 2001;11:171–5. doi: 10.1089/105072501300042901. [DOI] [PubMed] [Google Scholar]
- 34.Wang XB, Zhao X, Giscombe R, Lefvert AK. A CTLA-4 gene polymorphism at position -318 in the promoter region affects the expression of protein. Genes Immun. 2002;3:233–4. doi: 10.1038/sj.gene.6363869. [DOI] [PubMed] [Google Scholar]
- 35.Magistrelli G, Jeannin P, Herbault N, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, Fujimoto M, Hasegawa M, et al. Serum soluble CTLA-4 levels are increased in diffuse cutaneous systemic sclerosis. Rheumatology (Oxf) 2004;43:1261–6. doi: 10.1093/rheumatology/keh303. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GC, Esposito L, Barratt BJ, et al. Haplotype tagging for the identification of common disease genes. Nat Genet. 2001;29:233–7. doi: 10.1038/ng1001-233. [DOI] [PubMed] [Google Scholar]