Abstract
Neutrophils enter tissues including the uterus and are found in the endometrium in increased numbers prior to menses. In this environment, they are exposed to transforming growth factor (TGF)-β1 produced by endometrial stromal and epithelial cells. We observed that incubation of neutrophils in vitro with TGF-β1 at 1 pg/ml significantly reduced their secretion of lactoferrin in response to lipopolysaccharide (LPS). This effect was achieved with as little as 15 min of pretreatment with TGF-β1. Inhibition of lactoferrin release by TGF-β1 was observed irrespective of whether neutrophils were stimulated by ligands for Toll-like receptor (TLR)-2, TLR-4 or FPR, the G protein-coupled receptor for formylated peptides. Inhibition by TGF-β1 was negated by SB-431542, a small molecule inhibitor that specifically blocks the kinase activity of the type I TGF-β receptor (ALK5) In contrast to lactoferrin release, another important neutrophil function, interleukin (IL)-8 driven chemotaxis, was not affected by TGF-β1 at 1 pg/ml or 100 pg/ml. We conclude that in tissues of the female reproductive tract, TGF-β1 inhibition of neutrophil degranulation may prevent these cells from initiating an inflammatory response or releasing degradative enzymes that could potentially damage the oocyte or fetus.
Keywords: chemotaxis, lactoferrin, LPS, neutrophil, TGF-β
Introduction
Transforming growth factor (TGF)-β is a multi-functional cytokine produced by many different tissues and cell types [1]. Its pleiotrophic effects on different target cells regulate multiple functions including cell growth, differentiation, steroidogenesis, cell adhesion, regulation of extracellular matrix, angiogenesis and tissue remodelling. TGF-β exists in three isoforms: TGF-β1, TGF-β2 and TGF-β3 [2]. These TGF-β isoforms are highly conserved and display a complex pattern of interactions through multiple membrane receptor components. The most commonly occurring form of TGF-β is TGF-β1, the other forms being expressed in a more limited array of tissues and cell types [2].
All the phenomena associated with angiogenesis, tissue remodelling and differentiation take place in the human endometrium during the menstrual cycle. Therefore, it is not surprising to find that TGF-β1 is produced by endometrial epithelial and stromal cells [3–5]. In epithelial cells, its expression greatly increases during the proliferative to early mid-secretory phase and diminishes in the late secretory phase. In stromal cells, it is expressed most highly during the proliferative phase [3, 5]. This suggests that the production of TGF-β in the female reproductive tract is regulated by hormones.
Neutrophils, like almost all cells of the body, express receptors for TGF-β. Approximately 350 high-affinity TGF receptors per cell were detected by binding of radiolabelled TGF-β [6], which is roughly equivalent to the number expressed by monocytes [7]. Neutrophils residing in reproductive tract tissue are exposed to TGF-β at a site that is vulnerable to invasion by microorganisms because it is connected directly to the exterior of the body. It was therefore of interest to examine the effect of TGF-β on the innate protective function of neutrophils.
Neutrophils are found in the endometrium and other tissues of the female reproductive tract. During most of the menstrual cycle, neutrophils occur in endometrium in low numbers [8]. That they are present at all is somewhat surprising, as it is generally thought that neutrophils enter tissues in response to infections or tissue damage [9]. However, the presence of endometrial neutrophils does not produce an inflammatory condition under normal circumstances. Immediately prior to menses, the number of neutrophils in endometrium rises dramatically [10]. This correlates with endometrial epithelial cell production of the neutrophil attractant interleukin (IL)-8, which increases throughout the menstrual cycle and is highest at the late secretory phase [11]. At menses, endometrial neutrophils localize to regions of tissue breakdown [12] and are thought to contribute to the shedding of the functionalis.
Degranulation is an important property of neutrophils that fulfils two vital roles in innate immunity. It releases the microbicidal contents of neutrophil granules and adds adhesion molecules to the surface of the plasma membrane [13, 14]. Among granule constituents, lactoferrin is a major product that is stored in secondary (specific) granules [15]. It is an important microbicide, preventing bacterial growth by sequestering essential iron [16]. Sites of infection are high in both neutrophils and their secreted lactoferrin [17] and individuals deficient in neutrophil lactoferrin suffer from recurrent infections [18]. Degranulation may be triggered both by exudation into tissue [13] or by microbial stimuli [19]. Our studies focused on the effects of TGF-β on lactoferrin release as a measure of the possible effects of TGF-β on neutrophil innate immune function.
Materials and methods
Neutrophil separation and culture
Neutrophils were isolated as described previously [20] from venous blood from healthy laboratory volunteers. Our protocol for using blood donors was reviewed by the Dartmouth College Committee for Protection of Human Subjects and all participants gave informed consent prior to donating. All manipulations were performed in a sterile, laminar flow tissue culture hood with sterile reagents. Briefly, red cells were sedimented by addition of 1 ml of Hetasep (Stem Cell Technologies Inc., Vancouver, Canada) per 5 ml of blood. The upper leucocyte-rich fraction was layered onto a discontinuous density gradient of Histopaque 1·077 g/ml (Sigma, St Louis, MO, USA) over Optiprep 1·095 g/ml (Axis Shield, Oslo, Norway) and centrifuged at 40 g for 25 min at room temperature. The neutrophil fraction was recovered from the interface between the Histopaque and Optiprep layers and washed three times in RPMI-1640 medium (Mediatech, Inc., Herndon, VA, USA) at room temperature. Purity of the neutrophil preparation was routinely 95% or greater. All separation and culture reagents contained less than 0·01 ng/ml lipopolysaccharide (LPS).
Neutrophil degranulation
Neutrophils were resuspended at 3 × 106/ml in RPMI-1640/2% charcoal/dextran-stripped fetal bovine serum (FBS) (HyClone, Logan, UT, USA)/5 mM HEPES and 50 µg/ml gentamicin (Gibco, Carlsbad, CA, USA) (complete medium.) Stripped serum was used to avoid any hormone effects on the system. Neutrophil suspension (120 µl) was added to flat-bottomed 96-well microtitre plates and biologically active, recombinant human TGF-β1 (Peprotech, Rocky Hill, NJ, USA) added as indicated. The cells were cultured at 37°C in a 5% CO2 incubator for the indicated time-periods. Ultrapure LPS, peptidoglycan (Invivogen, San Diego, CA, USA), formylated MetLeuPhe (fMLP) (Sigma) or medium was added and the cells incubated for a further 2 h. The plates were then centrifuged and an aliquot of the supernatant removed for analysis by enzyme-linked immunosorbent assay (ELISA). Results are shown as one experiment representative of three or more separately conducted experiments. Data are shown as mean and standard deviation of three replicate cultures per test condition.
TGF-β1
The biological activity of each batch of recombinant TGF-β1 was determined at source, by testing the ability to inhibit the IL-4-dependent proliferation of mouse HT-2 cells. All batches were active at < 0·05 ng/ml, corresponding to a specific activity of 2 × 107 units/mg. Upon receipt on ice the TGF-β1 was aliquoted and stored at −20°C. Aliquots were thawed once and discarded.
ALK-5 inhibitor
The inhibitor SB431542 (ALK-5 inhibitor) was obtained from Sigma-Aldrich (St Louis, MO, USA). This compound is a selective and potent inhibitor of the ALK-5 kinase activity associated with the TGF type I receptor. The compound was dissolved in dimethylsulphoxide (DMSO) at 100 µM prior to use. Neutrophils were aliquoted into flat-bottomed microtitre wells and ALK-5 inhibitor added to the final concentration as indicated. Control neutrophils were treated with the same volume of DMSO without inhibitor. The cells were incubated at 37°C for 15 min, after which TGF-β1 or medium were added and the cells incubated for a further 45 min before assaying lactoferrin release in response to LPS.
ELISA assay
Neutrophil lactoferrin secretion was measured using a lactoferrin ELISA development kit (Jackson Immunoresearch Laboratories, West Grove, PA, USA), according to the manufacturer's instructions. Briefly, ELISA plates were coated overnight at room temperature with affinity-purified, rabbit anti-human lactoferrin at 1 µg/ml. After blocking with bovine serum albumin (BSA) and washing according to the manufacturer's protocol, 100 µl of neutrophil supernatants, medium or human milk lactoferrin standards (Sigma) were added and incubated for 2 h at room temperature. After washing, bound lactoferrin was detected using alkaline phosphatase-conjugated, affinity-purified, rabbit anti-human lactoferrin and p-nitrophenyl phosphatephosphatase substrate (Sigma). The reaction was stopped by addition of 1 N H2SO4 and read on a microtitre plate reader at 450 nm. Lactoferrin concentration was determined using linear regression from the standard curve.
Chemotaxis assay
The membranes of Transwell inserts (Falcon Plastics, Beckton Dickinson, Franklin, NJ, USA) with 3 µm pores were coated with a thin layer of Matrigel (BD Bioscience, Bedford, MA, USA) by adding 100 µl of Matrigel diluted 1 : 4 in cold RPMI-1640, and drawing off non-solidified Matrigel after 1 min on ice. Neutrophils were purified and resuspended in RPMI-1640 containing 5 mM HEPES and 0·5% human albumin (Aventis Behring, King of Prussia, PA, USA) at 1 × 107/ml. Neutrophils were then incubated for 30 min at 37°C with TGF-β1 or medium. Chemoattractants (600 µl) or medium were added to the bottom chamber as indicated. Neutrophils (2 × 106 cells) in 200 µl were added to the top chamber, and the plates incubated at 37°C in a 5% CO2 incubator for 1·5 h. The plates were chilled on ice and neutrophils that had traversed the membrane to the bottom chamber were collected and counted using a haemocytometer.
Statistical analysis
Lactoferrin release is expressed as mean and standard deviation of triplicate cultures. Comparison between TGF-β1-treated and non-treated cultures was performed using a two-tailed paired t-test with P ≤ 0·05, indicating a statistically significant difference. The subdivisions of statistical differences are represented by asterices as follows: *P < 0·05; **P < 0·01; ***P < 0·001.
Results
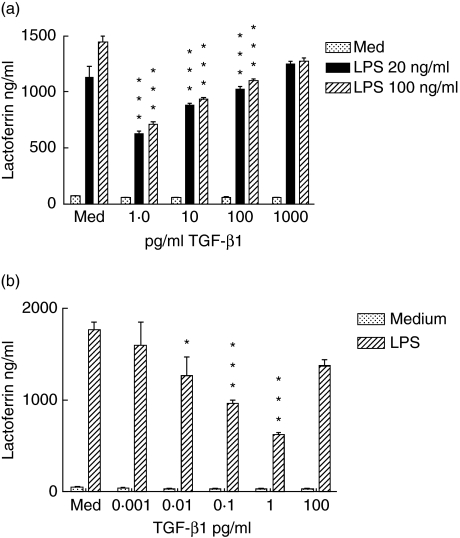
Our first objective was to examine the effects of TGF-β1 on neutrophil function. We observed that neutrophils pretreated for 1 h with a low dose of TGF- β1, 1 pg/ml, released significantly less lactoferrin than medium-treated control cells when incubated for 2 h with LPS (Fig. 1a) The degree of reduction did not vary to any great extent between low (20 ng/ml) or high (100 ng/ml) concentrations of LPS. Higher concentrations of TGF-β1 produced successively smaller amounts of inhibition. This reverse concentration effect of TGF-β1 has been reported in other instances, including a report on TGF-β modulation of IL-12 responsiveness in T cells [21]. In further experiments we observed that the TGF-β1 was no longer inhibitory when it was diluted to levels below 0·01 pg/ml (Fig. 1b).
Fig. 1.
(a) Pretreatment of neutrophils with transforming growth factor (TGF)-β1 diminishes degranulation in response to lipopolysaccharide (LPS). Neutrophils at 3 × 106/ml were incubated for 1 h with the indicated concentrations of TGF-β1 and then challenged with LPS at 20 ng/ml (black bars) or 100 ng/ml (striped bars) or were unstimulated (stippled bars.) After 2 h, supernatants were harvested and lactoferrin content determined by enzyme-linked immunosorbent assay. Results are representative of eight separately performed experiments. (b) The inhibitory effect of TGF-β1 can be diluted out. Neutrophils were incubated with TGF-β1 and challenged with 100 ng/ml LPS (striped bars) or no stimulus (stippled bars) and lactoferrin secretion determined. Results are representative of three separately performed experiments. In both figures, error bars indicate mean and standard deviation of three replicate cultures. Differences between cultures with LPS only and LPS plus TGF were analysed by paired t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
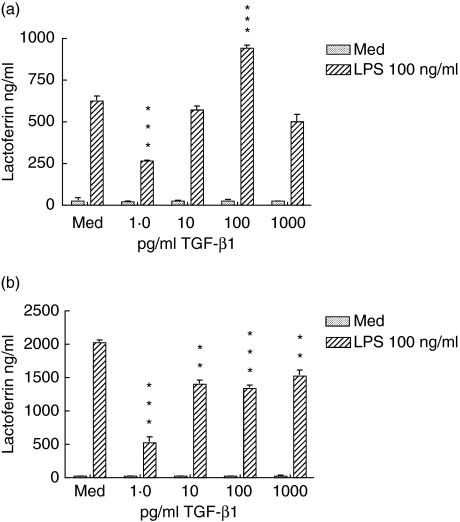
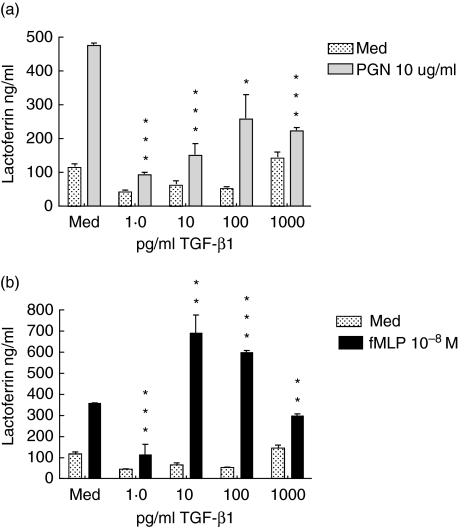
Further experiments demonstrated that the inhibitory effect of TGF-β1 on LPS-induced neutrophil degranulation could be detected with as little as 15 min of exposure (Fig. 2a). In these experiments, lactoferrin secretion was reduced by 60% by a 15-min pretreatment with TGF-β1 and by 75% by a 1-h treatment (Fig. 2b). Overall, neutrophils cultured for 1 h showed higher levels of lactoferrin release than neutrophils cultured for 15 min. We then compared the ability of TGF-β1 to inhibit lactoferrin release mediated by Toll-like receptor (TLR) and non-TLR ligands (Fig. 3). Both LPS and peptidoglycan (PGN) bind through TLR-4 and TLR-2, respectively, and signal through the MyD88 pathway, whereas the receptor for fMLP is coupled to G-proteins. As we observed previously with LPS, the release of lactoferrin in response to both PGN and fMLP was inhibited at 1 pg/ml of TGF-β1 and less significantly at higher TGF-β1 concentrations.
Fig. 2.
The transforming growth factor (TGF)-β1 effect on neutrophils is rapid. Neutrophils were incubated with the indicated concentrations of TGF-β1 for 15 min (a) or 1 h (b). They were then challenged with 100 ng/ml of lipopolysaccharide (LPS). After 2 h, supernatants were harvested and lactoferrin content determined by enzyme-linked immunosorbent assay. Results are representative of three separately conducted experiments. Error bars indicate mean and standard deviation of three replicate cultures. Differences between cultures with LPS alone and LPS plus TGF were analysed by paired t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 3.
Transforming growth factor (TGF)-β1 diminishes degranulation in response to Toll-like receptor (TLR) and non-TLR bacterial ligands. Neutrophils were incubated with the indicated concentrations of TGF-β1 for 1 h. They were then challenged with 10 µg/ml peptidoglycan (a) or 10−8 M formylated MetLeuPhe (fMLP) (b). After 2 h, supernatants were harvested and lactoferrin content determined by enzyme-linked immunosorbent assay. Results are representative of three separately conducted experiments. Differences between cultures with ligands only and ligands plus TGF were analysed by paired t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
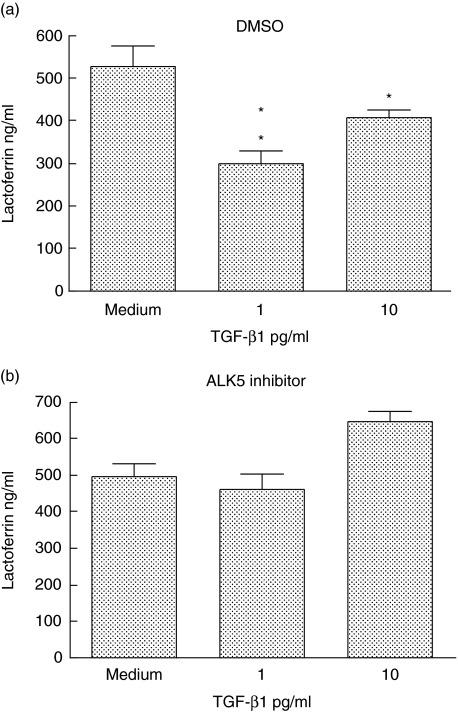
To demonstrate that the inhibitory effect was mediated through specific TGF receptors, neutrophils were pretreated with SB-431542 a specific inhibitor of the ALK5 kinase activity associated with the TGF receptor or the DMSO vehicle, then exposed to TGF-β1 and stimulated with LPS. As shown in Fig. 4, the inhibitory effect of TGF-β at 1 pg/ml (Fig. 4a) was negated in the presence of SB-431542 (Fig. 4b).
Fig. 4.
An inhibitor of ALK5 reverses the effect of transforming growth factor (TGF)-β1. Neutrophils were incubated for 15 min with (a) dimethylsulphoxide or (b) 1 µM SB431542. Medium or TGF-β1 at 1 or 10 pg/ml was then added and the cells cultured for a further 45 min. They were then challenged with lipopolysaccharide and supernatants harvested for lactoferrin determination after 2 h. Results are representative of three separately conducted experiments. Differences between cultures with and without TGF were analysed by paired t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
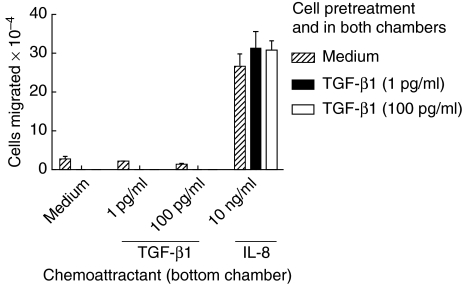
One of the important properties of neutrophils is chemotaxis towards sites of injury or infection. Because TGF-β is produced by stromal and other tissue cells it was of interest to determine how TGF-β1 might affect neutrophil chemotaxis. We performed chemotaxis using transwell membranes coated with extracellular matrix, to simulate more effectively the environment of migrating neutrophils. We observed that pretreatment of neutrophils with TGF-β1 at 1 pg/ml, the concentration that reduced lactoferrin release, did not alter their ability to migrate towards IL-8, even in the continuous presence of TGF-β1 (Fig. 5). High concentrations of TGF-β1 did not affect migration. In addition, TGF-β1 itself did not appear to mediate chemotaxis at 1 pg/ml or 100 pg/ml. Results were essentially the same, whether or not transwells were coated with extracellular matrix, and also in the Matrigel-free, under-agarose chemotaxis assay (data not shown); thus we think it is unlikely that the lack of TGF-β1 effect was due to sequestration of TGF-β1 in extracellular matrix. Overall, our data suggest that the effect of TGF-β1 on neutrophils appeared limited to inhibition of some responses, such as degranulation, whereas other functions such as chemotaxis were unaffected.
Fig. 5.
Transforming growth factor (TGF)-β1 does not inhibit chemotaxis at a concentration that diminishes degranulation. Striped bars: neutrophils (2 × 106) were incubated with medium for 1 h and chemotaxis performed with medium, TGF-β1 or 10 ng/ml interleukin (IL)-8 in the bottom chamber of the transwell, as indicated in the figure. Solid bars: neutrophils (2 × 106) were incubated for 1 h with TGF-β1 at 1 pg/ml and chemotaxis performed with IL-8 in the bottom chamber and 1 pg/ml of TGF-β1 in both upper and lower chambers. Open bars: neutrophils (2 × 106) were incubated for 1 h with TGF-β1 at 100 pg/ml and chemotaxis performed with IL-8 in the bottom chamber and 100 pg/ml of TGF-β1 in both upper and lower chambers. Results are representative of four separately conducted experiments. Results are shown as mean and standard deviation of three replicate chemotaxis chambers per group. There were no significant differences in IL-8-mediated chemotaxis between medium-pretreated or TGF-β1-pretreated neutrophils.
Discussion
We have shown that incubation of neutrophils with TGF-β1 at 1 pg/ml diminishes secretion of lactoferrin significantly in response to ligands for TLR-2, TLR-4 and the G protein-coupled FPR. This suppressive effect could be achieved with as little as 15 min of pretreatment with TGF-β1. TGF-β induces cellular responses by binding to two highly conserved transmembrane receptors, type I and type II receptors that have serine/threonine kinase domains and cooperate in signalling. The binding of TGF-β to the type I receptor, associated with the kinase ALK5, initiates its phosphorylation on threonine residues by the associated type II receptor. This activates ALK5 kinase activity, allowing it to phosphorylate Smad proteins that then mediate intracellularsignalling to the nucleus [22]. SB-431542 is a specific, small molecule inhibitor of ALK5 and does not interfere with other signalling pathways such as those mediated through C-Jun kinase (JNK), extracellular-regulated kinase (ERK) or p38 mitogen-activated protein kinase (MAPK) [23]. SB-431542 reversed the inhibition produced by 1 pg/ml of TGF-β1, thus demonstrating that inhibition was mediated through TGF receptor signalling. However, not all neutrophil functions were affected by TGF-β1. Another important process, IL-8-driven chemotaxis, was not affected by TGF-β1 at 1 pg/ml or 100 pg/ml. Nor were we able to demonstrate direct stimulation of neutrophil chemotaxis with these concentrations of TGF-β1. Our data show an inhibitory effect of TGF-β1 on neutrophil degranulation and are consistent with anti-inflammatory effects of TGF-β1 reported by others. TGF-β has been shown to inhibit the oxidative burst function of activated macrophages [24]. The ability of cytokine-stimulated macrophages to synthesize MMP12, a matrix-degrading protease involved in inflammation, was also inhibited by TGF-β [25]. Similarly, TGF-β inhibited both the chemotaxis of monocytes through extracelluar matrix and the synthesis and release of another matrix-degrading enzyme, MMP9 [26]. Such studies have led to the suggestion that TGF-β acts in vivo to moderate the inflammatory response, as demonstrated by the observation that animals lacking the ability to synthesize TGF-β suffer extensive inflammatory disease [27]. With respect to neutrophils, TGF-β is reported to exert an anti-inflammatory effect by inhibiting adhesiveness to endothelial cells [28] and by reducing neutrophil migration through activated endothelium [29].
We observed that low, but not high, concentrations of TGF-β1 were effective in inhibiting lactoferrin release. This bimodal effect of TGF-β1 has parallels in other systems. TGF-β has been shown to have both inhibitory and stimulatory effects on the growth of smooth muscle cells, depending on concentration and cell density [30]. Observations on production of IFN-γ by T cells in response to IL-12 showed that low, but not high, levels of TGF-β inhibited the maintenance of IL-12 responsiveness [21]. The reasons for the bimodal action of TGF-β are complex and understood incompletely. TGF-β effects are thought to be modulated by the nature of the target cell, the conditions of cell culture and the presence of other regulatory factors.
In a similar vein to these divergent observations, it is reported that neutrophil and monocyte function also can be stimulated by TGF-β. For instance, neutrophil chemotaxis was observed to be mediated by 1 pg/ml TGF-β [31], a concentration we observe to inhibit lactoferrin release. Monocyte chemotaxis has also been noted in this concentration range of TGF-β [32]. However, we have been unable to induce neutrophil chemotaxis, either across Matrigel-coated or non-coated membranes in Boyden chambers, or in the under-agarose assay, over a wide range of TGF-β levels (unpublished data). It could be argued that a lack of migration in presence of Matrigel (a secretion of a murine tumour line) is due to its TGF-β content that overwhelms the chemotactic gradient. While plausible, this does not explain why we did not observe TGF-β1-mediated chemotaxis across non-coated membranes. Moreover, TGF-β is secreted from cells in a biologically inactive form, known as latent TGF, and becomes associated with matrix betaglycans as latent TGF [33]. Thus it is unclear how much of the TGF-β in Matrigel is biologically active. As with the varied effects of TGF-β on cell growth, subtle and undocumented differences in cell preparation, culture conditions and other factors may be at the root of differences in observations on the chemotactic activity of TBF-β1. For instance, it is now recognized that traces of endotoxin can prime neutrophils, resulting in functional enhancement [34]. While we have been stringent in screening our separation and culture reagents for endotoxin, some reports do not mention whether endotoxin content was taken into consideration. Another agent that modulates neutrophils and yet is used widely in neutrophil preparation is dextran. This compound promotes red cell sedimentation but has also been reported to make neutrophils more responsive to a variety of stimuli [35]. For this reason we avoid the use of dextran in preparing neutrophils. These are examples of some of the differences at the technical level that may result in neutrophils at different activation levels, contributing to disparate responses to agents such as TGF-β1.
We observed that the overall amount of lactoferrin released by neutrophils was variable between different donors, and that while TGF-β1 at 1 pg/ml was consistently inhibitory in all donors, its effects at higher concentrations were somewhat varied. As discussed above, this cytokine exerts stimulating as well as inhibiting effects on many cell types, including those of myeloid lineage. Indeed, TGF-β has been described as a ‘switch’ that promotes cellular functions that are inactive but can also suppress the same functions [36]. It is possible that the outcome of its effect on neutrophils and other cells is the sum of a balance between these inhibitory and stimulatory pathways. The varied effects of TGF-β1 at concentrations above 1 pg/ml may result from a slight tip of the balance in one or the other direction, depending on donor or experimental situation.
Neutrophil degranulation can be both protective and detrimental. This process enables neutrophils to release potent microbicidal compounds that are essential to combat infections [37]. On the other hand, tissue-degrading enzymes and inflammatory cytokines are also secreted, exacerbating conditions such as arthritis [38]. It is possible that tissue TGF-β acts to moderate the ability of neutrophils to provoke inflammation within tissue. In particular, in the endometrium it would be important to prevent these cells from initiating an inflammatory response or releasing degradative enzymes that could potentially damage the oocyte or fetus. However, a side effect may be to reduce the microbicidal activities of neutrophils. Indeed, in a murine model, constitutively elevated levels of TGF-β were associated with inability of neutrophils to reduce bacterial growth in vivo [39]. Our data suggest that low levels of TGF-β may reduce neutrophil secretion, while still allowing them to migrate towards destinations such as the IL-8 secreting mucosal surface [20] where microbes initiate their attack.
Acknowledgments
This work was supported by the following grant from the National Institutes of Health: 1 P01 AI/NS 51877. J. M. Smith was supported by the National Institute of Allergy and Infectious Disease T32 training grant AI07363.
References
- 1.Massague J, Cheifetz S, Laiho M, Ralph DA, Weis FM, Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- 2.Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–74. [PubMed] [Google Scholar]
- 3.Mashburn PB, Casey ML, Arici AM. Expression of transforming growth factor-beta 1 messenger ribonucleic acid and the modulation of deoxyribonucleic acid synthesis by transforming growth factor-beta 1 in human endometrial cells. Am J Obstet Gynecol. 1994;170:1152–8. doi: 10.1016/s0002-9378(94)70112-1. [DOI] [PubMed] [Google Scholar]
- 4.Kanzaki H, Hatayama H, Narukawa S, Kariya M, Fujita J, Mori T. Hormonal regulation in the production of macrophage colony-stimulating factor and transforming growth factor-beta by human endometrial stromal cells in culture. Horm Res. 1995;44(Suppl. 2):30–5. doi: 10.1159/000184658. [DOI] [PubMed] [Google Scholar]
- 5.Polli V, Bulletti C, Galassi A, et al. Transforming growth factor-beta 1 in the human endometrium. Gynecol Endocrinol. 1996;10:297–302. doi: 10.3109/09513599609012815. [DOI] [PubMed] [Google Scholar]
- 6.Brandes ME, Mai UE, Ohura K, Wahl SM. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J Immunol. 1991;147:1600–6. [PubMed] [Google Scholar]
- 7.Brandes ME, Wakefield LM, Wahl SM. Modulation of monocyte type I TGF-β rceptors by inflammatory stimuli. J Biol Chem. 1991;266:19697–703. [PubMed] [Google Scholar]
- 8.Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999;44:1–27. doi: 10.1016/s0165-0378(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 10.Poropatich C, Rojas M, Silverberg SG. Polymorphonuclear leukocytes in the endometrium during the normal menstrual cycle. Int J Gynecol Pathol. 1987;6:230–4. doi: 10.1097/00004347-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1783–7. doi: 10.1210/jcem.83.5.4754. [DOI] [PubMed] [Google Scholar]
- 12.Kamat BR, Isaacson PG. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol. 1987;127:66–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–65. [PubMed] [Google Scholar]
- 14.Videm V, Strand E. Changes in neutrophil surface-receptor expression after stimulation with FMLP, endotoxin, interleukin-8 and activated complement compared to degranulation. Scand J Immunol. 2004;59:25–33. doi: 10.1111/j.0300-9475.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Masson PL, Heremans JF, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–58. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullen JJ. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–38. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson A, Asman B, Bergstrom K. Elastase and lactoferrin in gingival crevicular fluid: possible indicators of a granulocyte-associated specific host response. J Periodont Res. 1994;29:276–82. doi: 10.1111/j.1600-0765.1994.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 18.Breton-Gorius J, Mason DY, Buriot D, Vilde JL, Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am J Pathol. 1980;99:413–28. [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn WA, Raetz CRH, Qureshi N, Golenbock DT. Lipopolysaccharide-induced stimulation of CD11b/CD18 expression on neutrophils. Evidence of specific receptor-based response and inhibition by lipid A-based antagonists. J Immunol. 1991;147:3072–9. [PubMed] [Google Scholar]
- 20.Shen L, Fahey JV, Hussey SB, Asin S, Wira CW, Fanger MW. Synergy between IL-8 and GM-CSF in reproductive epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 2004;230:23–32. doi: 10.1016/j.cellimm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Gorham JD, Guler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-β attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–70. [PubMed] [Google Scholar]
- 22.Piek E, Heldin CH, ten Dijke P. Specificity, diversity and regulation in TGF-β superfamily signaling. FASEB J. 1999;13:2105–24. [PubMed] [Google Scholar]
- 23.Inman GJ, Nicolas FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily Type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg MW, Jain MK, Werner F, et al. Transforming growth factor-β1 inhibits cytokine-mediated induction of human metallo elastase in macrophages. J Biol Chem. 2000;275:25766–73. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen J, Knapnougel P, Lesavre P, Bauvois B. Inhibition of matrix metalloproteinase-9 by interferons and TGF-β-1 through distinct signalings accounts for reduced monocyte invasiveness. FEBS Lett. 2005;579:5487–94. doi: 10.1016/j.febslet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor β-1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamble JR, Vadas MA. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988;242:97–9. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- 29.Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996;157:360–8. [PubMed] [Google Scholar]
- 30.Battegay EJ, Raines EW, Selfert RA, Bowen-Pope DF, Ross R. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515–24. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 31.Parekh T, Saxena B, Reibman J, Cromstein BN, Gold LI. Neutrophis chemotaxis in response to TGF-beta isoforms (TGF-beta1, TGF-beta2, TGF-beta3) is mediated by fibronectin. J Immunol. 1994;152:2456–66. [PubMed] [Google Scholar]
- 32.Wahl SH, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–92. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J. Latent transforming growth factor-β binding proteins (LTBPs) − structural extracellular matrix proteins for targeting TGF-β action. Cytokine Growth Factor Rev. 1999;10:99–117. doi: 10.1016/s1359-6101(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 34.Forehand JR, Pabst MJ, Phillips WA, Johnston RB., Jr Lipopolysaccharide priming of human neutrophils for an enhanced respiratory burst-role of intra-cellular free calcium. J Clin Invest. 1989;83:74. doi: 10.1172/JCI113887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkow RL, Weisman SJ, Tzeng D, et al. Comparative responses of human polymorphonuclear leukocytes obtained by counterflow centrifugal elutriation and Ficoll-Hypaque density centrifugation. II. Membrane potential changes, membrane receptor analysis, membrane fluidity, and analysis of the effects of the preparative techniques. J Lab Clin Med. 1984;104:698–710. [PubMed] [Google Scholar]
- 36.Sporn MB, Roberts AB. TGF-β: problems and prospects. Cell Reg. 1990;1:875–82. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganz T, Selsted ME, Szklarek D, et al. Defensins: natural peptide antibiotics of human neutrophils. J Clin Inves. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Lowrance JH, O'Sullivan FX, Caver TE, Waegell W, Gresham H. Spontaneous elaboration of transforming growth factor β suppresses host defense against bacterial infection in autoimmune MRL-lpr mice. J Exp Med. 1994;180:1693–703. doi: 10.1084/jem.180.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]