Abstract
We present a case with subacute limbic encephalitis (LE) and thymoma. Neither classical onconeural antibodies nor antibodies to voltage gated potassium channels (VGKC) were detected, but the serum was positive for anti-glutamic acid decarboxylase (GAD). The patient serum also stained synaptic boutons of pyramidal cells and nuclei of granule cells of rat hippocampus. The objective of the study was to identify new antibodies associated with LE. Screening a cDNA expression library identified collapsin response mediator protein 3 (CRMP3), a protein involved in neurite outgrowth. The serum also reacted with both CRMP3 and CRMP4 by Western blot. Similar binding pattern of hippocampal granule cells was obtained with the patient serum and rabbit anti-serum against CRMP1–4. The CRMP1–4 antibodies stained neuronal nuclei of a biopsy from the patient's temporal lobe, but CRMP1–4 expression in thymoma could only be detected by immunoblotting. Absorption studies with recombinant GAD failed to abolish the staining of the hippocampal granule cells. Our findings illustrate that CRMP3–4 antibodies can be associated with LE and thymoma. This has previously been associated with CRMP5.
Keywords: cDNA library, CRMP proteins, limbic encephalitis, onconeural antibodies, paraneoplastic syndromes
Introduction
Limbic encephalitis (LE) can be paraneoplastic or non-paraneoplastic. Paraneoplastic LE is associated with tumours such as small cell lung cancer and thymoma and one or more of the classical onconeural antibodies Hu, collapsin response mediator protein 5 (CRMP5) or Ma2 [1]. Antibodies to voltage gated potassium channels (VGKC) may also associate with paraneoplastic LE due to thymoma or small cell lung cancer, but more frequently with non-paraneoplastic LE [2]. Recently, LE was described in patients with various tumours and antibodies against the neuropil of the hippocampus or the cerebellum [3, 4]. One of these patients had thymoma, neuropil antibodies and antibodies to glutamic acid decarboxylase (GAD) [3]. GAD antibodies have been detected in 54% of patients with neurological complications of thymoma other than myasthenia gravis [5]. To our knowledge, LE has not been reported with other CRMP antibodies than CRMP5. In the present study we report a case with LE, thymoma and antibodies to CRMP3–4 and GAD. CRMP3–4 antibodies may be of importance both in the diagnosis and pathogenesis of LE.
Patient and methods
Case
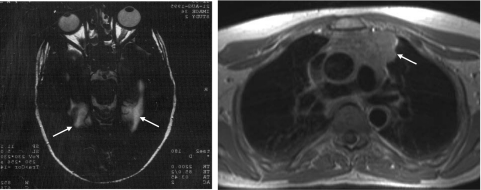
A previously healthy 52-year-old man developed a subacute cognitive decline, agitation and hallucinations. On admission, he was afebrile and had normal vital signs. Cognitive assessment showed loss of short-term memory, but motor and sensory examination, cerebellar assessment, deep tendon reflexes and plantar responses were normal. The following blood tests were normal or negative: haemoglobin and red cell indices, thyroid function, vitamin B12, fasting glucose, immunoelectrophoresis, anti-nuclear antibodies (ANA), antibodies against borrelia, HIV and syphilis and IgM antibodies against herpes and varicella zoster. Hu, Yo, Ri, amphiphysin, CRMP5 and Ma2 antibodies were not detected using immunohistochemistry of formalin-fixed rat cerebellum and immunoblot with recombinant proteins (Ravo Diagnostika GmbH, Freiburg, Germany). However, the patient serum stained the molecular and granular layer of rat cerebellum weakly. GAD antibodies were demonstrated by radioimmunoassay (33 U/ml), whereas VGKC antibodies were not. The urine was normal, as was examination of the cerebrospinal fluid (CSF) (no leucocytes, total protein 0·38 g/l and normal electrophoresis). Cerebral magnetic resonance imaging (MRI) showed hyperintense signal changes of the limbic system bilaterally (Fig. 1a), suggestive of LE. Electroencephalogram (EEG) showed bilateral temporal slow waves. The patient was treated initially with acyclovir and subsequently phenytoin after developing focal and then generalized seizures. Biopsy of the right temporal lobe showed gliosis and small, fresh cortical haemorrhages, but no perivascular infiltrates of lymphocytes. He was treated with prednisolone and cyclophosphamide. Subsequently, an invasive thymoma (Gett stadium IV-A) was found (Fig. 1b). This was not considered operable and he was treated with doxorubicin, cyclophosphamide and cisplatin, which reduced the tumour size. His short-term memory loss and neurological status remained stable. A follow-up MRI scan 8 years later showed atrophy of the limbic system.
Fig. 1.
Magnetic resonance images of patient showed hyperintense signal changes of the limbic system bilaterally (a) and a large thymoma (b) (arrows).
Sera and antibodies
Antibodies to CRMP1–4 were produced by immunizing a rabbit with the two keyhole limpet haemocyanin (KLH)-conjugated peptides AAFVTSPPLSPDPTT and GKMDENQFVAVTSTN (Eurogentec SA, Seraing, Belgium). The peptide sequences, corresponding to amino acid numbers 299–313 and 373–387, were present and identical in all the human CRMP1–4 proteins except for CRMP3, which differed in one and two amino acids, respectively. Serum from the same rabbit obtained before immunization was used as negative control. Rabbit anti-GAD65/67 antibody and rabbit anti-synaptophysin antibody were purchased from Chemicon International (Temecula, CA, USA) and Euro-Diagnostica (Malmo, Sweden), respectively. Secondary antibodies were Alexa fluor 488 goat anti-human IgG, Alexa fluor 488 goat anti-rabbit IgG and Alexa fluor 594 goat anti-rabbit IgG, all purchased from Molecular Probes (Eugene, OR, USA) and Rhodamine RedTM-X-conjugated goat anti-rabbit from Jackson ImmunoReserach (Baltimore, MD, USA). HRP-conjugated rabbit anti-human IgG (P0214), swine anti-rabbit immunoglobulins (P0217) as well as Dako EnVision System were all from Dako (Glostrup, Denmark).
Immunohistochemistry of rat cerebrum and cerebellum
Fifty µm vibratome sections of formaldehyde-fixed rat cerebrum were incubated with patient serum diluted 1 : 500 overnight at 4°C. The sections were then washed and incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG diluted 1 : 500 for 1 h at room temperature, washed and developed with diaminobenzidine (0·05%) and H2O2 (0·01%, both from Sigma, St Louis, MO, USA). Cryostat sections of snap-frozen rat cerebrum were incubated with patient serum or rabbit anti-CRMP1–4 (1 : 500) or rabbit anti-GAD (1 : 1000) in a moist chamber at 4°C overnight. The sections were subsequently washed and incubated with secondary antibodies Alexa fluor 488 goat anti-human IgG or Alexa fluor 488 goat anti-rabbit IgG, diluted 1 : 100 for 1 h at room temperature. Double-staining was performed by simultaneous incubation of sections with patient serum and rabbit anti-CRMP1–4 at various dilutions overnight, and subsequently washed and incubated with the secondary antibodies Alexa fluor 594 goat anti-human IgG and Alexa fluor 488 goat anti-rabbit diluted 1 : 100 in phosphate-buffered saline (PBS). After washing, the slides were mounted and examined with a Zeiss immunofluorescence microscope. In some experiments, patient serum was absorbed with excess recombinant GAD (Diamyd Diagnostics AB, Stockholm, Sweden) overnight at 4°C. The antibody–antigen complex was spun down and the supernatant applied to sections of rat cerebrum and analysed by immunofluorescence as described above.
Immunohistochemistry of human cerebrum, cerebellum, thymus and thymoma
Sections from cortex, hippocampus and cerebellum were collected at autopsy of a 78-year-old man with no central nervous system disease. Seven µm thick formalin-fixed sections were incubated with rabbit anti-CRMP1–4 diluted 1 : 1000 or rabbit anti-GAD diluted 1 : 500 for 30 min, stained using the Dako EnVision system with diaminobenzidine as chromogen and counterstained with haematoxylin. Staining using the same dilutions was also performed on formalin-fixed sections from one thymus, two benign thymomas, one malignant thymoma and on sections from the patient's brain biopsy and thymoma.
Hippocampal neuronal cultures
Primary hippocampal cultures containing both neurones and glial cells were prepared from the CA1-CA3 regions of 1–4-day-old rats (Wistar, Taconic M&B, Ry, Denmark), as described previously [6]. For immunohistochemistry, the cells were quenched with 0·1 M glycine in 0·1 M NaPi and subsequently permeabilized in 0·4% saponin, 1% bovine serum albumin (BSA) and 2% normal sheep serum in 0·1 M NaPi. The cultures were incubated with primary antibody and then secondary antibody in permeabilization solution. For double-labelling studies, the secondary antibodies were used separately. The cells were finally washed in 0·1 M NaPi, mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and analysed with a Leica TCS SP confocal laser-scanning microscope.
Western blots
Protein extracts of rat cerebrum and cerebellum, human thymus and three thymomas were prepared by incubating 100 mg of tissue with 0·5 ml extraction buffer (140 mM NaCl, 10 mM Tris-HCl pH 8·2, 2 mM ethylenediamine tetraacetic acid (EDTA), 1 mM phenylmethylsulphonyl fluoride (PMSF) and 2% Novidet P40) for 1 h at 4°C. The samples were then centrifuged at 1000 g for 10 min at 4°C, and the supernatant dialysed against PBS overnight. Twenty µg total protein per well was electrophoresed in 10% sodium dodecyl sulphate (SDS) polyacrylamide gels and electroblotted onto nitrocellulose membranes, and 6 µl of dual-colour prestained Precision Plus Protein standard (Bio-Rad, Sundbyberg, Sweden) was used for molecular weight determination. Immunoblots were incubated at 4°C overnight with patient serum, rabbit preimmune or anti-CRMP1–4 anti-serum (diluted 1 : 500 in PBS containing 0·05% Tween and 0·5% dry milk) or rabbit anti-GAD (diluted 1 : 1000). HRP-conjugated rabbit anti-human IgG or swine anti-rabbit immunoglobulins diluted 1 : 1000 were used as secondary antibodies, and the blots were developed subsequently with 4-chloro-1-naphtol (Sigma) and H2O2 in PBS. Western blot analysis of recombinant CRMP1, 2, 3, 4 and 5 proteins was performed as described previously [7]. Complete absorption of GAD antibodies from diluted patient serum was verified by Western blot using 5 µg recombinant GAD per well.
cDNA library screening
A rat cerebellar cDNA expression library was used for antibody screening in patient serum as described previously [8]. In brief, bacteria and the cDNA library were mixed on a Petri dish containing NZY agar. After about 3·5 h of culture, plaque appeared on the dish. Plaques were copied onto a Hybond C nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden) containing isopropyl–D-thiogalactoside (Sigma-Aldrich, Steinheim, Germany). Sera and secondary alkaline phosphatase-conjugated antibodies were added to the filter, followed by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Bio-Rad) to detect immune complexes. Positive clones were rescreened until pure isolates were obtained and sequenced and identified thereafter by a blast search (http://www.ncbi.nlm.nih.gov/BLAST).
Results
cDNA clones
Screening of the cDNA expression library with patient serum revealed two clones that were isolated and sequenced. One clone contained an insert of about 1400 base pairs (bp) identical to the 3′ end of Rattus norvegicus rCRMP-3 mRNA (GenBank Accession no. U52103), coding for 195 amino acids of the N-terminal end of CRMP3. Multiple sequence alignments using ClustalX revealed that this amino acid sequence was 93, 71, 64, 63 and 47% identical to the human variants of CRMP3, CRMP2, CRMP1, CRMP4 and CRMP5, respectively.
The other clone was approximately 1300 bp and contained a full-length open reading frame encoding a protein of 340 amino acids. blast search revealed that the insert was identical to the predicted R. norvegicus XAP-5 (GenBank Accession no. XM_215220), except for an additional six nucleotides. According to the SwissProt database, the protein is a potential DNA binding protein or transcriptional factor localized to the nucleus in all tissues examined.
Immunohistochemistry
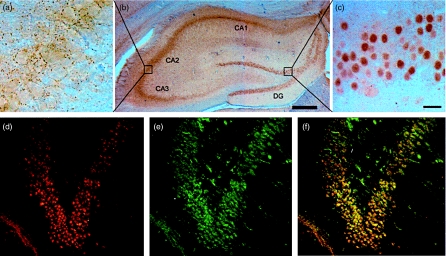
The patient serum stained the Purkinje cells and the granular layer of rat cerebellum with a titre of 4000, whereas the synaptic boutons in the CA1–CA3 area and nuclei of granule cells in the dentate gyrus of rat hippocampus was stained with a titre of 64 000 (Fig. 2a–c). Double-labelling experiments showed that the rabbit anti-CRMP1–4 stained the nuclei of granule cells in dentate gyrus in a similar manner to the patient serum (Fig. 2d–f). Similar staining of the hippocampus was seen after the patient serum had been absorbed with GAD. These results indicated that the staining of rat hippocampus was not due to GAD antibodies.
Fig. 2.
(a–c) Vibratome sections of rat hippocampus. The patient serum stained cellular regions of hippocampus (b), synaptic boutons in the CA1-CA3 region (a) and nuclei of cells in the dentate gyrus (c). Scale bars are 20 µm (a and c) and 500 µm (b). (d–f) Cryostat sections of rat hippocampus. Patient serum (d) stained the granule cells of dentate gyrus in a similar manner as rabbit anti-CRMP1–4 (e), as shown in the overlay image (f). Magnification × 400.
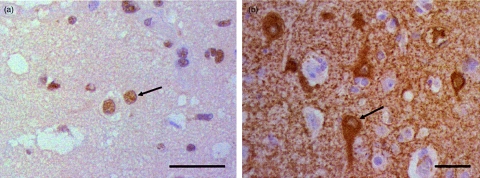
The rabbit anti-CRMP1–4 stained the nuclei of neurones and glia cells with the morphology of oligodendrocytes in the temporal lobe biopsy of the patient (Fig. 3a), and the rabbit anti-GAD stained the cytoplasm of some of the smaller neurones (Fig. 3b). The staining pattern of CRMP1–4 and GAD antibodies was similar to that seen in normal brain tissue obtained at autopsy.
Fig. 3.
Biopsy from the right temporal lobe. Rabbit anti-CRMP1–4 stained the nuclei of neurones and oligodendrocytes (a), whereas anti-glutamic acid decarboxylase (GAD) stained the cytoplasm of some of the neurones (b) (arrows). Scale bars are 40 µm.
No staining was observed by the rabbit CRMP1–4 or GAD antibodies in sections of human thymus and thymoma, including the patient's thymoma.
Neuronal cultures
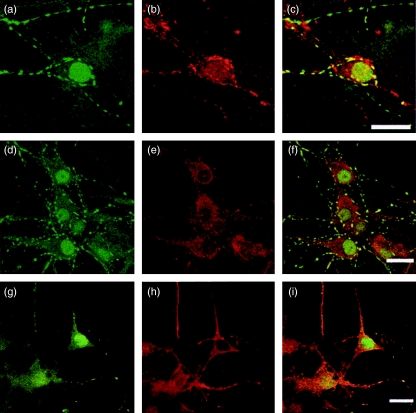
The patient serum showed strong staining of the nuclei and weak staining of the cytoplasm of rat hippocampal neurones in culture, as well as punctuate staining along the neuritis. Double-staining with the synaptic vesicle marker anti-synaptophysin showed that the punctuate staining observed by the patient serum represented synaptic boutons (Fig. 4a–c).
Fig. 4.
Patient serum stained the nucleus, cytoplasm and neurites in primary cultured neurones of rat hippocampus (a, d and g). The staining partially co-localizes with synaptophysin (b), GAD (e) and CRMP1–4 (h) as seen in the overlay images (c, f and i, respectively). Scale bars are 20 µm.
The rabbit anti-CRMP1–4 did not stain the nuclei of cultured neurones, as was observed for neurones in tissue sections. The CRMP1–4 staining was seen in the cytoplasm and along the neurites of the cells in culture, whereas GAD staining was seen mainly in the cytoplasm. Further double-staining experiments showed partial cytoplasmic co-localization of the patient serum and CRMP1–4, as well as GAD, while the punctuate staining along the neurites co-localized with CRMP1–4 (Fig. 4d–i).
Western blots
Western blots of rat cerebral and cerebellar extracts incubated with patient serum revealed two distinct bands of approximately 40 and 65 kDa. Rabbit anti-CRMP1–4 gave several bands in the range of 60–70 kDa, whereas the rabbit anti-GAD revealed a double band of approximately 65 kDa when blotted against the rat cerebral and cerebellar extracts.
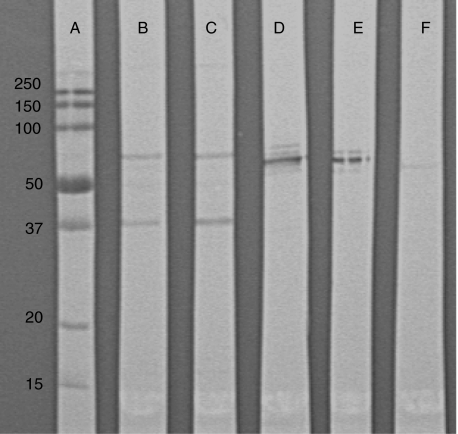
The rabbit anti-CRMP1–4 revealed a band of approximately 63 kDa, but no bands were detected by rabbit anti-GAD when blotted against the human thymus and thymoma extracts (Fig. 5).
Fig. 5.
Western blots. Lane (a) molecular weights standard (kDa), (b) rat brain extract incubated with patient serum, (c) rat cerebellar extract incubated with patient serum, (d) rat brain extract incubated with rabbit CRMP1–4 antibodies, (e) rat cerebellar extract incubated with rabbit CRMP1–4 antibodies and (f) human thymoma extract incubated with rabbit CRMP1–4 antibodies.
Western blots of the five recombinant proteins CRMP1–5 showed that the patient serum reacted with CRMP3 and CRMP4, but not with CRMP1, 2 and 5. The rabbit anti-CRMP1–4 was equally reactive with all the CRMP proteins, except CRMP5.
Discussion
LE is often associated with onconeural antibodies or VGKC antibodies [1, 2]. However, only anti-GAD was detected in the initial antibody screen of the present case. GAD is expressed widely in the central nervous system and catalyses the conversion of glutamic acid to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). GAD antibodies are found in patients with type 1 diabetes, stiff person syndrome, non-paraneoplastic cerebellar ataxia, idiopathic epilepsy [9], thymoma [5] and in patients with neurological symptoms related possibly to lung and breast carcinoma [10]. None of these diseases, except for thymoma, was found in the present case. Vernino et al. [5] reported that five of 12 patients with encephalopathy, including encephalitis, had GAD antibodies in addition to classical onconeural antibodies. Because in our case we did not detect classical onconeural antibodies, we screened the patient serum for other autoantibodies by including sections of rat hippocampus. Strong staining of hippocampal neurones was observed, and antibodies to CRMP3 were identified by cDNA library screening. Verification by Western blotting with recombinant CRMP proteins also identified antibodies to CRMP4 in the patient serum.
The CRMP1, 2, 3 and 4 proteins belong to a family of phosphoproteins expressed in the developing nervous system, and participate in the signal transduction pathway implicated in semaphorin-induced growth cone collapse and axonal guidance in developing neurones. In adult rat brain, the expression is down-regulated and restricted to the cortex and pyramidal cell layer of hippocampus (CRMP2), granular cells of the cerebellum (CRMP3) and regions that show neurogenesis, such as dentate gyrus of the hippocampus and the olfactory bulb (CRMP4) [11]. There is a high degree of homology between the different members in mouse (69–75%) [12]. The fifth member of the CRMP family, CRMP5, is quite different from CRMP1–4, with an amino acid identity of only 50% compared to the other CRMP proteins [13]. The CRMP5 antibody is a well-characterized onconeural antibody usually associated with paraneoplastic LE or subacute sensory neuronopathy and tumours such as small cell lung cancer or thymoma [1], but such antibodies were not detected in the present case.
The CRMP proteins have been described mainly as cytoplasmic proteins. We found that the patient serum and the rabbit CRMP1–4 antibodies stained the nuclei of rat and human neurones. The patient serum also stained neurites and synaptic boutons of cultured neurones, which is in accordance with previous CRMP4 localization [14]. The nuclear staining of the patient serum could be due partly to other nuclear antibodies, but the staining pattern observed by rabbit CRMP1–4 antibodies indicates that CRMP proteins are present both in the nucleus and the cytoplasm. This is also in accordance with a recent study, which showed that a cleavage product of CRMP3 can be translocated to neuronal nuclei [15]. The double-staining experiments performed on primary hippocampal cultures did not identify the nuclear staining as CRMP1–4. Our results therefore indicate that the subcellular distribution of CRMP proteins is different in adult neurones in situ versus fetal pyramidal cells in culture, and that the nuclear localization may be dependent on maturity of the neurones.
The rabbit CRMP1–4 and GAD antibodies both stained hippocampal neurones in culture as well as neurones of the temporal lobe biopsy of the patient. However, double-labelling experiments showed equal staining of the rabbit CRMP1–4 antibodies and the patient serum, and similar staining of dentate gyrus of the hippocamous was seen after the patient serum had been absorbed with GAD. These results indicated therefore that the staining of rat hippocampus was not due to GAD antibodies, but due probably to CRMP3–4 antibodies. However, the cDNA expression library screening also identified XAP5, which is a potential DNA binding protein or transcription factor. Whether anti-XAP5 or other unidentified antibodies in the patient serum also bind to hippocampal neurones is currently unknown.
Thymomas are associated commonly with myasthenia gravis, but may also be present in other neurological disorders such as LE [5]. CRMP1–4 staining was not observed in thymus or thymomas by immunohistochemistry, but a band of approximately 65 kDa was identified in Western blots of human thymus and thymomas incubated with rabbit CRMP1–4 antibodies, indicating that at least one of these CRMP proteins is expressed in thymomas. The difficulties in detecting CRMP staining by immunohistochemistry may be due to epitope masking by the fixative. This is in line with the results of Camdessanché et al. [16], who detected CRMP1, 2, 4 and 5 mRNA in thymus and thymoma by reverse transcription–polymerase chain reaction (RT–PCR), whereas no CRMP1–5 expression was detected by immunohistochemistry or Western blots.
We have screened samples from 58 patients with myasthenia gravis and thymoma for the presence of CRMP3 antibodies, but no such antibodies were detected (data not shown). The frequency of CRMP3–4 antibodies in thymoma and LE is currently unknown, but our results indicate that screening for such antibodies may be of importance in LE when classical onconeural antibodies are not found. Similar staining patterns of neuropils have been described in patients with LE and various tumours, including one patient with concomitant GAD antibodies [3]. If such neuropil antibodies also represent CRMP3–4 antibodies, the present results strengthen their importance in the diagnostics of paraneoplastic LE. Because CRMP proteins are present in thymoma, a possible cross-reactive autoimmune response may be causing LE.
Acknowledgments
We thank Mette Haugen at the Department of Neurology, Haukeland University Hospital and Camilla Haglerød at the Institute of Anatomy and Cell Biology, University of Oslo for technical help. This study was supported by grants from the University of Bergen and Haukeland University Hospital, Norway.
References
- 1.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 3.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res. 2004;10:7270–5. doi: 10.1158/1078-0432.CCR-04-0735. [DOI] [PubMed] [Google Scholar]
- 6.Vik-Mo EO, Oltedal L, Hoivik EA, et al. Sec6 is localized to the plasma membrane of mature synaptic terminals and is transported with secretogranin II-containing vesicles. Neuroscience. 2003;119:73–85. doi: 10.1016/s0306-4522(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 7.Honnorat J, Byk T, Kusters I, et al. Ulip/CRMP proteins are recognized by autoantibodies in paraneoplastic neurological syndromes. Eur J Neurosci. 1999;11:4226–32. doi: 10.1046/j.1460-9568.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 8.Bredholt G, Storstein A, Haugen M, et al. Detection of autoantibodies to the BTB-kelch protein KLHL7 in cancer sera. Scand J Immunol. 2006;64:325–35. doi: 10.1111/j.1365-3083.2006.01821.x. [DOI] [PubMed] [Google Scholar]
- 9.Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci. 2002;23:145–51. doi: 10.1007/s100720200055. [DOI] [PubMed] [Google Scholar]
- 10.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53:580–7. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 11.Wang LH, Strittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci. 1996;16:6197–207. doi: 10.1523/JNEUROSCI.16-19-06197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byk T, Ozon S, Sobel A. The Ulip family phosphoproteins − common and specific properties. Eur J Biochem. 1998;254:14–24. doi: 10.1046/j.1432-1327.1998.2540014.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukada M, Watakabe I, Yuasa-Kawada J, et al. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem. 2000;275:37957–65. doi: 10.1074/jbc.M003277200. [DOI] [PubMed] [Google Scholar]
- 14.Rosslenbroich V, Dai L, Franken S, et al. Subcellular localization of collapsin response mediator proteins to lipid rafts. Biochem Biophys Res Commun. 2003;305:392–9. doi: 10.1016/s0006-291x(03)00754-x. [DOI] [PubMed] [Google Scholar]
- 15.Hou ST, Jiang SX, Desbois A, et al. Calpain-cleaved collapsin response mediator protein-3 induces neuronal death after glutamate toxicity and cerebral ischemia. J Neurosci. 2006;26:2241–9. doi: 10.1523/JNEUROSCI.4485-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camdessanche JP, Lassabliere F, Meyronnet D, et al. Expression of the onconeural CV2/CRMP5 antigen in thymus and thymoma. J Neuroimmunol. 2006;174:168–73. doi: 10.1016/j.jneuroim.2006.01.018. [DOI] [PubMed] [Google Scholar]