Abstract
Reduced fibrinolytic activity has been described in primary anti-phospholipid syndrome (PAPS), and may be responsible for thrombotic events. Antibodies to tissue type plasminogen activator (t-PA) or plasminogen (PLG) might contribute to the hypofibrinolytic state in autoimmune diseases, but the clinical significance of these antibodies is still unclear in recurrent pregnancy loss (RPL). The aim of this study is to evaluate the prevalence and clinical significance of anti-PLG and anti-t-PA antibodies in 87 patients with a history of RPL: 54 women with well-defined PAPS (mean age 32·5 years; range 26–38) and 33 women with unexplained RPL (mean age 30 years; range 24–39). IgG anti-PLG antibodies were found in 20 and four patients from the group with RPL/PAPS and unexplained RPL, respectively; IgG anti-t-PA antibodies were found in 11 and two patients from the above two groups, respectively. IgG anti-PLG antibodies were associated with the high risk of RPL (OR 7·2, P = 0·004), especially with RPL/PAPS (OR 11·2, P < 0·001) evaluated by Fisher's exact test, while IgG anti-t-PA were associated with RPL/PAPS (OR 10·0, P = 0·01) but not with RPL (OR 6·8, P = 0·06). A significant inhibition of exogenous fibrinolysis was observed by IgG fractions from patients with anti-PLG or anti-t-PA antibodies on microplates and on the human umbilical vein endothelial cells, compared with those from healthy controls. The prevalence of IgG anti-PLG antibodies was high in RPL patients, especially in RPL/PAPS, while the prevalence of IgG anti-t-PA antibodies was high in RPL/PAPS but not in RPL, and some of them might inhibit fibrinolysis in patients.
Keywords: APS, fibrinolysis, plasminogen, recurrent pregnancy loss, t-PA
Introduction
Anti-phospholipid syndrome (APS) is a prothrombotic disorder characterized by vascular thrombosis and pregnancy morbidity with persistently positive circulating anti-phospholipid antibody (APA) [1]. According to the epidemiological studies focused on APS as a cause of recurrent spontaneous abortion, it is found that 7–25% of recurrent spontaneous abortions would have APS as the main risk factor [2]. APS is one of the most frequent causes of early and late pregnancy morbidity, and if properly managed is one of the few treatable causes of pregnancy loss [3]. The diagnosis and management of APS is important to achieve successful fetal outcomes and reduced maternal complications in pregnancy.
Although the association of APA with pregnancy morbidity is well established, the characteristics and pathogenic mechanisms of APA in obstetric APS remain difficult to resolve [4]. Fetal loss has been attributed to thrombosis of the uteroplacental vasculature and placental infarction [5]. A number of mechanisms by which APA may promote thrombotic events have been proposed, most of which involve the disturbance of the coagulation pathway, the regulatory systems and the cells which control them [6]. Fibrin formation and fibrinolysis are in dynamic balance, and abnormalities in either haemostasis or fibrinolysis will result in a prothrombotic state [7, 8]. Moreover, reduced fibrinolytic activity has also been described in APS patients and may be responsible for thrombotic events [9–11].
Several research groups suggest that antibodies to tissue type plasminogen activator (t-PA) or plasminogen (PLG) may be related with thrombosis in autoimmune diseases, but the clinical significance of these antibodies is still undetermined in recurrent pregnancy loss (RPL). It is unclear whether antibodies to PLG and t-PA are directly pathogenic, a result of the pregnancy losses or markers for an underlying and uncharacterized autoimmune disorder. The presence of anti-PLG antibody in patients with rheumatoid arthritis and systemic lupus erythematosus (SLE) was reported, and it was suggested that PLG might be a target of the immune response in autoimmune disease [12–14]. However, controversy exists with regard to the clinical significance of anti-PLG antibodies in patients with autoimmune diseases. Some investigators [15] suggest that anti-PLG antibodies are not a risk factor for thrombosis, although anti-PLG antibodies occur frequently in patients with systemic autoimmune disease. Whether or not anti-PLG antibody is related to thrombosis in patients with autoimmune disease remains a subject of debate.
t-PA plays a central role in the regulation of intravascular PLG activation [16]. The association of anti-t-PA antibodies with thrombosis has been reported to be related to APS and anti-t-PA antibodies interact specifically with the catalytic domain of t-PA in patients with APS, which may represent a possible cause of hypofibrinolysis [17, 18]. Even patients with pregnancy morbidity and thrombosis have been studied; however, so far there have been few studies analysing the presence of anti-t-PA antibodies in patients with RPL.
In this study, we hypothesize that antibodies to PLG and t-PA might lead to a hypofibrinolytic state. We investigated the prevalence and clinical significance of IgG anti-PLG and anti-t-PA in a cohort of patients with RPL distributed in two groups of patients: patients with well-defined primary anti-phospholipid syndrome (PAPS) and patients with unexplained RPL.
Materials and methods
Patients and healthy controls
Eighty-seven women with a history of RPL and 40 healthy women as controls were recruited from two Chinese hospitals (Guangzhou First Municipal People's Hospital and Guangzhou Second People's Hospital). Women were diagnosed with RPL on the basis of a history of three or more spontaneous losses fathered by the same partner prior to 20 weeks gestation. Patients were excluded if they had maternal anatomical or hormonal abnormalities, genetic abnormalities, systemic lupus erythematosus or other known immunological abnormalities (except APS). SLE was excluded according to the American Rheumatism Association criteria [19]. In this study, patients were divided into two groups: 54 women fulfilled the Sapporo classification criteria for definite PAPS [20] (mean age 32·5 years; range 26–38) and 33 women with unexplained RPL (mean age 30 years; range 24–39). Controls (mean age 29 years, range 20–39 years) were selected on the basis of clinical health, having at least one healthy child and being absent from various forms of reproductive failure. All women gave their written informed consent for participation in the study, which was approved by the Medical Ethics Committee in the corresponding hospitals.
All patients from the group with PAPS were positive for one of these antibodies: IgG/M anti-cardiolipin antibody (aCL), IgG/M anti-β2-glycoprotein 1 (β2GP1) and lupus anti-coagulant (LA) according to the Sapporo classification criteria for definite PAPS [20]. Forty-one of 54 PAPS patients were positive for IgG/M aCL, 12 patients had LA and 29 had IgG/M anti-β2GP1. Of the patients with PAPS, nine had a history of thrombosis (three arterial, eight venous and two patients with both arterial and venous events). In the group with unexplained RPL, none of these patients fulfilled the 1999 Sapporo criteria for the classification of APS, as only four patients were positive for IgG/M aCL and two for IgG/M anti-β2GP1 at lower titres. aCL IgG/M isotypes were detected semiquantitatively according to the manufacturer's instructions (Zeus Scientific, Inc., Raritan, NJ, USA). The method to detect anti-β2GP1 antibodies is described below. LA was screened using activated partial thromboplastin time (aPTT) and dilute Russell's viper venom time (dRVVT), and was confirmed according to the guidelines recommended by the Subcommittee on Lupus Anticoagulant/Phospholipid Dependent Antibodies [21]. These data are summarized in Table 1.
Table 1.
Anti-phospholipid antibody profile in patients with recurrent pregnancy loss (RPL).
| IgG/M aCL no. (%) | LA no. (%) | Anti-β2GP1 no. (%) | IgM anti-β2GP1 no. (%) | IgG anti-β2GP1 no. (%) | |
|---|---|---|---|---|---|
| PAPS/RPL (n = 54) | 41 (75·9) | 12 (22·2) | 29 (53·7) | 10 (18·5) | 27 (50) |
| Unexplained RPL (n = 33) | 4 (12·1) | 0 (0) | 2 (6·1) | 2 (6·1) | 0 (0) |
PAPS: primary anti-phospholipid syndrome; LA: lupus anti-coagulant; β2GP1: β2-glycoprotein 1.
The patients with PAPS had 190 miscarriages (mean 3·5, range 3–8) and 114 miscarriages for unexplained RPL (mean 3·4, range 3–6). These data are summarized in Table 2.
Table 2.
Characteristics of patients with recurrent pregnancy loss (RPL).
| Characteristics | PAPS/RPL (n = 54) | Unexplained RPL (n = 33) |
|---|---|---|
| Mean age (range) (years) | 32·5 (26–38) | 30 (24–39) |
| Thrombostic history no. (%) | 9 (16·7) | 3 (9·1) |
| Arterial events no. (%) | 3 (5·5) | 1 (3) |
| Venous events no. (%) | 8 (14·8) | 2 (6·1) |
| Total no. of miscarriages (mean; range) | 190 (3·5; 3–8) | 114 (3·4; 3–6) |
PAPS: primary anti-phospholipid syndrome.
Purification of β2GP1 from human plasma β2GP1 was purified from human plasma, as described previously [22]. Purified β2GP1 was dialysed in phosphate-buffered saline (PBS), and the purity was confirmed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), which revealed a single band of ∼50 kDa and was recognized by rabbit anti-human β2GP1 polyclonal antibodies (Chemicon International, Inc., Temecula, CA, USA).
Enzyme-linked immunosorbent assay (ELISA) for antibodies against β2GP1, Glu-PLG and t-PA
An ELISA for IgG against β2GP1, PLG and t-PA was performed as described previously, with a small modification [8, 17, 23]. High-binding plates (Costar, Cambridge, MA, USA) were coated with 10 µg/ml PLG (Enzyme Research Laboratories, South Bend, IN, USA), β2GP1 or t-PA (single-chain; Calbiochem, La Jolla, CA, USA) by incubation overnight at 4°C. After washing three times with PBS/0·05% Tween-20 (PBST) and blocking with PBS/1·2% gelatin at 37°C for 2 h, test plasma samples (1/50 dilution) or purified IgG samples in PBS/0·3% gelatin/0·05% Tween-20 were distributed to the corresponding wells in duplicate and incubated at 37°C for 1·5 h. After washing with PBST, bound human IgG was detected with peroxidase-conjugated goat anti-human IgG (gamma chain-specific; Sigma) and the peroxidase substrate tetramethylbenzidine (Sigma, Guangzhou, China). Human IgM was detected with peroxidase-conjugated goat anti-human IgM (Sigma) and the peroxidase substrate. IgG anti-β2GP1 antibody b1606, IgM anti-β2GP1 antibody b1718, IgG anti-t-PA antibody t2334 and IgG anti-PLG antibody p0617, collected from four patients fulfilling the Sapporo classification criteria for definite PAPS, were used as reference antibodies for detection of their corresponding antibodies, respectively. Anti-β2GP1 antibody b1606 and b1718 was determined to have high IgG and IgM anti-β2GP1 antibody titres (b1606 85 U/ml; b1718 76 U/ml), respectively, by commercially obtained ELISA kits from Inova Diagnostic, Inc. (San Diego, CA, USA). Anti-t-PA antibody t2334 and anti-PLG antibody p0617 were determined to have high IgG anti-t-PA antibody and IgG anti-PLG antibody titres, respectively, by immunoblot. Antibody levels were calculated by reference to the linear regression analysis of their corresponding standard line constructed from the serial dilutions of the reference antibody on the same plate. The results of anti-t-PA and anti-PLG antibody were expressed in abstract units (AU) with 1 AU equivalent to the OD of anti-t-PA antibody t2334 and anti-PLG antibody p0617 at 1 µg/ml, respectively. The mean interassay variation and intra-assay variation of the method for β2GP1, Glu-PLG and t-PA antibody were lower than 9%.
Immunoblot for IgG against PLG and t-PA
Human PLG or t-PA (2 ng per well) were subjected to SDS-PAGE in 7·5–10% polyacrylamide gels and transblotted onto a NC membrane at 70 V for 1 h and 45 min using Bio-Rad Mini Trans-Blot Cell. Afterwards, the membrane was blocked with 5% non-fat dry milk/Tris-buffered saline (TBS) (10 mM Tris-HCl, pH 7·5, 150 mM NaCl) overnight at 4°C. Lanes were probed with various IgG (0·1 mg/ml in TBST (10 mM Tris-HCl, pH 7·5, 150 mM NaCl, 0·05% Tween-20) containing 2% non-fat dry milk) or controls (anti-t-PA antibody 2A153 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), 0·2 µg/ml; anti-PLG antibody 3C2 (Santa Cruz Biotechnology, Inc.), 0·2 µg/ml). After incubation for 1 h at room temperature, the lanes were washed six times with TBST and then incubated with horseradish peroxidase (HRP)-conjugated second antibodies (0·05 µg/ml) for 50 min at room temperature. After this, the membrane was washed six times with TBST and visualized using a LumiGlo HRP system (KPL, Shenzhen, China) and exposure to autoradiographic film (Kodak, Shenzhen, China).
Affinity purification of IgG from patients' plasma
One hundred and twenty-seven human plasma samples, prebuffered with 10 mM phosphate buffer (PB, pH 6·8) at a ratio of 1 : 4, were precipitated using 18% (w/v, final concentration) polyethylene glycol (PEG). The IgG fractions were then purified from the precipitates using protein G-agarose columns. Protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA), according to the manufacturer's protocol.
Effects of IgG fractions on fibrinolytic activity
The effects of IgG fractions on fibrinolytic activity were determined using a sensitive plasmin fluorogenic substrate (I-1390, H-D-Val-Leu-Lys-AMC; Bachem Bioscience Inc., King of Prussia, PA, USA). The assay was performed using mixtures of t-PA, Glu-PLG and IgG fractions or 0·5% bovine serum albumin (BSA) (control). Briefly, mixtures of PLG (100 nM) and IgG fractions (0–100 µg/ml) were incubated at room temperature for 15 min, at which point plasmin substrate I-1390 (200 µM) was added. Then, 50 µl of each test mixture was aliquoted to 96 individual microplate wells to which t-PA (10 nM) was added. After mixing, substrate hydrolysis was measured immediately and at regular intervals as relative fluorescence units (I360/465nm) in a microplate reader (TECAN GENios, Beijing, China). Initial rates of plasmin generation were determined by linear regression analysis of plots of I360/465nm versus time2 (K) using Prism® version 4·0 (GraphPad Software, San Diego, CA, USA) [24]. The initial rate of plasmin generation of each test mixture was converted to the percentage of the total initial rate by dividing the initial rate in the absence of purified IgG.
The effects of anti-t-PA containing IgG fractions on t-PA amidolytic activity were determined in the absence or presence of anti-t-PA containing IgG fractions using a sensitive fluorogenic t-PA substrate (I-1195, Glutaryl-Gly-Arg-AMC; Bachem Bioscience Inc., King of Prussia, PA, USA). Assays were performed in 96-well black plates (Costar) containing mixtures of t-PA and IgG fractions or PBS/0·5% BSA as control. Briefly, mixtures of t-PA (10 nM) and anti-t-PA containing IgG fractions (50 µg/ml) were incubated at room temperature for 15 min, at which point t-PA substrate I-1195 (200 µM) was added. Substrate hydrolysis was measured and calculated as above. The initial rate of t-PA amidolysis of each test mixture was converted to a percentage of the total initial rate by dividing the initial rate in the absence of purified IgG. Normal human IgG1 and IgG3 were used as negative controls.
The effects of anti-PLG or anti-t-PA antibody on activation of Glu-PLG on human umbilical vein endothelial cells (HUVEC)
We evaluated the effects of anti-PLG antibodies on PLG activation at the surface of HUVEC (a gift from Dr Yaou Zhang, Tsinghua University, China), cultured in plastic 96-well culture plates as described previously [25]. Plasmin activity was estimated as described previously with a small modification [25]. HUVEC were incubated with 250 nM Glu-PLG and various concentrations of anti-PLG antibodies containing IgG fractions (0–150 µg/ml) at 4°C for 18 h. After extensive washing, a mixture containing 10 nM t-PA and 200 µM plasmin substrate I-1390 was added. As control, parallel assays were performed in the presence/absence of normal IgG. Substrate hydrolysis was measured and calculated as above. The initial rate of plasmin generation of each test mixture was converted to the percentage of the total initial rate by dividing the initial rate in the absence of purified IgG.
The effects of anti-t-PA antibodies on PLG activation on the endothelial cells were evaluated as mentioned above, with a small modification. HUVEC were incubated with 10 nM t-PA and various concentrations of anti-t-PA containing IgG fractions (0–150 µg/ml) at 4°C for 18 h. After extensive washing, the mixture containing 100 nM Glu-PLG and 200 µM plasmin substrate I-1390 was added.
Statistical analysis
The 99th percentile of the 40 healthy controls was used as the cut-off, and samples with values consistently higher than the cut-off in two separate experiments were considered positive [20]. The comparisons of mean values between patients and controls and between RPL patients with and without APS were performed by the Mann–Whitney U-test using the prism 4·0 program. Associations between antibody occurrence and a history of RPL or RPL/PAPS were assessed with Fisher's exact test using the spss 12 program. Differences in effects of purified IgG on fibrinolytic activity between patients and healthy control groups were analysed by the unpaired t-test using prism 4·0. Differences in effects of anti-t-PA containing IgG fractions on t-PA amidolytic activity was analysed by paired t-test using prism 4·0. A value of P < 0·05 was considered statistically significant.
Results
Detection of IgG against PLG and t-PA in the sera of patients with RPL
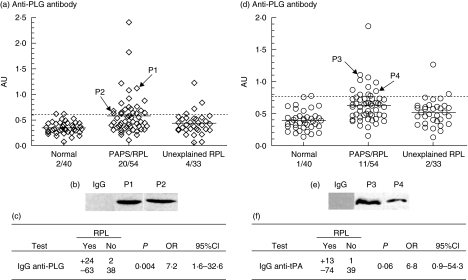
The prevalence of IgG anti-PLG and anti-t-PA antibodies detected in the different groups is detailed in Fig. 1. No significant differences were found between the patients with RPL/PAPS and unexplained RPL in age and mean number of pregnancies and miscarriages (Table 2). IgG anti-PLG and anti-t-PA antibodies were present in 24 of 87 (27·6%) and 13 of 87 (15%) patients with RPL, respectively (Fig. 1). In the 54 patients with RPL/PAPS, the prevalence rates for IgG anti-PLG and anti-t-PA antibody were 37·0% (20 of 54) and 20·4% (11 of 54), respectively. In the 33 unexplained RPL patients, the prevalence rates for these two antibody were 12·1% (four of 33) and 6·1% (two of 33), respectively. The mean values of IgG anti-PLG were higher in the serum of patients with RPL/PAPS (P < 0·0001) and unexplained RPL patients (P = 0·03) than in the healthy control group by means of the Mann–Whitney U-test. Although the mean values of IgG anti-PLG in patients with PAPS were higher than in the groups with unexplained RPL, the difference was not statistically significant (P = 0·05). Higher mean values of IgG anti-t-PA were also observed in the serum of patients with RPL/PAPS (P < 0·0001) and unexplained RPL patients (P = 0·005) than in the healthy control group. The mean values of IgG anti-t-PA in patients with PAPS were significantly higher than in the groups with unexplained RPL (P = 0·01).
Fig. 1.
Prevalence of IgG against plasminogen (PLG) and tissue type plasminogen activator (t-PA) in recurrent pregnancy loss (RPL) patients. (a, d) Enzyme-linked immunosorbent assay was developed to detect IgG against PLG and t-PA in 127 purified IgG samples from 87 RPL patients [54 patients with RPL/anti-phospholipid syndrome (APS) and 33 unexplained RPL patients] and 40 healthy controls. The respective ratio was established in all purified IgG samples from the corresponding group assayed as described in Materials and methods. The results of anti-t-PA and anti-PLG antibodies were expressed in abstract units (AU), with 1 AU equivalent to the OD of their relative internal standard anti-t-PA antibodies t2334 and anti-PLG antibodies p0617 at 1 µg/ml, respectively. The dashed line represents the cut-off, which means 99th percentile of the 40 healthy controls, and the solid line indicates the mean value for each group. (b, e) The antibody reactivity of affinity-purified IgG from patient plasma samples (two positive for IgG anti-PLG antibodies, designated P1 and P2; two positive for IgG anti-t-PA antibodies, designated P3 and P4) or a normal human IgG with PLG or t-PA were analysed by immunoblot assays. (c, f) Associations between antibody occurrence and a history of RPL were assessed with Fisher's exact test using the spss 12 program.
Associations between the occurrence of IgG anti-PLG and anti-t-PA antibodies and a history of RPL were evaluated with Fisher's exact test. In the RPL patients, the prevalence of IgG anti-PLG was higher than that of IgG anti-t-PA (27·6% versus 15%). Furthermore, IgG anti-PLG antibodies were associated with the high risk of RPL (OR 7·2, P = 0·004) (Fig. 1), while IgG anti-t-PA antibodies were not associated significantly with RPL in our detected patients groups (OR 6·8, P = 0·06) (Fig. 1). Associations between the occurrence of IgG anti-PLG and anti-t-PA antibodies and a history of RPL/PAPS were also evaluated with Fisher's exact test. Among 54 PAPS patients, IgG anti-PLG prevalence was higher than that of IgG anti-t-PA (37·0% versus 20·4%). Both IgG anti-PLG antibodies (OR 11·2, P < 0·001) and IgG anti-t-PA antibodies (OR 10·0, P = 0·01) were associated with the high risk of RPL/PAPS.
We purified IgG from the sera of patients and healthy controls, and analysed further their binding to the corresponding antigenic targets. The binding of anti-t-PA or anti-PLG containing IgG fractions to their corresponding antigens were evaluated by immunoblot assays (Fig. 1). We observed the specific binding of anti-t-PA or anti-PLG antibodies to the corresponding antigens, while no non-specific bindings were found in normal control IgG. In this way, the presence of IgG anti-PLG and anti-t-PA antibody in patients was confirmed.
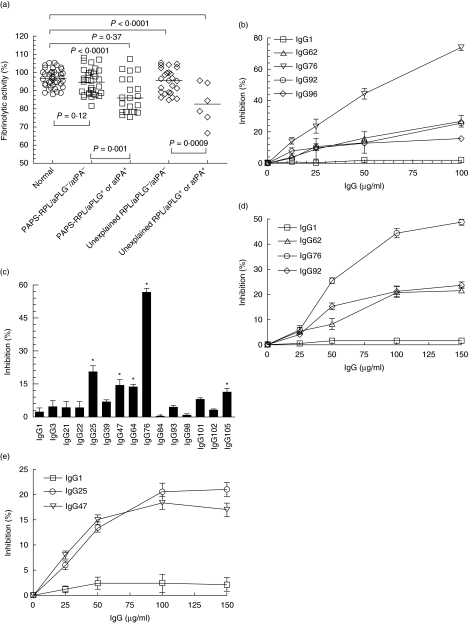
The effects of IgG fractions on extrinsic fibrinolytic activity
To explore further the significance of the presence of anti-PLG and anti-t-PA antibodies in patients with RPL, the effects of IgG fractions on the fibrinolytic activity were examined using a plasmin fluorogenic substrate assay. IgG fractions were prepared from 87 patients with RPL and 40 control subjects. The distribution of effects of these IgG fractions on fibrinolytic activities is shown in Fig. 2. Compared with those of the healthy control group and the patient group without anti-PLG and anti-tPA antibodies, exogenous fibrinolysis was inhibited significantly by IgG fractions from the patients with anti-PLG or anti-t-PA antibodies. Furthermore, significant inhibition of fibrinolytic activity was also observed by IgG fractions from patients with any one of the two antibodies, compared with that of the healthy control group and patients without the corresponding antibodies (data not shown). Afterwards, we found that anti-PLG containing IgG from patients significantly inhibited t-PA-dependent PLG activation in a concentration-dependent manner (Fig. 2). We then investigated further anti-t-PA containing IgG fractions on t-PA amidolytic activity using a t-PA fluorogenic substrate assay. Compared with healthy control IgG, t-PA amidolytic activity was inhibited significantly by five anti-t-PA containing IgG fractions (IgG25, IgG47, IgG64, IgG76 and IgG105) (P < 0·05) (Fig. 2), while the inhibitory effects of the others was not statistically significant. Furthermore, the inhibitory effects of the five anti-t-PA containing IgG fractions on t-PA activity were concentration-dependent, while the other IgGs at lower or higher concentrations still had no significant inhibitory effects on t-PA activity (data not shown). Thus, both anti-t-PA and anti-PLG may be responsible for the inhibition of exogenous fibrinolysis in patients.
Fig. 2.
Inhibition of exogenous fibrinolysis by IgG fractions purified from the plasma of patients with recurrent pregnancy loss (RPL). (a) The effects of IgG fractions from patients with RPL on exogenous fibrinolysis were measured using a fluorogenic substrate for plasmin (I-1390). The assay was performed using mixtures of tissue type plasminogen activator (t-PA) (10 nM), Glu-plasminogen (PLG) (100 nM), IgG fractions (50 µg/ml) or 0·5% bovine serum albumin (BSA) (control) and plasmin substrate (200 µM), as described in Material and methods. The initial rate of plasmin generation of each test mixture was converted to the percentage of the total initial rate by dividing the initial rate in the absence of purified IgG. atPA indicates anti-t-PA antibodies, and aPLG indicates anti-PLG antibodies. (b) The effects of IgG fractions from patients with RPL on exogenous fibrinolysis were measured with increasing amounts (0–100 µg/ml) of anti-PLG containing IgG fractions (IgG62, IgG76, IgG92 and IgG96) using a plasmin substrate as mentioned above. Exogenous fibrinolysis was inhibited significantly by anti-PLG containing IgG fractions in a concentration-dependent manner compared with normal human IgG1 at the same concentrations. (c) The effects of anti-t-PA containing IgG fractions on t-PA amidolytic activity were determined in the absence or presence of anti-t-PA containing IgG fractions using a sensitive fluorogenic t-PA substrate (I-1195). Assays were performed in 96-well black plates containing mixtures of t-PA (10 nM), anti-t-PA containing IgG fractions (50 µg/ml) or phosphate-buffered saline (PBS)/0·5% bovine serum albumin (BSA) (control), and t-PA substrate (200 µM) as described in Material and methods. The number represents the label for the corresponding plasma sample. IgG1 and IgG3 are healthy control IgG, and others are IgG from patients. The initial rate of substrate hydrolysis of each test sample was converted to a percentage of the total initial rate by dividing the relative initial rate in the absence of purified IgG. *P < 0·05 versus healthy control IgG by paired t-test. (d) The effects of anti-PLG antibodies on PLG activation were evaluated at the surface of human umbilical vein endothelial cells (HUVEC). Plasmin activity was estimated as described in Material and methods. HUVEC, cultured in plastic 96-well culture plates, were incubated with 250 nM Glu-PLG and various concentrations of anti-PLG containing IgG fractions from patients (IgG62, IgG76, IgG92, 0–150 µg/ml) at 4°C for 18 h. After extensive washing, the mixture containing 10 nM t-PA and 200 µM plasmin substrate I-1390 was added. The initial rate of plasmin generation of each test mixture was converted to the percentage of the total initial rate by dividing the initial rate in the absence of purified IgG. IgG1 are healthy control IgG. (e) The effects of anti-t-PA antibodies on PLG activation were evaluated at the surface of HUVEC. Plasmin activity was estimated as above with a small modification. HUVEC were incubated with 10 nM t-PA and various concentrations of anti-t-PA antibodies containing IgG fractions from patients (IgG25, IgG47, 0–150 µg/ml) at 4°C for 18 h. After extensive washing, the mixture containing 100 nM PLG and 200 µM plasmin substrate I-1390 was added. IgG1 are healthy control IgG.
The effects of anti-PLG or anti-t-PA antibodies on PLG activation were evaluated further at the surface of HUVEC. Non-specific interference was avoided by extensively washing away the unbound proteins after incubation of PLG and anti-PLG containing IgG fractions (when the effects of IgG anti-PLG were assayed), or t-PA and anti-t-PA containing IgG fractions (when the effects of IgG anti-t-PA were assayed) with HUVEC. We observed a decrease in plasmin generation at the cell surface level as a function of the concentration of anti-PLG and anti-tPA containing IgG fractions added compared with that obtained in the presence of normal IgG (Fig. 2). This may suggest that anti-t-PA or anti-PLG antibodies against some antigenic epitopes can inhibit PLG activation at the endothelial cell surface.
Discussion
In this study, we evaluated the prevalence and clinical significance of IgG anti-PLG and anti-t-PA in a cohort of patients with RPL distributed in two groups of patients: patients with well-defined PAPS and patients with unexplained RPL. Overall, our data showed that IgG anti-PLG and anti-t-PA antibodies were present in 24 of 87 (27·6%) and 13 of 87 (15%) patients with RPL, respectively (Fig. 1). The presence of IgG anti-PLG has been reported in patients with rheumatoid arthritis and SLE, and it was suggested that PLG may be a target of the immune response in autoimmune disease [12–14]. The association of anti-t-PA antibodies with thrombosis was also reported to be related to APS [17, 18]. In our patient groups, IgG anti-PLG antibodies were associated with the high risk of RPL (OR 7·2, P = 0·004) evaluated by Fisher's exact test, while IgG anti-t-PA antibodies were not associated significantly with RPL (OR 6·8, P = 0·06), although higher mean values of IgG anti-t-PA antibodies were observed in the sera of patients with RPL/APS (P < 0·0001) and unexplained RPL patients (P = 0·005) than in the healthy control group (Fig. 1). However, both IgG anti-PLG antibodies (OR 11·2, P < 0·001) and IgG anti-t-PA antibodies (OR 10·0, P = 0·01) were found to be associated with the high risk of RPL/PAPS. Of these anti-t-PA or anti-PLG antibody-positive patients with PAPS, three were found to have a history of thrombosis (two were positive for anti-PLG antibodies, two had anti-t-PA antibodies and one had both). These data may indicate that IgG anti-PLG and/or anti-t-PA antibodies might (to a lesser degree) play a role in the pathogenesis of RPL.
In animal models, the infusion of APA during pregnancy causes placental insufficiency accompanied with pregnancy loss [26, 27]. Thrombotic events at the placental level might be related to endothelial cell activation, inhibition of the protein C/S system and fibrinolysis as well as to annexin V displacement [28]. A reduction in fibrinolysis has been described in association with thrombosis in the PAPS [9–11]. In our study, we measured anti-t-PA and anti-PLG antibodies as possible causes of hypofibrinolysis in 87 patients with RPL. We investigated the effects of anti-PLG or anti-t-PA antibodies on t-PA-dependent PLG activation, using plasmin or t-PA fluorogenic substrate assay. A significant inhibition of exogenous fibrinolysis was observed by IgG fractions from patients with anti-PLG antibodies or anti-t-PA antibodies compared with those of the healthy control group and the patient groups without these two antibodies (Fig. 2). We found that both anti-t-PA and anti-PLG may be responsible for the inhibition of exogenous fibrinolysis in patients, which was shown further on the HUVEC surface (Fig. 2). Previous studies have shown that the patients with abnormally high plasma levels of anti-t-PA antibodies had lower levels of t-PA activity than healthy controls [18]. By binding the catalytic or kringle-2 domains of t-PA, anti-t-PA antibodies could lead to hypofibrinolysis and contribute to the prothrombotic state [18, 29]. Because a defect of t-PA has been associated with thrombosis [30], APS patients with abnormally high levels of anti-t-PA and anti-PLG antibodies may have an additional risk for thrombosis. The role of fibrinolytic components in trophoblast invasiveness has been suspected in animals [31]. t-PA may play a key role in the early stages of placentation and when placenta separates from maternal tissue at term [32]. An impaired plasmin-dependent proteolysis in women might favour recurrent abortion by promoting fibrin deposition in early placental circulation or by limiting trophoblast development, or both [31]. Furthermore, anti-t-PA antibodies may limit trophoblast invasiveness by binding to t-PA [33]. Thus, hypofibrinolysis due to anti-t-PA or anti-PLG antibodies could be added to the list of possible pathological processes responsible for the clinical symptoms in APS.
In conclusion, the prevalence of both IgG anti-PLG and IgG anti-t-PA antibodies was high in patients with RPL/PAPS, but only the prevalence of IgG anti-PLG antibodies was high in patients with RPL, and some of them might play a role in the pathogenesis of these patients.
Acknowledgments
This research work was supported by the grants from the National Natural Science Foundation of China (no. 39970703). We thank Qiaoer Chen for recruiting patients and obtaining blood samples.
References
- 1.de Groot PG, Derksen RH. Pathophysiology of the antiphospholipid syndrome. J Thromb Haemost. 2005;3:1854–60. doi: 10.1111/j.1538-7836.2005.01359.x. [DOI] [PubMed] [Google Scholar]
- 2.Vinatier D, Dufour P, Cosson M, Houpeau JL. Antiphospholipid syndrome and recurrent miscarriages. Eur J Obstet Gynecol Reprod Biol. 2001;96:37–50. doi: 10.1016/s0301-2115(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 3.Branch DW, Khamashta MA. Antiphospholipid syndrome: obstetric diagnosis, management, and controversies. Obstet Gynecol. 2003;101:1333–44. doi: 10.1016/s0029-7844(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 4.Marai I, Zandman-Goddard G, Shoenfeld Y. The systemic nature of the antiphospholipid syndrome. Scand J Rheumatol. 2004;33:365–72. doi: 10.1080/03009740410010290. [DOI] [PubMed] [Google Scholar]
- 5.Caruso A, De Carolis S, Di Simone N. Antiphospholipid antibodies in obstetrics: new complexities and sites of action. Hum Reprod Update. 1999;5:267–76. doi: 10.1093/humupd/5.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Mackworth-Young CG. Antiphospholipid syndrome: multiple mechanisms. Clin Exp Immunol. 2004;136:393–401. doi: 10.1111/j.1365-2249.2004.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esmon CT. Regulation of blood coagulation. Biochim Biophys Acta. 2000;1477:349–60. doi: 10.1016/s0167-4838(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 8.Yang CD, Hwang KK, Yan W, et al. Identification of anti-plasmin antibodies in the antiphospholipid syndrome that inhibit degradation of fibrin. J Immunol. 2004;172:5765–73. doi: 10.4049/jimmunol.172.9.5765. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda S, Atsumi T, Ieko M, Koike T. Beta2-glycoprotein 1, anti-beta2-glycoprotein 1, and fibrinolysis. Thromb Res. 2004;114:461–5. doi: 10.1016/j.thromres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Ieko M, Sawada KI, Koike T, et al. The putative mechanism of thrombosis in antiphospholipid syndrome: impairment of the protein C and the fibrinolytic systems by monoclonal anticardiolipin antibodies. Semin Thromb Hemost. 1999;25:503–7. doi: 10.1055/s-2007-994958. [DOI] [PubMed] [Google Scholar]
- 11.Ieko M, Ichikawa K, Atsumi T, et al. Effects of beta2-glycoprotein 1 and monoclonal anticardiolipin antibodies on extrinsic fibrinolysis. Semin Thromb Hemost. 2000;26:85–90. doi: 10.1055/s-2000-9808. [DOI] [PubMed] [Google Scholar]
- 12.Stefanescu M, Szegli G, Cremer L, et al. The presence and significance of some anti-enzyme antibodies (anti-plasminogen, anti-trypsin, anti-phospholipase C) in rheumatoid arthritis (RA) and reactive arthritis (rA) Arch Roum Pathol Exp Microbiol. 1989;48:47–53. [PubMed] [Google Scholar]
- 13.Gonzalez-Gronow M, Cuchacovich M, Grigg DM, Pizzo SV. Analysis of autoantibodies to plasminogen in the serum of patients with rheumatoid arthritis. J Mol Med. 1996;74:463–9. doi: 10.1007/BF00217522. [DOI] [PubMed] [Google Scholar]
- 14.Kozmin LD, Shirokova IE, Lisitsina TA, et al. Anti-plasminogen autoantibodies from plasma of patients with systemic lupus erythematosus having anti-phospholipid antibody syndrome: isolation and some immunochemical properties. Biochemistry (Mosc) 2003;68:339–45. doi: 10.1023/a:1023066503209. [DOI] [PubMed] [Google Scholar]
- 15.Simmelink MJ, De Groot PG, Derksen RH. A study on associations between antiprothrombin antibodies, antiplasminogen antibodies and thrombosis. J Thromb Haemost. 2003;1:735–9. doi: 10.1046/j.1538-7836.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- 16.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–21. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 17.Cugno M, Dominguez M, Cabibbe M, et al. Antibodies to tissue-type plasminogen activator in plasma from patients with primary antiphospholipid syndrome. Br J Haematol. 2000;108:871–5. doi: 10.1046/j.1365-2141.2000.01948.x. [DOI] [PubMed] [Google Scholar]
- 18.Cugno M, Cabibbe M, Galli M, et al. Antibodies to tissue-type plasminogen activator (tPA) in patients with antiphospholipid syndrome: evidence of interaction between the antibodies and the catalytic domain of tPA in 2 patients. Blood. 2004;103:2121–6. doi: 10.1182/blood-2003-07-2422. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 21.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 22.Cai G, Guo Y, Shi J. Purification of apolipoprotein H by polyethylene glycol precipitation. Protein Expr Purif. 1996;8:341–6. doi: 10.1006/prep.1996.0109. [DOI] [PubMed] [Google Scholar]
- 23.Reber G, Tincani A, Sanmarco M, de Moerloose P, Boffa MC. Proposals for the measurement of anti-beta2-glycoprotein 1 antibodies. J Thromb Haemost. 2004;2:1860–2. doi: 10.1111/j.1538-7836.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 24.Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–203. [PubMed] [Google Scholar]
- 25.Hajjar KA, Harpel PC, Jaffe EA, Nachman RL. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986;261:11656–62. [PubMed] [Google Scholar]
- 26.Branch DW, Dudley DJ, Mitchell MD, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am J Obstet Gynecol. 1990;163:210–16. doi: 10.1016/s0002-9378(11)90700-5. [DOI] [PubMed] [Google Scholar]
- 27.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci USA. 1991;88:3069–73. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meroni PL, di Simone N, Testoni C, D'Asta M, Acaia B, Caruso A. Antiphospholipid antibodies as cause of pregnancy loss. Lupus. 2004;13:649–52. doi: 10.1191/0961203304lu2001oa. [DOI] [PubMed] [Google Scholar]
- 29.Saibeni S, Ciscato C, Vecchi M, et al. Antibodies to tissue-type plasminogen activator (t-PA) in patients with inflammatory bowel disease. high prevalence, interactions with functional domains of t-PA and possible implications in thrombosis. J Thromb Haemost. 2006;4:1510–16. doi: 10.1111/j.1538-7836.2006.01970.x. [DOI] [PubMed] [Google Scholar]
- 30.Juhan-Vague I, Valadier J, Alessi MC, et al. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb Haemost. 1987;57:67–72. [PubMed] [Google Scholar]
- 31.Gris JC, Neveu S, Mares P, Biron C, Hedon B, Schved JF. Plasma fibrinolytic activators and their inhibitors in women suffering from early recurrent abortion of unknown etiology. J Lab Clin Med. 1993;122:606–15. [PubMed] [Google Scholar]
- 32.Hu ZY, Liu YX, Liu K, et al. Expression of tissue type and urokinase type plasminogen activators as well as plasminogen activator inhibitor type-1 and type-2 in human and rhesus monkey placenta. J Anat. 1999;194:183–95. doi: 10.1046/j.1469-7580.1999.19420183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gris JC, Perneger TV, Quere I, et al. Antiphospholipid/antiprotein antibodies, hemostasis-related autoantibodies, and plasma homocysteine as risk factors for a first early pregnancy loss: a matched case–control study. Blood. 2003;102:3504–13. doi: 10.1182/blood-2003-01-0320. [DOI] [PubMed] [Google Scholar]