Abstract
Innate immune system deficiency may predispose to severe infections such as Legionnaires' disease. We have investigated the role of mannose-binding lectin (MBL) deficiency in the Melbourne Aquarium Legionnaires' disease outbreak. Serum samples from patients and controls that were exposed but shown to be uninfected from the Melbourne Aquarium Legionnaires' disease outbreak were tested for MBL function (C4 deposition) and level (mannan-binding). MBL function was lower in Legionnaires' disease cases than in age- and sex-matched uninfected, exposed controls. The frequency of MBL deficiency with C4 deposition < 0·2 U/µl was significantly higher in Legionnaires' disease cases than in controls. This also applied to Legionnaires' disease cases requiring hospital care. There was no difference in MBL mannan-binding levels between Legionnaires' disease patients and controls. There was no significant interval change in MBL function or level after a mean of 46 days. MBL complement activation functional deficiency appears to predispose to Legionnaires' disease.
Keywords: deficiency, innate immunity, Legionella pneumophila, mannose-binding lectin
Introduction
Newly accumulating understanding of the innate immune system shows us that subtle deficiency states may predispose to infectious diseases. Cellular and soluble effector mechanisms of the innate immune system recognize and kill pathogens quickly and efficiently. When there are defects in their action patients may be more prone to severe infection. Legionnaires' disease due to infection with Legionella pneumophila is seen predominantly in elderly patients with comorbidities such as cigarette abuse, diabetes mellitus or renal impairment. Community-acquired Legionnaires' disease continues to have a significant mortality of up to 5%, despite advances in diagnosis and treatment [1]. Additional risks for Legionnaires' disease may be present in patients with innate immune deficiency where alterations in L. pneumophila opsonophagocytosis and complement activation may represent as-yet-undefined susceptibility factors.
Mannose-binding lectin (MBL) is a central player in the innate immune response. It is a C-type serum lectin [2] produced by the liver that binds microbial surface carbohydrates and mediates opsonophagocytosis directly and by activation of the lectin complement pathway [3]. A wide range of clinical isolates of bacteria, fungi, viruses and parasites are bound by MBL [4]. MBL deficiency is common, arising from mutation in the MBL2 structural gene and polymorphisms in the promoter sequence, and such deficiency appears to predispose to serious infection [5]. MBL deficiency has been defined variously on the basis of MBL2 genotypes that lead to production of low amounts of MBL [6], low MBL blood levels [7] and low MBL complement activation function [8].
MBL has been shown to bind to L. pneumophila [9]. Antibody-independent phagocytosis of L. pneumophila that is not mediated by typical cell surface receptors [10] and complement activation in excess of that attributable to classical and alternative pathways [11] have been demonstrated, yet neither of these have been investigated further to determine the contribution played by MBL. Similarly, there are no data on whether deficiency in MBL predisposes to Legionnaires' disease. To address this issue we have studied individuals involved in a case–control (uninfected, exposed) study performed after the Legionnaires' disease outbreak at the Melbourne Aquarium in 2000 [12]. Stored serum was tested for MBL function and levels but MBL2 genotypes could not be determined due to a lack of stored cellular material.
Materials and methods
Patients with proven Legionnaires' disease (positive urinary L. pneumophila serogroup 1 antigen or at least a fourfold rise in L. pneumophila antibodies or culture of L. pneumophila from respiratory secretions) and uninfected exposed controls had been recruited as part of the investigation of the Melbourne Aquarium Legionella outbreak in April/May 2000 [12]. This was the largest outbreak of Legionnaires' disease in Australia, with 125 patients. The 201 controls were part of a study to define risk factors for acquisition, having visited the Melbourne Aquarium during the outbreak but been shown to be serologically negative for L. pneumophila infection. This project was approved by the Human Research Ethics Committees of Melbourne Health and the Victorian Department of Human Services. Informed consent for study participation was provided by 102 Legionnaires' disease case patients, with 10 patients opting out of the study. Contact details were not available for 13 patients who were excluded, therefore, from the study. Informed consent was also provided by all 201 controls. A sufficient sample for study assays was available for 76 case patients and 181 controls, samples having been exhausted by previous tests in the other individuals. Of the 76 case patients, detailed clinical data from hospital in-patient stays were available in 46 instances from an earlier study [13] that documented 71 of the 95 Legionnaires' disease patients who were admitted to hospital. We are able to account for all 71 of these known in-patients among our results. Seven were known not to have been tested and 18 could not be matched to MBL results on age, sex and initials. Because of incomplete database details we are unable to unable to discriminate accurately between the other 30 tested patients, a number of whom will represent some of the remaining 24 in-patients whose treating clinicians had not responded to requests for clinical information in the previous study [13] or the 30 patients known not to require in-patient treatment.
MBL C4 deposition and mannan-binding enzyme-linked immunosorbent assays (ELISAs)
Serum samples had been stored optimally at −80°C and freeze-thawed minimally. During this project, freeze-thawing was restricted to a maximum of four runs. Where multiple patient samples were available, the specimen closest to the time of diagnosis was used for the main analysis. Follow-up specimens were available for testing for 31 patients. Only single samples were available for controls. MBL function was measured using a C4 deposition ELISA and MBL levels were measured using a mannan-binding ELISA, as described previously [14]. C4 deposition ELISA quantifies C4 deposited onto a solid phase mannan surface. After MBL binding to mannan, subsequent C4 deposition from added MBL-deficient human serum was measured using a biotinylated anti-C4 antibody (Sigma, Castle Hill, Australia). This assay is specific for MBL mediated C4 deposition with no activation of the classical or alternative complement pathways [15]. MBL-deficient serum is added to the assay providing complement factors and ensuring that there is no artefactual decrease in C4 deposition due to consumption in septic patients. One ml of Statens Serum Institute Standard (Statens, Denmark) was arbitrarily assigned 1000 U C4 deposition. MBL level was determined using a mannan-binding ELISA. MBL is bound to a solid-phase mannan surface and quantified using a biotinylated anti-MBL monoclonal HYB-131–01B (The Antibody Shop, Gentofte, Denmark). MBL functional deficiency was defined as C4 deposition < 0·2 U/µl, as this has been shown to be a highly discriminative predictor of low MBL2-producing genotypes [8].
Statistical analysis
Comparisons of discrete outcomes, including frequency of MBL-deficient individuals among cases and exposed, uninfected controls and the relationship between MBL deficiency and Legionnaires' disease severity indices, were performed using χ2 test. Comparisons of continuous values such as C4 deposition and mannan binding were performed using the Mann–Whitney U-test. Statistical analyses were performed using Minitab® release 14 (Minitab Inc., State College, PA, USA).
Results
Case patients
Results from 76 Legionnaires' disease patients were compared with a group of 181 exposed controls that had no evidence of L. pneumophila infection. Randomly selected controls matched for sex and age within 2 years of each Legionnaires' disease patient were included. Another five of the 76 MBL-tested Legionnaires' disease patients needed to be excluded because of the absence of appropriate age- or sex-matched controls. Legionnaires' disease patients excluded from the final analysis by the absence of consent (n = 23), samples (n = 26) or controls (n = 5) did not differ from the remaining study patients in age or sex distribution.
C4 deposition and MBL levels in cases and healthy exposed controls
MBL function, as shown by C4 deposition, was significantly lower in Legionnaires' disease case patients (0·09 versus 0·13 U/µl, P = 0·01) and in hospitalized Legionnaires' disease patients (0·09 versus 0·14 U/µl, P < 0·002) than in age- and sex-matched controls. Defining MBL functional deficiency as C4 deposition < 0·2 U/µl [8] showed that significantly more Legionnaires' disease cases were MBL-deficient than the age- and sex-matched controls [57/71 versus 44/71, P = 0·02, odds ratio (OR) = 2·5, 95% confidence intervals (CI) 1·1–5·7]. More in-patient Legionnaires' disease cases were MBL-deficient than the age- and sex-matched controls (40/45 versus 26/45, P < 0·001, OR = 5·9, 95% CI 1·8–25·1). Significantly lower C4 deposition (0·09 versus 0·11 U/µl, P = 0·02) and higher frequency of MBL functional deficiency (62/76 versus 116/181, P < 0·01; OR = 2·5, 95% CI 1·2–5·0) was also seen in Legionnaires' disease cases when compared with all controls, including those who were unmatched. There was no difference, however, in the MBL mannan-binding levels between Legionnaires' disease patients and matched controls (0·85 versus 0·67 µg/ml, P = 0·75), hospitalized Legionnaires' disease patients and matched controls (0·97 versus 1·02 µg/ml, P = 0·48) and Legionnaires' disease patients and all controls, matched or unmatched (0·81 versus 0·86 µg/ml, P = 0·81). There was no difference in the frequency of MBL mannan-binding deficiency in Legionnaires' disease cases or controls using previous MBL deficiency definitions based on mannan-binding levels of < 0·5 µg/ml [7] (32/71 versus 25/71, P = 0·23) or < 1·0 µg/ml [16] (38/71 versus 38/71, P = 1·0).
MBL function and to a lesser extent, levels, were lower in both case and control specimens than our previous studies [8, 14]. This is due possibly to prolonged sample storage. However, function and level remain correlated strongly in the Legionnaires' disease controls analysed in this study (Pearson's correlation coefficient 0·82, P < 0·001), as in Australian blood donor controls analysed previously (Pearson's correlation coefficient 0·83, P < 0·001) [14]. A similar distribution of function across the control populations persists, albeit with the Legionnaires' disease control results at a lower level. While it is possible that Legionnaires' disease specimens may have been thawed and refrozen more than control specimens, this would have applied to a small, random minority of specimens used for evaluating new diagnostic kits.
Temporal change in C4 deposition and MBL levels
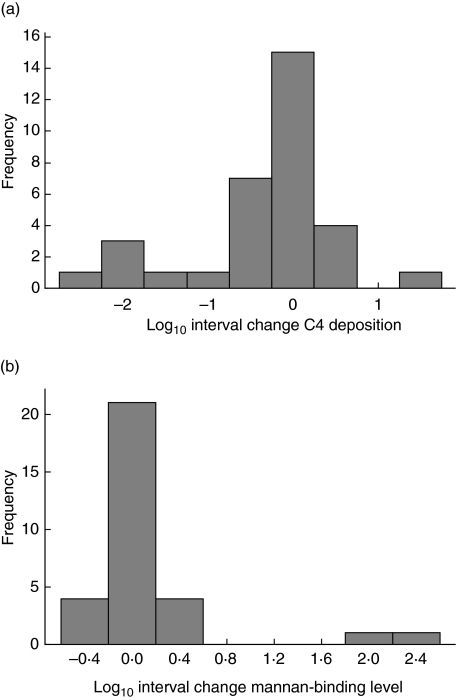
An unselected subset of 31 Legionnaires' disease patients had follow-up blood available for sequential analysis. These samples were taken a minimum of 17 days after the initial sample used for diagnosis of Legionnaires' disease (mean 46 days, range 17–86 days, interquartile range 31–63 days). There was no consistent or significant change in MBL assays with time. MBL function trended downwards with resolution of Legionnaires' disease (median C4 deposition diagnostic specimens 0·13 U/µl versus median convalescent specimens 0·06 U/µl, P = 0·08), while MBL level trended upwards (median diagnostic specimens 1·15 µg/ml versus median convalescent specimens 1·52 µg/ml, P = 0·93). Only three of the 14 patients with deficient MBL levels < 0·5 µg/ml showed any increase, with only one of these increasing to >0·5 µg/ml. The distribution of percentage change in C4 deposition and MBL level between the convalescent and acute stage tests are shown in Fig. 1. This figure emphasizes that in most instances there was less than 1 log interval change in C4 deposition with greater than 1 log interval change seen in only six of 31 patients. Only two patients had greater than 1 log interval change in MBL level, both being greater than 2 logs.
Fig. 1.
Interval change in (a) mannan-binding lectin (MBL) function (C4 deposition) and (b) MBL mannan-binding level between diagnostic and convalescent specimens.
Comparisons between Legionnaires' disease cases
Assessment of known Legionnaires' disease risk factors in the hospitalized cases was undertaken by dividing these patients into groups with and without recognized comorbidities (diabetes, chronic pulmonary disease, smoking, chronic renal impairment, malignancy and steroid use) that predispose to the disease. There was no difference in MBL function, level or age between these two groups of hospitalized patients (data not shown). Seven patients who did not have samples available for study and one unmatched case patient had clinical data recorded and they were no different from study patients in terms of age, sex or comorbidity.
Legionnaires' diseases severity and outcome comparisons in relation to MBL
The following disease severity indicators − pneumonia severity index risk classes 4 or 5 [17], need for intensive care unit admission, evidence of hypoxaemia or tachypnoea on admission as well as outcome measures, prolonged fever >3 days or hospital stay >5 days − were used to stratify hospitalized patients. There was no difference in MBL function, frequency of MBL functional deficiency or mannan-binding level between hospitalized Legionnaires' disease patients according to any of these parameters. The seven patients with clinical information who were known not to have MBL studies performed did not differ from study patients in any of these disease severity parameters. There were insufficient deaths for meaningful analysis of MBL's contribution to this outcome.
Discussion
The Melbourne Aquarium Legionnaires' disease outbreak that occurred in 2000 represents a natural experiment for investigation of susceptibility to L. pneumophila infection. This was a substantial Legionnaires' disease outbreak involving 125 patients, being the largest recorded in Australia. The clinical data that were available in the 46 hospitalized patients [13] show that these patients have similar characteristics to those from other outbreaks of Legionnaires' disease [18]. We have shown an association between MBL functional deficiency and predisposition to Legionnaires' disease by demonstrating lower MBL complement activation function and a higher frequency of MBL functional deficiency in cases than in age- and sex-matched uninfected controls that were also exposed to L. pneumophila. Our control group MBL function and mannan-binding level results are lower than our previously studied controls, raising the possibility of decay with long-term storage. However, we have shown that the expected strong relationship between C4 deposition function and mannan-binding levels persists. Additionally, the C4 deposition assay is specific for MBL activation and is not affected by consumption of complement in sepsis as it includes addition of fresh MBL-deficient serum ensuring sufficient complement and MBL-associated serine proteases.
As in our previous study of bloodstream infection [19], we have shown that MBL complement activation function and mannan-binding level do not change significantly in the setting of severe infection. This reinforces the absence of acute phase change in MBL [20] and questions recent data showing MBL consumption in Gram-negative sepsis [21].
The absence of stored cellular material meant that we were unable to determine the association between MBL2 genotype and Legionnaires' disease susceptibility. There was no demonstrable association between MBL mannan-binding level and Legionnaires' disease susceptibility. MBL functional deficiency has been shown previously to be associated strongly with severe bloodstream infection when neither MBL mannan-binding level nor MBL2 genotype were associated with increased risk [8]. This may be due to MBL-associated serine protease 2 (MASP-2) deficiency, as described recently [22, 23]. MASP-2 deficiency has been shown recently to be associated with susceptibility to invasive fungal infection [24]. MASP-2 mutation, associated with reduced MBL complement activation function, is present in 5·5% of healthy Danes [23], so this alone is unlikely to explain the very high frequency of low MBL complement activation we have documented in the healthy controls described herein (64%) and previously in healthy blood donors (28%) [8]. MASP-2 mutation rates are yet to be determined in Australians. Non-functional MBL can still be measured in the mannan-binding assay, albeit at a low efficiency [25]. This might also contribute to the absence of association between MBL level and Legionnaires' disease. There is also a wide range of MBL function and level within the different MBL2 genotypes [14].
MBL has been shown to bind to L. pneumophila and activate complement [9]. Additional observations of L. pneumophila phagocytosis and complement activation indicate the potential role of MBL in these processes. L. pneumophila is phagocytosed by alveolar macrophages via a partially complement-mediated process [26]. A study of phagocytosis of L. pneumophila by monocytes that investigated non-complement-mediated mechanisms failed to identify alternative uptake receptors using typical cell surface receptors. The macrophage mannose receptor used by MBL was not studied, however [10]. Previous investigations of L. pneumophila C3 binding have demonstrated total activity not accounted for by the contributions of the classical and alternative pathways, suggesting additional lectin pathway complement activation [11].
At present we are able to conjecture only about possible contributions of MBL deficiency to Legionnaires' disease pathogenesis. MBL deficiency has been thought to be protective against intracellular pathogens such as Mycobacterium tuberculosis [27, 28], although there are conflicting data [29, 30]. However, our data support a protective role of MBL against L. pneumophila infection. Tumour necrosis factor (TNF) induction is required for control of experimentalL. pneumophila infection [31]. In other infective models, such as Cryptococcus neoformans, MBL has been shown to increase binding of cryptococcal mannoprotein to human peripheral blood mononuclear cells and enhance production of TNF [32]. There are few data to support complement killing of L. pneumophila [33]. MBL-mediated opsonophagocytosis via non-complement receptors may be shown to increase intracellular killing potentially through the efficient delivery of L. pneumophila to lysosomes. Additionally, lectin pathway complement activation may directly killL. pneumophila. We are currently studying both these pathways.
Toll-like receptor (TLR) polymorphisms have been investigated in relation to Legionnaires' disease susceptibility. The TLR5 stop codon mutation affects bacterial flagellin recognition and has been associated with increased Legionnaires' disease susceptibility in humans [34]. Conversely, TLR4 receptor polymorphism may reduce susceptibility to Legionnaires' disease through lipopolysaccharide hyporesponsiveness [35]. Just as we are unable to determine MBL2 genotypes in our case–control investigation, we are also unable to compare the effect of MBL deficiency and TLR4/5 polymorphisms due to the lack of subject DNA. A direct comparison of MBL deficiency and TLR4/5 polymorphisms in an outbreak cohort such as the Netherlands Legionnaires' disease cohort [34] would be instructive.
Greater understanding of the innate immune system is driving the development of new therapeutics that may be useful for the therapy of severe infection such as Legionnaires' disease. MBL therapy may be shown to be a useful adjunct to anti-microbial therapy for severe sepsis as a number of studies have now shown an association between MBL deficiency and this condition. We have shown in this study that individuals who are functionally deficient in this potent pattern recognition molecule may be at increased risk of Legionnaires' disease.
Acknowledgments
Janine Stubbs was supported by a grant from the Cooperative Research Centre for Vaccine Technology. We wish to thank Emma McBryde and Melinda Dean for critical review of the manuscript.
References
- 1.Mykietiuk A, Carratala J, Fernandez-Sabe N, et al. Clinical outcomes for hospitalized patients with Legionella pneumonia in the antigenuria era: the influence of levofloxacin therapy. Clin Infect Dis. 2005;40:794–9. doi: 10.1086/428059. [DOI] [PubMed] [Google Scholar]
- 2.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 3.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 4.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 6.Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–53. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 7.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 8.Eisen DP, Dean MM, Thomas P, et al. Low mannose-binding lectin function is associated with sepsis in adult patients. FEMS Immunol Med Microbiol. 2006;48:274–82. doi: 10.1111/j.1574-695X.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers S, Aerts PC, van Dijk H. Differential microorganism-induced mannose-binding lectin activation. FEMS Immunol Med Microbiol. 2003;36:33–9. doi: 10.1016/S0928-8244(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 10.Weissgerber P, Faigle M, Northoff H, Neumeister B. Investigation of mechanisms involved in phagocytosis of Legionella pneumophila by human cells. FEMS Microbiol Lett. 2003;219:173–9. doi: 10.1016/S0378-1097(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 11.Mintz CS, Arnold PI, Johnson W, Schultz DR. Antibody-independent binding of complement component C1q by Legionella pneumophila. Infect Immun. 1995;63:4939–43. doi: 10.1128/iai.63.12.4939-4943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greig JE, Carnie JA, Tallis GF, et al. An outbreak of Legionnaires' disease at the Melbourne Aquarium, April 2000: investigation and case–control studies. Med J Aust. 2004;180:566–72. doi: 10.5694/j.1326-5377.2004.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 13.Howden BP, Stuart RL, Tallis G, Bailey M, Johnson PD. Treatment and outcome of 104 hospitalized patients with Legionnaires' disease. Intern Med J. 2003;33:484–8. doi: 10.1046/j.1445-5994.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 14.Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56:630–41. doi: 10.1046/j.1365-3083.2002.01167.x. [DOI] [PubMed] [Google Scholar]
- 15.Super M, Levinsky RJ, Turner MW. The level of mannan-binding protein regulates the binding of complement-derived opsonins to mannan and zymosan at low serum concentrations. Clin Exp Immunol. 1990;79:144–50. doi: 10.1111/j.1365-2249.1990.tb05170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–8. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 17.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 18.Sabria M, Alvarez J, Dominguez A, et al. A community outbreak of Legionnaires' disease: evidence of a cooling tower as the source. Clin Microbiol Infect. 2006;12:642–7. doi: 10.1111/j.1469-0691.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- 19.Dean MM, Minchinton RM, Heatley S, Eisen DP. Mannose binding lectin acute phase activity in patients with severe infection. J Clin Immunol. 2005;25:346–52. doi: 10.1007/s10875-005-4702-1. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Castellano M, Penaranda M, Payeras A, et al. Mannose-binding lectin does not act as an acute-phase reactant in adults with community-acquired pneumococcal pneumonia. Clin Exp Immunol. 2006;145:228–34. doi: 10.1111/j.1365-2249.2006.03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumestre-Perard C, Doerr E, Colomb MG, Loos M. Involvement of complement pathways in patients with bacterial septicemia. Mol Immunol. 2007;44:1642–9. doi: 10.1016/j.molimm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Stengaard-Pedersen K, Thiel S, Gadjeva M, et al. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N Engl J Med. 2003;349:554–60. doi: 10.1056/NEJMoa022836. [DOI] [PubMed] [Google Scholar]
- 23.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Meth. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Granell M, Urbano-Ispizua A, Suarez B, et al. Mannan-binding lectin pathway deficiencies and invasive fungal infections following allogeneic stem cell transplantation. Exp Hematol. 2006;34:1435–41. doi: 10.1016/j.exphem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Dean MM, Heatley S, Minchinton RM. Heteroligomeric forms of codon 54 mannose binding lectin (MBL) in circulation demonstrate reduced in vitro function. Mol Immunol. 2006;43:950–61. doi: 10.1016/j.molimm.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Bellinger-Kawahara C, Horwitz MA. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–10. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–82. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 28.Hoal-Van Helden EG, Epstein J, Victor TC, et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45:459–64. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Selvaraj P, Narayanan PR, Reetha AM. Association of functional mutant homozygotes of the mannose binding protein gene with susceptibility to pulmonary tuberculosis in India. Tuber Lung Dis. 1999;79:221–7. doi: 10.1054/tuld.1999.0204. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy R, Ruwende C, McAdam KP, et al. Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. Q J Med. 1998;91:13–8. doi: 10.1093/qjmed/91.1.13. [DOI] [PubMed] [Google Scholar]
- 31.Friedman H, Yamamoto Y, Klein TW. Legionella pneumophila pathogenesis and immunity. Semin Pediatr Infect Dis. 2002;13:273–9. doi: 10.1053/spid.2002.127206. [DOI] [PubMed] [Google Scholar]
- 32.Chaka W, Verheul AF, Vaishnav VV, et al. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–85. [PubMed] [Google Scholar]
- 33.Friedman H, Klein TW, Widen R, Newton C, Blanchard DK, Yamamoto Y. Legionella pneumophila immunity and immunomodulation: nature and mechanisms. Adv Exp Med Biol. 1988;239:327–41. doi: 10.1007/978-1-4757-5421-6_32. [DOI] [PubMed] [Google Scholar]
- 34.Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signalling and is associated with susceptibility to Legionnaires' disease. J Exp Med. 2003;198:1563–72. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires' disease. Proc Natl Acad Sci USA. 2005;102:2487–9. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]