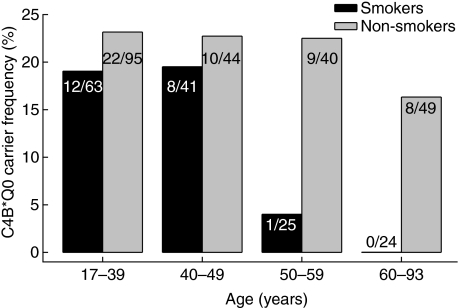Abstract
We have demonstrated previously that carriers of a genotype called C4B*Q0 (silent allele of the C4B gene) have a substantially increased risk to suffer from myocardial infarction or stroke, and are selected out from the healthy elderly population. Because smoking carries a major risk for cardiovascular disease (CVD), it seemed worthwhile to study if these two factors interact. Study 1 involved 74 patients with angina pectoris (AP), 85 patients with recent acute myocardial infarction (AMI) and 112 survivors of a previous AMI and 382 controls from Iceland. Study 2 involved 233 patients with severe CVD and 274 controls from Hungary. Smoking habits were registered for each subject. The number of C4A and C4B genes was determined by phenotyping or genotyping. Compared to controls, C4B*Q0 carrier frequency was significantly higher at diagnosis in Icelandic smokers with AP (P = 0·005) and AMI (P = 0·0003) and Hungarian smokers with severe coronary artery disease (P = 0·023), while no such difference was observed in non-smoking subjects. Age-associated decrease in C4B*Q0 observed previously in two remote Caucasian populations was found, in the present study, to be associated strongly with smoking, and to already occur in smokers after age 50 years both in Iceland and Hungary. Our findings indicate that the C4B*Q0 genotype can be considered as a major covariate of smoking in precipitating the risk for AMI and associated deaths.
Keywords: C4B*Q0, cardiovascular mortality, coronary artery disease, myocardial infarction, smoking
Introduction
Atherosclerotic cardiovascular disease (CVD) is the single most common cause of death in all regions of the world except sub-Saharan Africa, with >30% of all global mortalities and US prevalence estimates of 85% at age 50 years [1, 2]. The aetiology of CVD results from an interplay of multiple heritable and environmental risk factors, such as dyslipidaemia, male gender, smoking, hypertension, diabetes, obesity and inflammation [3].
Among CVD risk factors, smoking stands out as the leading cause of avoidable deaths, with US estimates of 18% of all deaths annually and a cost of tens of billions of dollars per year [4, 5]. Globally, about 1·1 billion people (one in three adults) smoke today, with prevalence values still rising [6], and it has been estimated that 50% of smokers will die as a result of their addiction to tobacco, with half of them dying in middle age [7]. On a global scale, smoking causes more deaths from CVD than from cancer [7, 8].
Family history has been identified as a strong independent risk factor for CVD [9], implying the presence of one or more as yet unidentified contributing genes. Our previous findings suggest that the so-called silent allele (C4B*Q0) of the C4B gene encoding the fourth component of human complement C4 could account for part of the unexplained genetic association and identify this genotype as a risk factor for early morbidity and/or mortality in two remote European populations [10, 11]. C4 is encoded by two genes (C4A and C4B) located in the RCCX module of the central major histocompatibility complex (MHC) region of human chromosome 6 [12]. Up to six C4 genes can be present in the genome, and each C4 gene may code for C4A or C4B. When there are fewer C4B than C4A genes, the concentration of C4B protein is also less than that of the C4A protein, which is known traditionally as C4B*Q0. We demonstrated that the frequency of the C4B*Q0 genotype drops dramatically with age in Hungarian (16–5%; P < 0·001) and Icelandic (24–5%; P < 0·001) healthy populations [10, 11]. Consistent with this, the carrier frequency of C4B*Q0 is many times higher in elderly Hungarian patients with AMI (38%; P < 0·0001) [13] and stroke (11·3%; P = 0·0003) [14] than in age- and gender-matched healthy individuals (8% and 5%, respectively). Moreover, the short-term mortality of AMI patients was significantly higher (P = 0·024) in the C4B*Q0-carriers than non-carriers [13].
Tobacco smoking severely affects health and survival in both Hungary and Iceland, and we thus tested the working hypothesis that the increased risk of early morbidity and of CVD and CVD-related mortalities observed in carriers of C4B*Q0, could be associated with smoking. Carrier frequencies of C4B*Q0 were analysed in relation to age and smoking history of Icelandic and Hungarian patients with cardiovascular disease and healthy controls.
Materials and methods
Subjects
Study 1 involved 271 consecutive patients admitted to the emergency room of Landspitali University Hospital in Iceland between 1995 and 1998, with chest pain as their main complaint (Table 1). Of these, 85 patients had their first lifetime acute myocardial infarction (AMI). The diagnosis of AMI was based on typical electrocardiographic changes and increased serum activities of relevant enzymes and confirmed by echocardiography and coronary angiography. A total of 112 patients were survivors of a previous AMI that occurred 7 years (4·3–11·0) earlier, while 74 patients had angina pectoris (AP) due to coronary artery disease with no AMI at admission or in their case history. A plasma sample was obtained at admission and detailed information was collected on disease history, including risk factor profile. This information was checked against hospital records where available. Data on Icelandic patients are summarized in Table 1. The 382 healthy Icelandic subjects of this study (117 males, 265 females, 17–90 years old) included 250 controls from a previous study [11] and 132 subjects admitted to the Landspitali University Hospital between 1995 and 1998 with chest pain, which on further examination was found to be unrelated to cardiovascular disease (study control). These two subgroups were considered identical for the purpose of the study after a detailed comparison showing no statistical difference. All participants gave informed consent and the study was approved by the Ethical Committee of Landspitali University Hospital, the Icelandic Data Protection Authority and the National Bioethics Committee of Iceland. Samples were aliquoted and stored throughout at −70°C. Plasma samples were thawed only once, i.e. just before C4 allotyping performed 1·5 years after blood collection.
Table 1.
Basic clinical data of the Icelandic and Hungarian patients with cardiovascular diseases.
| Icelandic patients with | ||||
|---|---|---|---|---|
| Variable | Angina pectoris | New AMI | Survivors of a previous AMI | Hungarian patients with documented significant CAD* |
| No. of patients | 74 | 85 | 112 | 233 |
| Date of collection | 1995–97 | 1995–97 | 1995–97 | 1994–96 |
| Age, years, mean ± s.d. | 65·9 ± 10·2 | 63·6 ± 13·3 | 65·3 ± 12·7 | 58·4 ± 8·9 |
| Males/females | 44/30 | 58/27 | 74/38 | 155/78 |
| AMI in case history, yes/no | 0/74 | 0/85 | 112/0 | 125/108 |
| Never/quit/current smoker | 20/34/20 | 24/25/36 | 27/57/28 | 77/138/18 |
| Years of smoking, mean ± s.d. | 34·6 ± 13·5 | 32·9 ± 14·7 | 33·3 ± 13·7 | 34·7 ± 11·3 |
| Hypertension, yes/no | 47/27 | 48/36 | 75/37 | 115/118 |
| DM or pre-DM, yes/no* | 18/56 | 23/62 | 35/77 | 53/180 |
| Serum total cholesterol, mmol/l, mean ± s.d. | 6·25 ± 1·3 | 6·01 ± 1·14 | 5·98 ± 1·12 | 6·17 ± 1·35 |
| Serum triglycerides mmol/l, mean ± s.d. | 1·87 ± 0·89 | 1·92 ± 0·88 | 1·95 ± 1·06 | 1·91 ± 0·86 |
| Serum LDL-cholesterol, mmol/l, mean ± s.d. | 4·37 ± 1·29 | 4·15 ± 1·00 | 4·05 ± 1·05 | 4·21 ± 1·06 |
| Serum HDL-cholesterol, mmol/l, mean ± s.d. | 1·06 ± 0·35 | 0·97 ± 0·36 | 1·03 ± 0·33 | 1·24 ± 0·25 |
| Pecutaneous transluminal coronary angioplasty, yes/no | 25/49 | 6/78 | 25/84 | 15/218 |
| Coronary artery bypass grafting (CABG), yes/no | 20/54 | 1/83 | 28/81 | 233/0 |
| Coronary angiography (CAG), yes/no | 62/12 | 7/77 | 73/36 | 233/0 |
Coronary artery disease. AMI: acute myocardial infarction; DM: diabetes mellitus; LDL: low density pipoprotein; HDL: high density lipoprotein.
Study 2 involved 233 Hungarian patients with documented significant coronary artery disease (Table 1) and 274 healthy Hungarian volunteers (175 males, 99 females) [15, 16]. Basic characteristics of the Hungarian patients are summarized in Table 1. The study was approved by the Ethical Committee of the Semmelweis University, and the patients gave informed consent to participate.
Registration of smoking habits
Smoking behaviour was registered by a physician at study entry, and analysed as described previously [15]. Originally four groups of subjects were identified: current smokers, never smokers, former smokers (quit 3 or more years ago) and recent quitters (quit < 3 years ago). In order to simplify the analysis, the groups of current smokers and recent quitters were combined as smokers.
Measurements
C4 allotypes were determined by visual scoring of protein bands after high-voltage agarose electrophoresis followed by immunofixation and staining [16, 17]. In the Hungarian confirmatory study the number of the C4A and C4B genes were determined in DNA samples by a newly developed real-time polymerase chain reaction (PCR) [18]. Each person with less C4B than C4A gene dosage was considered as a C4B*Q0 carrier.
Statistical analysis
The nonparametric Mann–Whitney U-test was used for group comparisons. Categorical data were compared using Fisher's exact test or χ2 test for trend. Multiple logistic regression was used to evaluate potential confounders and correct P-values of univariate analyses. All tests were two-tailed. Statistical analysis was performed using GraphPad Prism version 3·0 (GraphPad Software Inc., San Diego, CA, USA; http://www.graphpad.com) and spss version 13·0 (SPSS Inc., Chicago, IL, USA) software. Power analysis was made using GraphPad StatMate (http://www.graphpad.com) software.
Results
Controls
Because smoking and C4B*Q0 have been identified previously as risk factors for early morbidity and mortality, we studied the possible interaction of these inherited and acquired risk factors in 382 Icelandic healthy controls with known smoking habits. The results are shown in Fig. 1. A strong relationship was observed between smoking and the age-associated drop in C4B*Q0, as the decrease turned out to be confined to smokers (χ2 test for trend P = 0·0079). In the smokers, the C4B*Q0 frequency had already decreased in the 50–59-year age group. For this reason, all further analysis was performed with dichotomization at 50 years of age. By contrast, no appreciable age-associated decrease occurred in the group of never smoking or former smoking individuals (P = 0·395). The age- and gender-adjusted odds ratio [OR, 95% confidence interval (CI)] of carrying C4B*Q0 for ≥ 50-year-old smokers compared to non-smokers was 0·083 (0·011–0·660), P = 0·019. According to power analysis with the indicated sample size the study had a power between 85% and 90% to yield a statistically significant (α = 0·05) result.
Fig. 1.
Carrier frequencies of C4B*Q0 in smoking (current smokers + quitters in < 3 years) and non-smoking (never smokers + quitters in ≥ 3 years) healthy Icelandic people at different ages. The decrease in smokers with age is significant (P = 0·008, χ2 test for trend), while no significant changes (P = 0·395) occurred in the group of non-smokers (never smokers and former smokers).
Patients with a first-time acute myocardial infarction
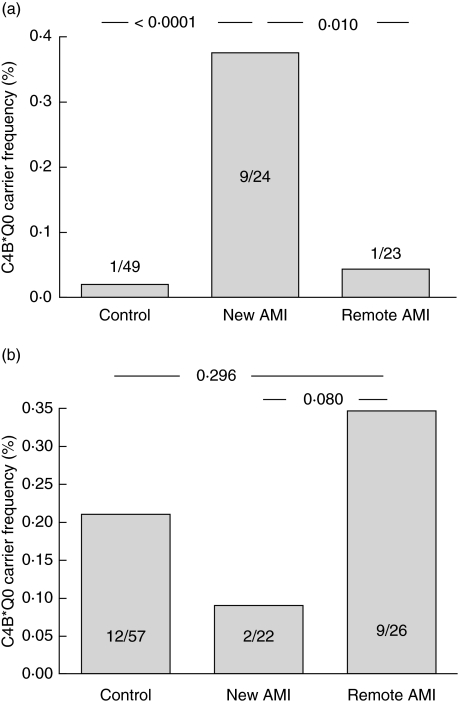
The age-associated decrease in C4B*Q0 in smokers together with the previously established link between C4B*Q0 and AMI in Hungary suggested that C4B*Q0 could increase the risk of AMI in smokers. Consistent with this hypothesis, the frequency of C4B*Q0 was significantly higher (P = 0·0003) at age ≥ 50 years in current smokers with AMI than in age-matched controls (Fig. 2a, Table 2). By contrast, the frequency of C4B*Q0 was not increased in never smokers or former smokers with AMI (Fig. 2b). Among the ≥ 50-year-old smokers, C4B*Q0 carriers, with respect to non-carriers, had an age- and gender-adjusted OR of 22·66 (2·45–206·59; P = 0·006) to have AMI. Instead, among the ≥ 50-year-old former or never smokers, the OR to have AMI was 0·677 (0·245–1·867; P = 0·451) for the C4B*Q0 carriers, with respect to non-carriers. According to power analysis the study had a power between 85% and 90% to yield a statistically significant result.
Fig. 2.
Carrier frequency of C4B*Q0 in currently smoking (a) and never smoking Icelandic patients (b) compared to controls; new acute myocardial infarction (AMI): patients entering the study with their first lifetime acute myocardial infarction; remote AMI: survivors of a myocardial infarction that occurred 7 years (4·3–11·0) previously. Only ≥ 50-year-old subjects were included in the analysis.
Table 2.
C4B*Q0 carrier frequency in ≥ 50-year-old Icelandic cardiovascular disease (CVD) patients and healthy controls with different smoking habits†.
| No. (%) of C4B*Q0-carriers | |||||
|---|---|---|---|---|---|
| Group | n | Age mean ± s.d. | Never smokers | Former smokers | Smokers |
| Controls | 139 | 62·8 ± 10·9 | 12/57 (21%) | 5/32 (16%) | 1/50 (2%) |
| AP | 69 | 67·5 ± 8·7 | 4/17 (24%) | 7/33 (21%) | 5/19 (26%)* |
| AMI | 70 | 67·9 ± 10·2 | 2/22 (9%) | 4/24 (17%) | 9/24 (38%)** |
| Remote AMI | 104 | 70·5 ± 10·1 | 9/26 (35%) | 12/55 (22%) | 1/23 (4%) |
AP: angina pectoris; AMI = acute myocardial infarction; remote AMI: survivors of an AMI that occurred 7 years (4·3–11·0) previously; current smokers includes recent quitters (quit < 3 years earlier). Statistical comparisons with controls
P = 0·005
P = < 0·0001 (Fisher's exact test).
Survivors of a previous acute myocardial infarction
To address the question of long-term survival of C4B*Q0 carriers, we compared the frequencies of C4B*Q0 in smokers who had survived a previous AMI to the frequency at diagnosis. Conspicuously, C4B*Q0 was carried by only one of 23 still smoking survivors of a previous AMI (Fig. 2a), indicating a marked decrease from the high carrier frequency of C4B*Q0 found among smokers at AMI diagnosis (P = 0·01); the age- and gender-adjusted OR of carrying C4B*Q0 of patients with new AMI compared to AMI survivors was 15·18 (1·66–139·10; P = 0·016). By contrast, no such differences were observed with the non-smoking subjects (Fig. 2b) [OR: 0·45 (0·17–1·23); P = 0·118].
The incentive for smoking cessation is very high after AMI, and the group of AMI survivors thus included a high proportion of former smokers (57 of 112). The carrier frequency of C4B*Q0 in this group (23%) was similar to non-smoking controls and significantly different from both smoking (4%) and non-smoking (35%) AMI-survivors (χ2 test for trend, P = 0·010). These observations indicate effective risk reduction after smoking cessation. Most of these patients quit smoking at (n = 19) or previous to (n = 28) their first AMI, but six patients smoked for extended periods (4–18 years) after the event and none of these carried C4B*Q0; in marked contrast, the four carriers of C4B*Q0 who were survivors of a previous myocardial infarction while continuing to smoke all quickly reduced their smoking and continued only for short periods after AMI (0·5, 1·5, 2 and 3 years); the patient who continued for 3 years only smoked two to five cigarettes/day after the event.
Patients with angina pectoris
The frequency of C4B*Q0 in current smokers with angina pectoris was increased significantly at age ≥ 50 (P = 0·005) (Table 2) compared to age-matched controls who smoked. As calculated by multiple regression analysis among the ≥ 50-year-old-smokers, C4B*Q0 carriers with respect to non-carriers had an age- and gender-adjusted OR of 30·07 (1·93–469·15) (P = 0·015) to have angina pectoris. Instead, among the ≥ 50-year-old never smokers, the odds ratio to have angina pectoris was 1·38 (0·55–3·45) (P = 0·488) for the C4B*Q0 carriers with respect to non-carriers.
Confirmatory study in Hungary
We determined the carrier frequency of C4B*Q0 in Hungarian controls and patients with documented significant CVD by using a newly developed genotyping method [18]. In 203 controls of age < 50 years the frequency of the C4B*Q0 carriers was about the same (P = 0·100) in non-smokers (48 of 155, 31%) and smokers (nine of 48, 19%). By contrast, in ≥ 50-year-old subjects the frequency of the C4B*Q0 carriers was significantly (P = 0·015) lower in smokers than non-smokers (Table 3). The gender-adjusted OR (95% CI) of carrying C4B*Q0 for ≥ 50-year-old smokers compared to non-smokers was 0·106 (0·013–0·861), P = 0·036. In addition, the carrier frequency of C4B*Q0 was significantly (P = 0·023) higher in the smoking ≥ 50-year-old patients with severe CVD than in smoking controls of the same age (Table 3). Among the ≥ 50-year-old smoking subjects the C4B*Q0 carriers compared to the non-carriers had an age- and gender-adjusted OR of 8·897 (1·115–70·976), P = 0·039 to have severe CVD. In contrast, in the never smokers, the odds ratio to have AP were not raised (P = 0·885) in carriers compared to non-carriers (Table 3).
Table 3.
Carrier frequency of C4B*Q0 in ≥ 50-year-old smoking and non-smoking Hungarian controls and patients with severe cardiovascular disease (CVD).
| No. (%) of C4B*Q0 carriers | |||
|---|---|---|---|
| Group | Controls | CVD patients | P-value* |
| Age, years | 59·6 ± 11·0 | 60·7 ± 6·4 | |
| Non-smokers | 20/54 (37%) | 28/72 (39%) | 0·855 |
| Smokers | 1/17 (6%) | 43/130 (33%) | 0·023 |
| P-value* | 0·015 | 0·440 | |
Fisher's exact test.
Correlation between the results obtained by phenotyping and genotyping
Because, in the two studies, C4 allotypes were determined by phenotyping and genotyping, respectively, it was necessary to compare the two methods. We thus performed the C4 protein allotyping in parallel with C4A/C4B genotyping in plasma and DNA (respectively) from the 231 of 233 Hungarian CVD patients from whom samples were available. According to phenotyping, 50 (21·6%) and nine (3·9%) patients, respectively, were heterozygote and homozygote carriers of the C4B*Q0 phenotype. By genotyping, all homozygote and 48 of 50 (96%) of the heterozygote carriers were found to have less C4B than C4A gene dosage, and thus could also be considered C4B*Q0 carriers by this method. Less concordant results were found in the case of samples with C4A*Q0 or ‘normal’ C4 phenotypes (data to be reported elsewhere)
Discussion
We have shown previously that C4B*Q0 carriers are removed prematurely from the pool of healthy individuals in two remote Caucasian populations [10, 11]. We now demonstrate for both populations that this is related to their smoking status, i.e. it occurs in persistent smokers but cannot be demonstrated for non-smokers or people who quit smoking. The more rapid removal of C4B*Q0-carriers than non-carriers from the group of healthy smokers suggests that they may be especially sensitive to the well-known health-reducing effect of smoking, and as our previous findings had already suggested a role of C4B*Q0 in promoting CVD [10, 11], our attention was turned to this disease. The age-associated decrease in carriage of C4B*Q0 in the present study was shown to already occur at age 50, and as it is well known that age is the strongest risk factor of CVD, we dichotomized our patient group at this age limit for optimal sensitivity of comparison. This approach showed a strong association of C4B*Q0 with CVD in the > 50 years smoking subjects. This is consistent with our previous findings in Hungarian C4B*Q0 patients with AMI [13] as well as stroke [14], and the overall results suggest that smokers carrying the C4B*Q0 genotype are selected out from the population of healthy > 50-year-old subjects due to increased morbidity and/or mortality from CVD, stroke or other devastating disease. The possibility that a part of this effect occurs through increased mortality of patients after diagnosis of CVD is supported by comparison of patients with their first lifetime AMI with the group of patients who entered the study with a previous history of AMI. The proportion of the C4B*Q0 carriers dropped from 33% to 5% among the current smokers, while no significant difference could be observed in the case of never or former smokers (Table 2, Fig. 2)
CVD risk factors have traditionally been identified originally through cross-sectional association studies such as the present one. The overall results suggest that C4B*Q0 can be added to the list of CVD risk factors, and this could have far-reaching implications. For instance, they suggest that the smoking-related risk of morbidity and mortality from CVD may be higher in a subset of individuals (C4B*Q0 carriers) who can be identified at an early age, before the onset of CVD. For this reason, it seems imperative that the question of cause and effect, already established for conventional risk factors, is addressed in prospective studies. The most important findings of the present study, i.e. the age- and smoking-related decrease in carrier frequency of C4B*Q0 in the general population and the association between CVD morbidity and the co-existence of the smoking and C4B*Q0 phenotypes, were based on observations in two remote European populations, and the finding of increased mortality of C4B*Q0 carriers in the Icelandic cohort confirms our earlier finding in Hungary [13]. Each result was based on a null-hypothesis suggested by our previous studies, together with our observations described in Fig. 1; however, the need to correct the results for multiple hypothesis testing was met additionally with multivariate analysis. Although some subgroups were small, statistical power turned out to be high for all comparisons, and most P-values were lower than 0·01. The overall power of our observations is high, as a significant association observed in two independent populations in the same direction increases the power substantially. Notwithstanding, and especially considering the far-reaching implications of the results, it appears important to repeat the findings quickly in more populations and to extend them by prospective studies. Studies should also be designed to examine if the age-associated decrease of C4B*Q0 in smokers could result in part from the other major smoking-related diseases, chronic obstructive pulmonary disease (COPD), lung cancer and stroke. Such studies are in progress in our laboratories.
For technical reasons, we used a newly developed genotyping method for the confirmatory study. This method forms a very convenient alternative to the traditional phenotyping by serum analysis, and it seems probable that it will become the method of choice in future studies on C4B and C4A null alleles.
At present we are not able to provide a definitive explanation for the increased susceptibility for AP and AMI that we observed in smokers with C4B*Q0. It is possible, however, that this association is due to increased concentrations of circulating immune complexes in smokers [19]. This theory presumes that low levels of C4B lead to an impaired ability to ensure safe elimination of immune complexes, and a wealth of data suggest this possibility. First, a surrogate marker of in vivo immune complex handling, prevention of immune precipitation exhibited a very strong positive correlation with serum levels of C4B in patients with different autoimmune diseases [20]. The increased prevalence of C4B*Q0 in the IgA immune complex disease Henoch–Schoenlein purpura [21] is also consistent with this assumption. It has been suggested that systemic inflammation and soluble immune mediators such as immune complexes may play a role in atherosclerosis by accelerating vessel pathology [22, 23]. Thirdly, an association was observed between smoking and the prototype immune complex disease, systemic lupus erythematosus (SLE) [24] and smoking, together with complement deficiency (C2, C4), also carries an increased risk for cutaneous LE in males [25]. Fourthly, the significant association between C2 deficiency and atherosclerosis [26] and raised levels of circulating immune complexes in patients with early CVD [27] lend further credibility to the above hypothesis. Studies to test these possibilities and determine further the mechanism responsible for the strong association observed between smoking, C4B*Q0 and premature cardiovascular morbidity are in progress in our laboratories.
It is important to note that most consequences of C4B*Q0 carrier state were manifested only in smokers, i.e. those who still smoked at the time of study or quit smoking less than 3 years previously. This observation indicates that the strong detrimental effect of C4B*Q0 carriage can be weakened quickly and effectively by cessation of smoking, and this is in keeping with reports showing that the smoking-related risk of cardiovascular disease diminishes strongly or disappears after 3 years of smoke cessation [28–30].
In conclusion, findings in two remote Caucasian populations indicate that due to increased risk of AP, AMI and AMI-associated mortalities, smoking carriers of C4B*Q0 have dramatically diminished expectancy for a long and healthy life compared to non-carriers; this prospect can, however, be improved greatly if the carriers quit smoking. The possibility that the removal of C4B*Q0-carriers from the pool of healthy individuals may occur also in part through other smoking-related diseases (e.g. COPD, cancer or stroke) will be addressed in future studies.
Acknowledgments
We are greatly indebted to the vast number of medical students and doctors who assisted in gathering patient data and plasma samples, but in particular we thank the participants themselves. This study was supported by the Science Fund of Landspitali University Hospital (G. J. A.) as well as by the National Office for Research and Technology, Hungary, the OTKA T049266 grant of Hungarian Research Fund and the National Office for Research and Technology (G. F.). We thank Adalgeir Arason for critical reading.
References
- 1.Bonow RO, Smaha LA, Smith SC, Mensah GA, Jr, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106:1602–5. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 2.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 5.Leistikow BN. The human and financial costs of smoking. Clin Chest Med. 2000;21:189–1xi. doi: 10.1016/s0272-5231(05)70017-4. [DOI] [PubMed] [Google Scholar]
- 6.Prabhat I, Chaloupka FJ. Governments and Economics of Tobacco Control. Washington D.C.: The World Bank; 1999. World Bank: Curbing the epidemic. [Google Scholar]
- 7.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case–control studies. BMJ. 2000;321:323–9. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaher C, Halbert R, Dubois R, George D, Nonikov D. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung Dis. 2004;8:1423–8. [PubMed] [Google Scholar]
- 9.Murabito JM, Nam BH, D'Agostino RB, Lloyd-Jones DM Sr, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–40. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kramer J, Fulop T, Rajczy K, Nguyen AT, Fust G. A marked drop in the incidence of the null allele of the B gene of the fourth component of complement (C4B*Q0) in elderly subjects: C4B*Q0 as a probable negative selection factor for survival. Hum Genet. 1991;86:595–8. doi: 10.1007/BF00201547. [DOI] [PubMed] [Google Scholar]
- 11.Arason GJ, Bodvarsson S, Sigurdarson ST, et al. An age-associated decrease in the frequency of C4B*Q0 indicates that null alleles of complement may affect health or survival. Ann NY Acad Sci. 2003;1010:496–9. doi: 10.1196/annals.1299.091. [DOI] [PubMed] [Google Scholar]
- 12.Yu CY, Chung EK, Yang Y, et al. Dancing with complement C4 and the RP-C4-CYP21-TNX (RCCX) modules of the major histocompatibility complex. Prog Nucl Acid Res Mol Biol. 2003;75:217–92. doi: 10.1016/s0079-6603(03)75007-7. [DOI] [PubMed] [Google Scholar]
- 13.Kramer J, Rajczy K, Hegyi L, et al. C4B*Q0 allotype as a risk factor for myocardial infarction. BMJ. 1994;309:313–14. doi: 10.1136/bmj.309.6950.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer J, Harcos P, Prohaszka Z, et al. Frequencies of certain complement protein alleles and serum levels of anti-heat-shock protein antibodies in cerebrovascular diseases. Stroke. 2000;31:2648–52. doi: 10.1161/01.str.31.11.2648. [DOI] [PubMed] [Google Scholar]
- 15.Fust G, Arason GJ, Kramer J, et al. Genetic basis of tobacco smoking: strong association of a specific major histocompatibility complex haplotype on chromosome 6 with smoking behavior. Int Immunol. 2004;16:1507–14. doi: 10.1093/intimm/dxh152. [DOI] [PubMed] [Google Scholar]
- 16.Awdeh ZL, Alper CA. Inherited structural polymorphism of the fourth component of human complement. Proc Natl Acad Sci USA. 1980;77:3576–80. doi: 10.1073/pnas.77.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim E, Cross SJ. Phenotyping of human complement component C4, a class-III HLA antigen. Biochem J. 1986;239:763–7. doi: 10.1042/bj2390763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szilagyi A, Blasko B, Szilassy D, Fust G, Sasvari-Szekely M, Ronai Z. Real-time PCR quantification of human complement C4A and C4B genes. BMC Genet. 2006;7:1. doi: 10.1186/1471-2156-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanski B, Zbikowska-Gotz M, Kakol J, Sinkiewicz W. The immunologic response to tobacco antigens in smokers. VI. Phagocytosis of tobacco antigens by peripheral blood polymorphonuclear leucocytes studied by immunofluorescence. Allergol Immunopathol (Madr) 1984;12:321–7. [PubMed] [Google Scholar]
- 20.Arason GJ, Kolka R, Hreidarsson AB, et al. Defective prevention of immune precipitation in autoimmune diseases is independent of C4A*Q0. Clin Exp Immunol. 2005;140:572–9. doi: 10.1111/j.1365-2249.2005.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefansson TV, Kolka R, Sigurdardottir SL, Edvardsson VO, Arason G, Haraldsson A. Increased frequency of C4B*Q0 alleles in patients with Henoch–Schonlein purpura. Scand J Immunol. 2005;61:274–8. doi: 10.1111/j.1365-3083.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 22.Chan RK, Ibrahim SI, Verna N, Carroll M, Moore FD, Hechtman HB., Jr Ischaemia–reperfusion is an event triggered by immune complexes and complement. Br J Surg. 2003;90:1470–8. doi: 10.1002/bjs.4408. [DOI] [PubMed] [Google Scholar]
- 23.Thorbjornsdottir P, Kolka R, Gunnarsson E, et al. Vaccinia virus complement control protein diminishes formation of atherosclerotic lesions. Complement is centrally involved in atherosclerotic disease. Ann NY Acad Sci. 2005;1056:1–15. doi: 10.1196/annals.1352.001. [DOI] [PubMed] [Google Scholar]
- 24.Costenbader KH, Kim DJ, Peerzada J, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 2004;50:849–57. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 25.Boeckler P, Milea M, Meyer A, et al. The combination of complement deficiency and cigarette smoking as risk factor for cutaneous lupus erythematosus in men; a focus on combined C2/C4 deficiency. Br J Dermatol. 2005;152:265–70. doi: 10.1111/j.1365-2133.2004.06308.x. [DOI] [PubMed] [Google Scholar]
- 26.Sjoholm AG, Jonsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–81. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 28.Kinjo K, Sato H, Sakata Y, et al. Impact of smoking status on long-term mortality in patients with acute myocardial infarction. Circ J. 2005;69:7–12. doi: 10.1253/circj.69.7. [DOI] [PubMed] [Google Scholar]
- 29.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. doi: 10.7326/0003-4819-137-6-200209170-00009. [DOI] [PubMed] [Google Scholar]
- 30.Serrano M, Madoz E, Ezpeleta I, et al. [Smoking cessation and risk of myocardial reinfarction in coronary patients: a nested case–control study] Rev Esp Cardiol. 2003;56:445–51. doi: 10.1016/s0300-8932(03)76898-5. [DOI] [PubMed] [Google Scholar]