Abstract
Lupus anti-coagulants (LA) are a variety of anti-phospholipid antibodies characterized by their capacity to interfere with phospholipid-dependent coagulation assays. LA are increasingly recognized as important predictors of thrombosis. However, the antigen specificity of LA is still poorly characterized. Growing evidence indicates that oxidized phospholipids are among the targets of anti-phospholipid antibodies. This prompted us to investigate the role of IgG directed against different oxidized phospholipids in 164 subjects without clotting factor defects that were tested for the presence of LA using a LA-sensitive activate partial thromboplastin time (aPTT-FSL) and a screening/confirmation assay based on diluted Russell's viper venom test (dRVVT-PL). The response to aPTT-FSL was significantly (P < 0·0005) associated with high titres of IgG against oxidized phosphatidylserine, phosphatidylethanolamine and phosphatidylinositol, whereas positivity to dRVVT-PL was associated with the elevation of IgG against oxidized phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine (P < 0·0005) and phosphatidylinositol (P < 0·01). No difference in reactivity against oxidized cardiolipin was evident between the different groups. Positivity to the dRVVT-PL test was also associated significantly (P < 0·005) with the elevation of anti-cardiolipin and anti-β2-glycoprotein-1 IgG. However, stepwise logistic regression demonstrated that IgG recognizing oxidized phosphatidylethanolamine and oxidized phosphatidylcholine were the only independent predictors of the response to dRVVT-PL assay, while IgG recognizing oxidized phosphatidylethanolamine and oxidized phosphatidylinositol were independent predictors of the response to aPTT-FSL test. In conclusion, autoantibodies against defined oxidized phospholipids are independent predictors of LA detection by aPTT-FSL or dRVVT-PL assays and might contribute to the variability often observed in the responses to the functional tests detecting LA.
Keywords: anti-phospholipid antibodies, β2-glycoprotein-1, cardiolipin, lipid peroxidation, thrombosis
Introduction
Anti-phospholipid antibodies (aPL) are a heterogeneous group of autoantibodies targeting cell membrane phospholipids as well as several phospholipid-binding proteins, particularly beta-2 glycoprotein 1 (β2GP-1) and prothrombin [1, 2]. The presence of these antibodies characterizes the anti-phospholipid syndrome and is often associated with autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis and systemic sclerosis [3, 4]. Current interest in the characterization of aPL relies not only on the diagnosis of anti-phospholipid syndrome, but is related to the association between aPL detection and an increased risk of stroke, myocardial infarction and deep venous thrombosis [4–7].
Two types of laboratory test are used currently for detecting aPL: solid phase enzyme-linked immunosorbent assays (ELISA) using cardiolipin and/or β2GP-1 and functional coagulation tests as antigen. These latter reveal the property of certain aPL, also known as lupus anti-coagulant (LA), to interfere with several phospholipid-dependent coagulation assays [8, 9]. Guidelines for the detection of LA require confirmation of the phospholipid specificity by neutralization of the antibodies with an excess of negatively charged phospholipids [10]. Although a positive response to LA tests has higher predictivity for thrombosis than the detection of anti-cardiolipin and anti-β2GP-1 antibodies by ELISA, the former suffer from a large interlaboratory variability and a number of pre-analytical and analytical biases [8, 9]. Antibodies targeting β2GP-1 and prothrombin have been shown to contribute to LA effects [11, 12]. However, in clinical laboratory practice, discrepancies are often observed between positive responses to LA-detecting assays and the identification of anti-β2GP-1 and anti-prothombin antibodies by ELISA [8]. Several reports indicate that aPL from SLE patients recognize oxidized cardiolipin in ELISA [13, 14] and cross-react with oxidized lipoproteins [15, 16]. Moreover, mice immunized with syngeneic apoptotic thymocytes develop aPL that specifically bind oxidized phosphatidylcholine [17]. Taken together, these observations suggest the possibility that aPL might recognize antigens derived from the oxidative modification of phospholipids. This prompted us to investigate the contribution of antibodies targeting different oxidized phospholipids in determining the response to the laboratory tests currently used for detecting LA.
Materials and methods
Patient recruitment
For this study we recruited 164 unselected subjects (66 male, 98 female, age range 25–83 years) who referred to the Clinical Chemistry Unit of the Ospedale Maggiore della Carità di Novara within the past 12 months for performing LA tests. In all the subjects, clotting function was assayed preliminarily using prothrombim time normalized ratio (PT-INR) (Thromborel-S; Dade Behring, Marburg, Germany) and an aPL-insensitive aPTT test (actin FS activated PTT reagent; Dade Behring, Marburg, Germany). Only the subjects with PT-INR and aPTT within the normal range were included in the study. Serum samples were collected at the time of LA testing and stored frozen until analysis. The study was planned according to the principles of the Declaration of Helsinki. All the subjects gave informed consent to the analysis.
Assay of lupus anti-coagulant antibodies
LA activity was evaluated by an automated procedure (Behring Coagulation System, Marburg, Germany) using a standardized activated partial thromboplastin time assay with increased sensibility for LA aPTT-FSL (Dade Behring) and the combination of screening and confirmation assays based on the diluted Russell's viper venom test (dRVVT-PL) (LA1/LA2 reagent; Dade Behring). Reference intervals for aPTT-FSL were 27–35 s, while the phospholipid correction ratios of dRVVT-PL were interpreted as follows: ratios below 1·3 no LA, ratios between 1·3 and 1·5 low LA activity, ratios between 1·5 and 2·0 medium LA activity and ratios above 2·0 high LA activity. All patients were also investigated for the presence of anti-cardiolipin and anti-β2GP-1 IgG by solid-phase immunoassays (DiaSorin Spa, Saluggia, Italy). Values above 14 U/ml were taken as positive. Anti-prothrombin and anti-annexin V IgG were determined by commercial kits (Hyphen Biomed, Neuville-sur-Oise, France) according to the manufacturer's instructions.
Phospholipid oxidation
Phospholipids were oxidized under controlled conditions by free radicals originating from the thermal decomposition of 2,2′-azobis(2-amidinopropane) hydrochloride (AAPH) (Polyscience Inc., Warrington, PA, USA). Briefly, cardiolipin (85 µg/ml), phosphatidylserine (125 µg/ml), phosphatidylcholine (85 µg/ml), phosphatidylethanolamine (85 µg/ml) and phosphatidylinositol (85 µg/ml) were suspended in phosphate-buffered saline (PBS) pH 7·4 and incubated up to 6 h at 37°C in the presence of 1 mmol/l AAPH. The kinetics of phospholipid oxidation was monitored continuously by measuring conjugated diene absorbance at 234 nm. The reaction was stopped by the addition of 0·1 mM N,N′-diphenyl-p-phenylenediamine (DPPD) when the relative difference of absorbance from the solution at t0 was 0·7 OD. Oxidized phospholipids were then extracted with an equal volume of chloroform–methanol (1 : 1 v/v) mixture, dried under nitrogen and resuspended at 100 µg/ml (final concentration) in either ethanol (cardiolipin and phosphatidylcholine) or methanol (phosphatidylserine, phosphatidylethanolamine and phosphatidylinositol).
Evaluation of the reactivity towards oxidized phospholipids
Immune reactivity towards both native and oxidized phospholipids was determined by solid-phase immunoassays using 96-microwell ELISA plates (Nunc-Immuno Poly Sorb, Nunc, S/A, Roskilde, Denmark). ELISA plates were coated by the addition of 30 µl of the different phospholipid solutions and the solvent was evaporated immediately under vacuum. The same amount of solvent was added to the reference wells. After two washes with PBS, non-specific binding sites were blocked by 1 h incubation at 37°C with 0·3 ml of 1% (v/v) solution of polyethyleneglycol compound in PBS pH 7·4, according to Kilpatrick [18]. The coated wells were then washed three times with PBS. Patient sera (1 : 50 dilution in PBS supplemented with 1% polyethyleneglycol compound were added in duplicate and incubated for 1 h at 37°C. After washing five times with PBS, peroxidase-linked goat anti-human IgG (dilution 1 : 6000) (Dako Spa, Milan, Italy) was added and incubated for 60 min at 37°C. Unbound antibodies were removed by five washes with PBS. The antibody binding was revealed by the addition of0·15 ml of a reaction mixture containing 0·4 mg/ml of 1-phenylenediamine, 0·4 µl/ml hydrogen peroxide (30%), 5·1 mg/ml citric acid, 6·1 mg/ml anhydrous Na2HPO4, pH 5·0. The reaction was stopped after 15 min by adding 50 µl 2 N H2SO4 and absorbance was measured at 490 nm using a Bio-Rad microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). The results were expressed by subtracting the background reactivity of each sera in the wells treated with the solvent alone.
Data analysis and statistical calculations
Statistical analyses were performed by spss statistical software (SPSS Inc., Chicago, IL, USA). Differences between groups were estimated by non-parametric Mann–Whitney U-test. Fisher's exact tests was used for the comparison of frequency data. Significance was taken at the 5% level. Differences between reactivity to native and oxidized forms of phospholipids were assessed by the non-parametric Wilcoxon's matched-pairs test. Confidence intervals were calculated by Newcombe's method using the CIA software version 2.1.1 (by T. Bryant, Southampton University, UK). Normality distribution was assessed preliminarily by the Kolmogorov–Smirnov and the Shapiro–Wilk tests. In the presence of a substantial deviation from normality hypothesis correction was performed by logarithmic transformation. The independent effect of significant variables was assessed using stepwise logistic regression analysis.
Materials
Cardiolipin, phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol, polyethyleneglycol compound and N,N′-diphenyl-p-phenylenediamine were supplied by Sigma Chemical Co. (St Louis, MO, USA). All other chemicals were of analytical grade and were supplied by Merck (Darmstadt, Germany).
Results
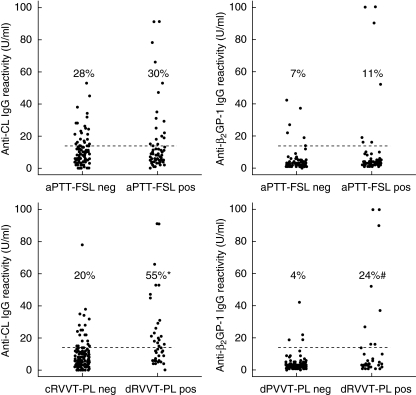
The contribution of aPL targeting oxidized phospholipids in determining the response to functional tests used currently in laboratory practice for the detection of LA was investigated in 164 subjects who referred to our clinical chemistry unit for performing LA tests. All the subjects had PT-INR, aPTT and aPTT-Actin FS ratios within the normal range (Table 1). LA activity was evaluated in all the subjects by both an aPL-sensitive standardized activated partial thromboplastin time (aPTT-FSL) test and a combination of screening and confirmation assays based on the diluted RVVT plus phospholipid supplementation (dRVVT-PL). Among the subjects investigated 85 (52%) had both aPTT-FSL and dRVVT-PL tests within the normal range, 41 (25%) had an abnormal aPTT-FSL test only, 15 (9%) an abnormal dRVVT-PL test only and 23 (14%) had both the tests above the normal range. The values of the different LA assays are reported in Table 1. The groups were not statistically different for gender and age distribution (not shown). By using commercial ELISA assays we detected anti-cardiolipin and anti-β2GP-1 IgG above the threshold values (14 U/ml) in, respectively, 47 (29%) and 13 (8%) of the individuals investigated. High (> 100AU/ml) titres of anti-prothrombin IgG were evident in only one subject, while none had detectable anti-annexin autoantibodies (not shown). The elevation of anti-cardiolipin or anti-β2GP-1 IgG was significantly (χ2 < 0·0001) associated only with the positivity to dRVVT-PL test (Fig. 1).
Table 1.
Values of the different clotting assay in the subjects with different response to activated partial thromboplastin time (aPTT-FSL) and diluted Russell's viper venom test plus phospholipid supplementation (dRVVT-PL) tests.
| Groups | PT (INR) normal range 0·8–1·2 | aPTT (s)* normal range 22–33 | aPTT (ratio)* normal range 0·8–1·2 | aPTT-FSL (s) normal range 23·5–35 | dRVVT-PL (s) normal values < 38 | Phospholipid correction ratio |
|---|---|---|---|---|---|---|
| aPTT-FSL and dRVVT-PL negative (n = 82) | 0·98 ± 0·07 | 26·83 ± 2·57 | 0·98 ± 0·09 | 31·13 ± 2·88 | 33·21 ± 3·04 | |
| 95% CI | 0·97–0·99 | 26·27–27·4 | 0·96–1 | 30·49–31·76 | 32·54–33·88 | |
| aPTT-FSL positive and dRVVT-PL negative (n = 44) | 1·02 ± 0·08 | 28·65 ± 2·40 | 1·05 ± 0·09 | 36·51 ± 3·16 | 33·37 ± 2·41 | 1·04 ± 0·07 |
| 95% CI | 0·99–1·05 | 27·92–29·38 | 1·02–1·08 | 35·60–37·50 | 32·64–34·12 | 1·02–1·07 |
| aPTT-FSL negative and dRVVT-PL positive (n = 15) | 0·95 ± 0·07 | 25·53 ± 3·15 | 0·94 ± 0·12 | 31·5 ± 3·42 | 45·09 ± 7·93 | 1·50 ± 0·25 |
| 95% CI | 0·92–0·99 | 23·79–27·28 | 0·88–1·01 | 29·61–33·39 | 40·70–49·48 | 1·34–1·61 |
| aPTT-FSL positive and dRVVT-PL positive (n = 23) | 1·02 ± 0·08 | 28·54 ± 2·18 | 1·05 ± 0·08 | 45·80 ± 10·58 | 53·77 ± 17·77 | 1·7 0 ± 0·45 |
| 95% CI | 0·99–1·06 | 27·60–29·49 | 1·01–1·08 | 41·20–50·35 | 46·09–61·45 | 1·50–1·86 |
The values are means ± s.d.; 95% CI denotes confidence intervals.
aPTT test and ratio refers to aPL-insensitive aPTT test (actin FS activated PTT reagent; Dade Behring, Marburg, Germany). The phospholipid correction ratios were obtained using screening and confirmation assays based on the diluted dRVVT-PL(LA1/LA2 Reagent; Dade Behring). Among the patients positive to dRVVT-PL the phospholipid correction ratios allowed us to establish that 22 had low activity, 11 medium activity and five high activity of LA antibodies (see Methods section for details).
Fig. 1.
Distribution of anti-cardiolipin (anti-CL) and anti-β2-glycoprotein-1 (β2GP-1) IgG in 164 patients without evidence of clotting defects evaluated for lupus anti-coagulant (LA) activity by both standardized activated partial thromboplastin time (aPTT-FSL) and a combination of screening and confirmation assays based on the diluted Russell's viper venom test plus phospholipid supplementation (dRVVT-PL). Abnormal aPTT-FSL was found in 74 subjects, while 48 had an abnormal dRVVT-PL test. The dotted lines represent the cut-off value for anti-cardiolipin and anti-β2GP-1 IgG. Values in parentheses are the percentage of subjects with anti-cardiolipin and anti-β2GP-1 IgG above the threshold. Differences of proportions calculated by Newcombe's method were: *35% (95% CI 17–51%) for anti-cardiolipin IgG; #21% (95% CI 9–37%) for anti-β2GP-1 IgG.
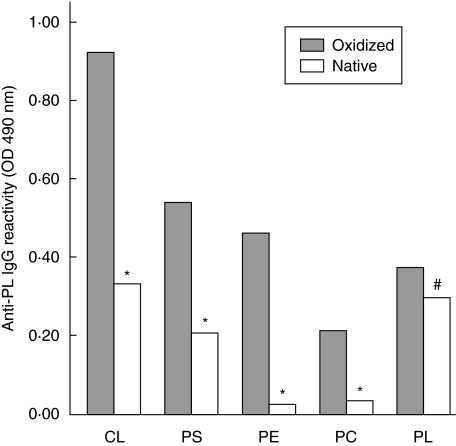
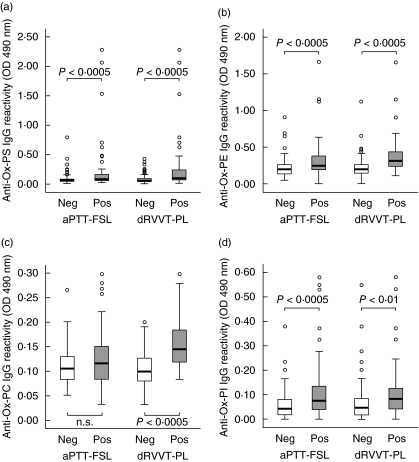
Solid-phase immunoassays using different oxidized phospholipids as antigens showed that individuals negative for both aPTT-FSL and dRVVT-PL had circulating IgG against oxidized phosphatidylserine (oxPS), phosphatidylcholine (oxPC), phosphatidylethanolamine (oxPE) and phosphatidylinositol (oxPI) significantly (P ≤ 0·005) lower than the subjects positive for one or both the LA assays (Table 2). Although some of the sera showed appreciable IgG binding to oxidized cardiolipid (oxCL), no difference in anti-oxCL reactivity was evident between the two groups (Table 2). Specific recognition of oxidized antigens was confirmed by comparing the reactivity of selected sera in ELISA plates coated with both the native and oxidized forms of the same phospholipid. As shown in Fig. 2, the binding of the different sera to oxCL, oxPS, oxPC and oxPE was three to eight times greater than that to the relative native phospholipids. A smaller, although significant, difference (P < 0·05) was observed in the recognition of the oxidized form of phosphatidylinositol (Fig. 2). By grouping the subjects according to the response to the different LA assays we observed that the IgG reactivity against oxPS, oxPC, oxPE and oxPI was significantly higher (P ranging from < 0·01 to < 0·0005) in the dRVVT-PL-positive than in the dRVVT-PL-negative individuals (Fig. 3). When the same subjects were grouped according to positivity to aPTT-FSL, significant differences were evident only for anti-oxPS, oxPE and oxPI IgG (Fig. 3). Univariate analysis confirmed these associations (Table 2). Multivariate analysis using a logistic regression model revealed that only the recognition of oxPE and oxPI was associated independently with the response to aPTT-FSL (P < 0·0005; r2 = 0·206), whereas reactivity against oxPE and oxPC was associated independently with the response to the dRVVT-PL test (P < 0·0005; r2 = 0·387) (Table 3). At univariate analysis the measure of anti-cardiolipin and anti-β2GP-1 IgG by commercial kits was also associated with positivity to the dRVVT-PL test (P < 0·005). However, the inclusion of anti-cardiolipin and anti-β2GP-1-values in the above logistic regression model did not verify their independent association with dRVVT-PL. Moreover, performing multivariate analysis, using as dependent variable the positivity to aPTT-FSL or dRVVT-PL alone or in combination, revealed that IgG recognizing oxPE remained the only independent predictor of the response to one or both of the tests (P = 0·006; r2 = 0·311). Taken together, these results indicate that the pattern of antibodies recognizing specific classes of oxidized phospholipids might influence the response to the different tests exploring LA reactivity.
Table 2.
IgG reactivity against different oxidized phospholipids in subjects investigated for the presence of lupus anti-coagulant (LA) activity by activated partial thromboplastin time (aPTT-FSL) and diluted Russell's viper venom test plus phospholipid supplementation (dRVVT-PL) tests.
| Negative to both aPTT-FSL and dRVVT-PL (n = 85) | Positive to aPTT-FSL and/or dRVVT-PL (n = 79) | Statistical significance | |
|---|---|---|---|
| Ox-CL | 0·141 ± 0·092 | 0·213 ± 0·328 | n.s. |
| Ox-PS | 0·074 ± 0·061 | 0·206 ± 0·384 | P < 0·0005 |
| Ox-PE | 0·208 ± 0·101 | 0·328 ± 0·249 | P < 0·0005 |
| Ox-PC | 0·105 ± 0·033 | 0·128 ± 0·054 | P = 0·0005 |
| Ox-PI | 0·055 ± 0·058 | 0·103 ± 0·116 | P < 0·0005 |
The values are expressed as optical density at 490 nm after subtracting the background reactivity of each sera in the wells treated with the solvent alone. All the sera were evaluated in duplicate. oxPS: oxidized phosphatidylserine; oxPC: phosphatidylcholine; oxPE: phosphatidylethanolamine; oxPI: phosphatidylinositol.
Fig. 2.
IgG reactivity against oxidized (filled bars) and native (empty bars) forms of cardiolipin (CL), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylinositol (PI) in sera of 10 randomly selected sera showing high titres of antibodies against the different oxidized phospholipids. Statistical significance was estimated by Wilcoxon's matched-pairs tests: *P < 0·005, # P < 0·05.
Fig. 3.
IgG reactivity against oxidized phosphatidylserine (a), phosphatidylethanolamine (b), phosphatidylcholine (c) and phosphatidylinositol (d) in the sera of subjects positive (empty bars) or negative (filled bars) for, respectively, activated partial thromboplastin time (aPTT-FSL) or diluted Russell's viper venom test plus phospholipid supplementation (dRVVT-PL) tests. Among the 164 subjects investigated 74 had abnormal aPTT-FSL, while 48 has abnormal dRVVT-PL values. The sera were tested at 1 : 50 dilution in microplate enzyme-linked immunosorbent assay plates coated with the different antigens and revealed with peroxidase-linked goat anti-human IgG anti-serum. The results are expressed as optical density (OD) at 490 nm after subtracting the background reactivity of each serum. Boxes include the values within 25th and 75th percentiles and the horizontal bars represent the medians. Eighty per cent of the values are comprised between the extremities of the vertical bars (10th−90th percentiles). The extreme values are represented by individual points. Statistical significance was evaluated by non-parametric Mann–Whitney U-test.
Table 3.
Association between the IgG reactivity against different oxidized phospholipids and the detection of lupus anti-coagulant (LA) activity by activated partial thromboplastin time (aPTT-FSL) and diluted Russell's viper venom test plus phospholipid supplementation (dRVVT-PL) tests.
| aPTT-FSL | dRVVT-PL | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Ox-CL | n.s. | n.s. | ||
| Ox-PS | P < 0·0005 | n.s. | P < 0·0005 | n.s. |
| Ox-PE | P < 0·0005 | P < 0·05 | P < 0·0005 | P < 0·005 |
| Ox-PC | n.s. | P < 0·0005 | P < 0·0005 | |
| Ox-PI | P < 0·0005 | P < 0·005 | P < 0·01 | n.s. |
oxPS: oxidized phosphatidylserine; oxPC: phosphatidylcholine; oxPE: phosphatidylethanolamine; oxPI: phosphatidylinositol.
Discussion
In recent years there has been growing consensus in considering the detection of LA antibodies to be a better predictor for the risk of thrombosis than determination of aPL by solid-phase immunoassays [19]. Moreover, the presence of LA is considered a specific risk factor for the occurrence of thrombotic complications in patients with a history of previous thrombosis and/or SLE [20]. However, the antigen specificity of the antibodies responsible for LA effect is still a matter of controversy. It is now well established that aPL detected by cardiolipin-based immunoassays and associated with thrombosis, fetal loss or autoimmune diseasesrecognize complexes between cardiolipin and β2GP-1 as antigens [21]. Moreover, elevated circulating anti-β2GP-1 IgG have been associated with an increased risk of thrombosis [22]. These observations, along with the capacity of monoclonal anti-β2GP-1 antibodies to interfere coagulation assays [11, 23], have led to the conclusion that anti-β2GP-1 autoreactivity is involved in causing an LA effect in vitro and might have prothrombotic activity in vivo. Antibodies recognizing prothrombin alone or in complex with phosphatidylserine also interfere with prothrombin functions and are associated with LA [24, 25]. This has led to the development of methods to discriminate between anti-β2GP-1-dependent and anti-prothrombin-dependent LA [19, 26]. However, the detection of LA in plasmas with low anti-β2GP-1 or anti-prothrombin autoreactivity remains unexplained.
Tests based on aPTT and dRVVT are considered to be reliable assays for the detection of LA [9, 10, 27]. However, because no single test is 100% sensitive, more than one test should be used to exclude the presence of LA [27]. By analysing the reactivity against different oxidized phospholipids in a group of individuals screened for the response to LA tests, we show that IgG targeting oxidized phosphatidylethanolamine and phosphatidylinositol are associated independently with positivity to aPTT-FSL, whereas IgG against oxidized phosphatidylcholine and phosphatidylethanolamine are associated independently with positivity to dRVVT-PL. This suggests the possibility that detection of LA antibodies by either aPTT-FSL or dRVVT-PL is influenced by the presence of antibodies recognizing oxidized phosphatidylethanolamine in combination with antibodies targeting either oxidized phosphatidylinositol or oxidized phosphatidylcholine. The capacity of phosphatidyl–ethanolamine alone or in combination with other phospholipids to neutralize the prolongation of clotting time due to LA antibodies is well recognized [28]. However, the association between the presence of anti-phosphatidylethanolamine IgG and LA activity still awaits further confirmation [29].
As discussed above, anti-β2GP-1 and anti-prothrombin IgG have been implicated in causing LA activity. In our hands, a significant association is evident between a positive response to dRVVT-PL and elevated anti-β2GP-1 or anti-cardiolipin IgG. However, such a relationship is not verified further in a multivariate analysis that also takes into account the values of IgG against the different oxidized phospholipids. This suggests that aPL targeting oxidized phospholipids might interfere with the clotting process more specifically than anti-β2GP-1 and anti-cardiolipin antibodies detected by commercial solid phase immunoassays.
Previous studies have suggested that antibodies recognizing oxidized cardiolipin might contribute to anti-phospholipid reactivity in humans [13, 14]. Although some of the sera investigated in this study show high reactivity towards oxidized cardiolipin, no association has been verified between anti-oxidized cardiolipin IgG and the response to both aPTT-FSL and dRVVT-PL. We have reported previously that antibodies against oxidized cardiolipin are evident in patients with chronic liver disease and oxidative stress without evidence of LA [30, 31]. Moreover, Schlame and colleagues [32] have observed that the oxidation state of cardiolipin does not influence its recognition by sera of patients with anti-phospholipid syndrome. Thus, we postulate that IgG recognizing oxidized cardiolipin might be a separate subset of anti-cardiolipin antibodies without relation with LA.
In conclusion, the results presented indicate that autoantibodies against defined oxidized phospholipids are independent predictors of the response to LA detection by either aPTT-FSL and dRVVT-PL tests, suggesting that different patterns of these autoantibodies might explain the variability often observed in the response to the functional assays used currently for detecting LA. Further studies are in progress to establish the clinical relevance of these observations.
Acknowledgments
This work has been supported by grants from the University of East Piedmont and the Regional Government of Piedmont. R. R. is the recipient of a fellowship from the Associazione Italiana contro le Leucemie, Linfomi e Mieloma, NovarAIL (Novara).
References
- 1.Bertolaccini ML, Hughes GR, Khamashta MA. Revisiting antiphospholipid antibodies: from targeting phospholipid to phospholipid binding proteins. Clin Lab. 2004;50:653–65. [PubMed] [Google Scholar]
- 2.Pierangeli SS, Harris EN. Clinical laboratory testing for antiphospholipid syndrome. Clin Chim Acta. 2005;357:17–23. doi: 10.1016/j.cccn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrom. N Engl J Med. 2003;346:752–63. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 4.McNeil HP, Chesterman CN, Krilis SA. Immunological and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 5.Veres K, Lakos G, Kerenyi A, et al. Antiphospholipid antibodies in acute coronary syndrome. Lupus. 2004;13:423–7. doi: 10.1191/0961203304lu1011oa. [DOI] [PubMed] [Google Scholar]
- 6.Katzav A, Chapman J, Shoenfeld Y. CNS dysfunction in the antiphospholipid syndrome. Lupus. 2003;12:903–7. doi: 10.1191/0961203303lu500oa. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C. Epidemiology of venous thromboembolism. Semin Vasc Med. 2001;1:7–26. doi: 10.1055/s-2001-14668. [DOI] [PubMed] [Google Scholar]
- 8.Passam FH, Krilis SA. Laboratory tests for the antiphospholipid syndrome: current concepts. Pathology. 2004;36:129–38. doi: 10.1080/00313020410001671966. [DOI] [PubMed] [Google Scholar]
- 9.Wong RC, Adelstein S, Gillis D, Favaloro EJ. Development of consensus guidelines for anticardiolipin and lupus anticoagulant testing. Semin Thromb Hemost. 2005;31:39–48. doi: 10.1055/s-2005-863804. [DOI] [PubMed] [Google Scholar]
- 10.Derksen HMW, de Groot PG. Tests for lupus anticoagulant revisited. Thomb Res. 2004;114:521–6. doi: 10.1016/j.thromres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Roubey RA, Pratt CW, Boyson JP, Winfield JB. Lupus anticoagulant activity of autoimmune antiphospholipid antibodies is dependent upon beta 2-glycoprotein. J Clin Invest. 1992;90:1100–4. doi: 10.1172/JCI115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oosting JD, Derksen RH, Bobbink IW, et al. Antiphospholipid antibodies directed against a combination of phospholipid with prothombin, protein C, or protein S: an explanation for their pathogenetic mechanisms? Blood. 1993;81:2618–25. [PubMed] [Google Scholar]
- 13.Hörkkö S, Miller E, Dudl E, et al. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. Recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J Clin Invest. 1996;98:815–25. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hörkkö S, Miller E, Branch DW, et al. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and β2-glycoprotein 1 (and other proteins) Proc Natl Acad Sci USA. 1997;94:10356–61. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaarala O, Alfthan G, Jauhiainen M, et al. Cross reaction between antibodies to oxidized low density lipoproteins and to cardiolipin in lupus erythematosus. Lancet. 1993;341:923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 16.Vaarala O, Puurunen M, Lukka M, et al. Affinity purified cardiolipin-binding antibodies show heterogeneity in their binding to oxidized low density lipoproteins. Clin Exp Immunol. 1996;104:269–74. doi: 10.1046/j.1365-2249.1996.21728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MK, Binder CJ, Miller YI, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;11:1359–70. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilpatrick DC. Factors affection cardiolipin antibody assays: modification with polyethylene glycol compound. Br J Haematol. 1998;100:52–7. doi: 10.1046/j.1365-2141.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 19.Greaves M. Antiphospholipid antibodies and thrombosis. Lancet. 1999;353:1348–53. doi: 10.1016/S0140-6736(98)10362-8. [DOI] [PubMed] [Google Scholar]
- 20.Galli M, Luciani D, Bertolini G, et al. Lupus anticoagulants are stronger risk factors of thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–32. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 21.Rand JH. Molecular pathogenesis of the antiphospholipid syndrome. Circ Res. 2002;90:29–37. doi: 10.1161/hh0102.102795. [DOI] [PubMed] [Google Scholar]
- 22.Zoghlami-Rintelen C, Vormittag R, Sailer T, et al. The presence of IgG antibodies against beta2-glycoprotein I predicts the risk of thrombosis in patients with the lupus anticoagulant. J Thromb Haemost. 2005;3:1160–5. doi: 10.1111/j.1538-7836.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- 23.Takeya H, Mori T, Gabazza EC, Kuroda F, et al. Anti-beta2glycoprotein I (beta2GPI) monoclonal antibodies with lupus anticoagulant like activity enhances the beta2GPI binding to phospholipids. J Clin Invest. 1997;99:2260–8. doi: 10.1172/JCI119401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amengual O, Atsumi T, Koike T. Antiprothrombin antibodies and the diagnosis of the antiphospholipid syndrome. Clin Immunol. 2004;112:144–9. doi: 10.1016/j.clim.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Atsumi T, Ieko M, Bertolaccini ML, et al. Association of autoantibodies against the phosphatidylserine–prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Reumatol. 2000;43:1982–93. doi: 10.1002/1529-0131(200009)43:9<1982::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Simmelink MJA, Derksen RMWM, Arnout J, et al. A simple methods to discriminate between β2-glycoprotein 1- and prothrombin-dependent lupus anticoagulants. J Thromb Haemost. 2003;1:740–7. doi: 10.1046/j.1538-7836.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 27.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 28.Rauch J, Tannenbaum M, Neville C, et al. Inhibition of lupus anticoagulant activity by hexagonal phase phosphatidylethanolamine in the presence of prothrombin. Thromb Haemost. 1998;80:936–41. [PubMed] [Google Scholar]
- 29.McIntyre JA, Wagenknecht DR. Anti-phosphatidylethanolamine (aPE) antibodies: a survey. J Autoimmun. 2000;15:185–93. doi: 10.1006/jaut.2000.0425. [DOI] [PubMed] [Google Scholar]
- 30.Rolla R, Vay D, Mottaran E, et al. Anti-phospholipid antibodies associated with alcoholic liver disease specifically recognize oxidized phospholipids. Gut. 2001;49:852–9. doi: 10.1136/gut.49.6.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigamonti C, Mottaran E, Reale E, et al. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology. 2003;38:42–9. doi: 10.1053/jhep.2003.50275. [DOI] [PubMed] [Google Scholar]
- 32.Schlame M, Haller I, Sammaritano LR, Blank TJJ. Effect of cardiolipin oxidation on solid-phase immunoassay for antiphospholipid antibodies. Thromb Haemost. 2001;86:1475–82. [PubMed] [Google Scholar]