Abstract
Successful bacille Calmette–Guérin (BCG) immunotherapy of bladder cancer depends on the proper induction of a T helper-type 1 (Th1) immune response. In this study we investigated the possible involvement of Th1-stimulating cytokines in BCG-induced interferon (IFN)-γ production as well as their potential roles in enhancing BCG-induced IFN-γ from human peripheral blood mononuclear cells (PBMCs). BCG efficiently induced IFN-γ production by PBMCs in a dose-dependent manner. Neutralization of endogenous cytokines interleukin (IL)-2, IL-12 and IFN-α reduced BCG-induced IFN-γ by 38%, 67% and 49%, respectively. Although single recombinant (r) IL-2, rIL-12 and rIFN-α induced no or a marginal amount of IFN-γ, a combination of any two or three cytokines increased IFN-γ production. When BCG (a subsaturated dose) was combined with mono, dual or triple cytokines, a synergy on IFN-γ production was observed. Such a synergy was readily achievable even when minimal or low doses of cytokines were used. No saturation of IFN-γ production was observed even when a subsaturated BCG dose was combined with very high doses of cytokines. A robust IFN-γ production was also observed when a minimal BCG dose was combined with minimal doses of triple cytokines. In addition, we demonstrated that IL-2- and IFN-α-expressing rBCGs were superior to wild-type BCG for PBMC IFN-γ induction and that combination of both rBCGs showed a synergy in IFN-γ production. Taken together, these results suggest that combination of BCG with certain exogenous or endogenous (expressed by rBCGs) Th1-stimulating cytokines is a rational candidate for further study in bladder cancer treatment.
Keywords: BCG, cytokine, IFN-γ, Th1
Introduction
Intravesical instillation of bacille Calmette–Guérin (BCG) has been used to treat superficial transitional cell carcinoma (TCC) of the bladder for three decades and demonstrated to be more effective than localized chemotherapy and radiotherapy [1–3]. However, approximately 30% of patients do not respond to BCG therapy and 50% of patients recur after the BCG therapy [4–6]. In addition, the side effects associated with BCG use are common and occasionally even life-threatening [7, 8]. Clearly, the current BCG therapy needs to be improved.
Although the exact mechanisms by which BCG mediates anti-tumour immunity remain unclear, a proper induction of a localized T helper 1 (Th1) immune response appears to be indispensable for successful BCG therapy. Following intravesical BCG instillation, immune cell infiltration in the bladder wall has been observed including T cells, natural killer (NK) cells and macrophages [9–12]. A large number of immune cells in patients' voided urine have also been reported, including neutrophils, T cells and macrophages [13–15]. Furthermore, a transit secretion of cytokines and chemokines in patients' urine after intravesical BCG therapy has been reported including interleukin (IL)-1, IL-2, IL-6, IL-10, IL-12, IL-18, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, granulocyte–macrophage colony-stimulating factor (GM-CSF), macrophage-derived chemokine (MDC), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, interferon-inducible protein (IP)-10 and eotaxin [15–23]. Although the specific role each of these proinflammatory mediators in orchestrating BCG-induced anti-tumour immunity is unclear, a high expression of Th1 cytokines (IL-2, IL-12 and IFN-γ) has been observed to be associated with BCG responders [17, 18, 24, 25]. Because IFN-γ is a major Th1 restricted cytokine, easily detectable in urine and culture, and a useful surrogate marker with prognostic value for BCG therapy, its expression was evaluated in this study to define the acquisition of a Th1 immune response.
To improve the clinical efficacy of intravesical BCG immunotherapy, BCG has been combined with certain Th1-stimulating cytokines in several studies [26–31]. Previously, we used BCG plus recombinant (r)IFN-α to treat bladder cancer patients who had failed BCG therapy and found that the combinational therapy resulted in a favourable tumour response [32, 33]. We also used a reduced BCG dose, in combination with rIFN-α or rIL-12, to treat bladder cancer patients and found that the combinational therapy induced a high level of urinary IFN-γ [29, 30, 33]. In addition, we also observed that Th1-stimulating cytokines rIL-2, rIL-12 and rIFN-α enhanced BCG-induced IFN-γ from human peripheral blood mononuclear cells (PBMCs) in culture [29, 30, 34]. In this study, we further investigated the dose–responses of BCG and Th1-stimulating cytokines for IFN-γ production by human PBMCs. We observed that exogenous rIL-2, rIL-12 or/and rIFN-α synergized with BCG for IFN-γ production in a dose-dependent manner. We also observed that a minimal dose of BCG was capable of inducing a high level of IFN-γ when it was combined with triple cytokines rIL-2, rIL-12 and rIFN-α. In addition, we also observed that rBCGs expressing IL-2 (rBCG–IL-2) or IFN-α (rBCG–IFN-α) were superior to BCG for PBMC IFN-γ induction. Our results suggest that supplementation of Th1-stimulating cytokines could enhance the effect of BCG on induction of a Th1 immune response and allow the use of lower and safer doses of BCG in the treatment of bladder cancer patients.
Materials and methods
BCG
MV261 BCG (BCG), a Pasteur strain transfected previously with the kanamycin resistance plasmid pMV261 [35], was used alone or in combination with Th1-stimulating cytokines (rIL-2, rIL-12 or/and rIFN-α) for PBMC stimulation. This BCG strain has demonstrated the similar immunostimulatory property to that of commercial lyophilized BCG preparations. BCG was cultured routinely at 37°C in Middlebrook 7H9 Bacto broth (Difco, Detroit, MI, USA) supplemented with 10% albumin dextrose catalase (ADC) [5% bovine serum albumin (BSA), 2% dextrose and 0·85% NaCl], 0·05% Tween 80 (Sigma, St Louis, MO, USA) and 30 µg of kanamycin per ml. One unit of absorbance at 600 nm for the BCG culture was calculated as 2·5 × 107 colony-forming units (CFU) per ml.
Both rBCG–IFN-α and rBCG–IL-2 were also used in PBMC IFN-γ induction. The rBCG–IFN-α strain had been developed previously [36]. The rBCG–IL-2 strain was developed similarly, as described for rBCG–IFN-α. Briefly, the mature IL-2 coding sequence was amplified by polymerase chain reaction (PCR) and placed downstream to the α antigen signal sequence of the Escherichia coli–BCG shuttle plasmid pMV261 [35]. The sequences of the PCR primer pair used were 5′-CAAG(ggatcc)GCACCTACTTCAAGTTCTACAAAG-3′ (sense primer; lower case for the BamH1 cutting site) and 5′-GCCG(gaattc)TTATCAAGTCAGTGTTGAGATGAT-3′ (anti-sense primer; lower case for the EcoR1 cutting site). After transformation, selection and amplification in E. coli competent XL1-Blue MR cells (Stratagene, La Jolla, CA, USA), the IL-2-expressing vector was transformed further into BCG competent cells (a Pasteur strain) and selected on 7H10 Bacto agar plates (Difco). Both rBCG–IFN-α and rBCG–IL-2 were cultured in the same conditions used for BCG. Both rBCGs typically secreted ∼5000–7000 pg/ml of the coding cytokines under the standardized conditions described previously [35].
Cytokines and neutralizing antibodies
Recombinant human cytokines were obtained from Genzyme (Cambridge, MA, USA) for rIL-2, Genetics Institute (Cambridge, MA, USA) for rIL-12 and Schering (Kenilworth, NJ, USA) for rIFN-α. Human cytokine-neutralizing antibodies were obtained from Schering for IFN-α (sheep polyclone) and from R&D Systems (Minneapolis, MN, USA) for IL-2 (clone 5334·21, mouse IgG1), IL-10 (clone 23738·111, mouse IgG2b) and IL-12 (goat polyclone). Species and isotype-matched control antibodies were obtained from BD PharMingen (San Diego, CA, USA) for mouse IgG1 (clone 107·3) and IgG2b (clone G11-59) and from Sigma for sheep and goat IgG.
Human PBMC culture
In accordance with the approved clinical protocol at our institution, blood samples were collected from both BCG-naive and BCG-vaccinated healthy donors with negative and positive skin test reactivity to the purified protein derivative (PPD). PBMCs were prepared from buffy coat leucocytes purified on Ficoll-Paque (Pharmacia, Uppsala, Sweden), as described previously [34]. Viability of PBMCs exceeded 95% by trypan blue exclusion. PBMCs were suspended in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) and 30 µg of kanamycin per ml, and incubated at 37°C in a humidified 5% CO2 incubator at a density of 5 × 105 cells/200 µl per well in 96-well tissue culture plates. To evaluate the effects of Th1-stimulating cytokines on PBMC IFN-γ induction, BCG-naive PBMCs were cultured in the presence or absence of BCG or/and rIL-2, rIL-12 and rIFN-α. BCG-naive PBMCs were also used to evaluate the effects of rBCGs for IFN-γ induction. PBMCs from BCG-vaccinated subjects were used to evaluate the role of endogenous cytokines in BCG-induced IFN-γ production, as these PBMCs produce higher IFN-γ in response to BCG stimulation and allow more accurate determination of the role in an antibody neutralization assay. The plates were incubated for 3 days and then frozen at −20°C until the enzyme-linked immunosorbent assay (ELISA) was performed.
ELISA analysis
Paired monoclonal capture (clone 2G1) and detecting (clone B133·5) antibodies for human IFN-γ ELISA were obtained from Endogen (Woburn, MA, USA). A sandwich format ELISA was performed according to the manufacturers' instructions.
Statistical analysis
All determinations were made in duplicate and each result was expressed as mean ± standard deviation (s.d). Statistical significance was determined by paired Student's t-test. A P-value of 0·05 was considered to be significant.
Results
Role of endogenous Th1 cytokines in BCG-induced IFN-γ production by PBMCs
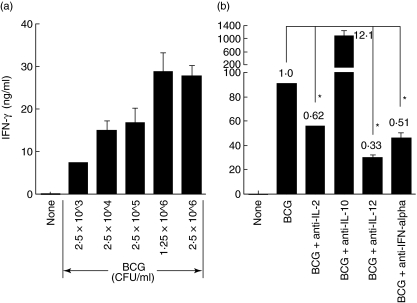
We observed previously that PBMCs produced a high level of IFN-γ in response to BCG stimulation at a dose of 2·5 × 105 CFU/ml for 3 days [23, 29, 30, 34]. In this study, we further evaluated PBMC IFN-γ production in response to five different BCG doses ranging from 2·5 × 103 to 2·5 × 106 CFU/ml. BCG efficiently induced IFN-γ production in a dose-dependent manner (Fig. 1a). Even at a minimal dose tested (2·5 × 103 CFU/ml), BCG was still capable of inducing a significant amount of IFN-γ (7·2 ng/ml). The IFN-γ production increased steadily in parallel with BCG dosing until reaching a plateau level (28·7 ng/ml) at a BCG dose of 1·25 × 106 CFU/ml. This BCG dose-dependent IFN-γ production was also observed for both rBCG–IL-2 and rBCG–IFN-α.
Fig. 1.
(a) Bacille Calmette–Guérin (BCG) dose-dependent interferon (IFN)-γ production by human peripheral blood mononuclear cells (PBMCs). PBMCs were incubated with medium or five different doses of BCG ranging from 2·5 × 103 to 2·5 × 106 colony-forming units (CFU)/ml for 3 days and then assayed for IFN-γ production in the conditioned media. (b) The roles of endogenous T helper 1 (Th1) and Th2 cytokines in BCG-induced IFN-γ production by human PBMCs. PBMCs were incubated with 2·5 × 105 CFU/ml of BCG in the presence or absence of indicated cytokine-neutralizing antibodies (3 µg/ml) for 3 days and then assayed for IFN-γ production in the conditioned media. Species and isotype-matched control antibodies had no effect on IFN-γ production (data not shown). *Significantly decreased.
In addition to IFN-γ, BCG is known to induce a number of other Th1-stimulating cytokines from PBMCs, including IL-2, IL-12 and IFN-α[34]. To evaluate the role of these BCG-induced endogenous cytokines in IFN-γ production, PBMCs were stimulated with BCG (2·5 × 105 CFU/ml) in the presence of neutralizing antibodies to IL-2, IL-12 or IFN-α (Fig. 1b). For comparison, a neutralizing antibody to Th2 cytokine IL-10 was also included. The functional blockage of Th1-stimulating cytokines reduced IFN-γ by 38% for IL-2, 67% for IL-12 and 49% for IFN-α, respectively. Conversely, neutralization of IL-10 increased IFN-γ by 12·1-fold. Control isotype antibodies showed no effects on PBMC IFN-γ production (data not shown). These results suggest that the endogenously produced Th1-stimulating cytokines play favourable roles in BCG-induced IFN-γ production and that supplementation of these cytokines may augment BCG for Th1 immune induction.
Exogenous rIL-2, rIL-12 and rIFN-α alone induce no or marginal IFN-γ but synergize with BCG for IFN-γ production by PBMCs
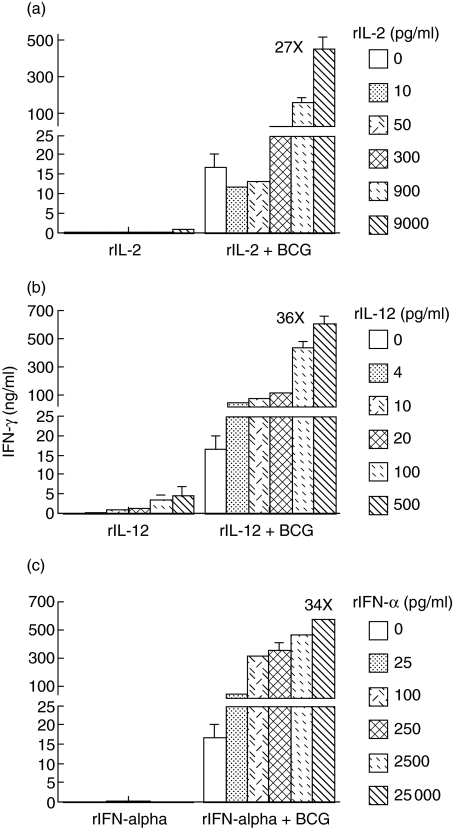
To determine the effects of exogenous Th1-stimulating cytokines rIL-2, rIL-12 and rIFN-α on BCG-induced IFN-γ production, we cultured PBMCs in the presence of these cytokines alone or in combination with BCG. All cytokines were tested in five different doses, ranging from a minimal dose to a high dose based on our previous studies [29, 30, 34]. BCG was used at a subsaturated dose (2·5 × 105 CFU/ml) for better determination of the supplemental effects of these cytokines. Both rIL-2 and rIFN-α alone induced no or a marginal amount of IFN-γ (Fig. 2a,c). Although rIL-12 alone also showed a weak effect, a clear dose-dependent induction of IFN-γ was observed (Fig. 2b). Despite no or weak effects when used alone, all Th1-stimulating cytokines tested potently enhanced BCG for IFN-γ production (Fig. 2a–c). A clear dose-dependent IFN-γ production was observed, starting at a dose of 300 pg/ml for rIL-2, 4 pg/ml for rIL-12 and 25 pg/ml for rIFN-α, respectively. The highest IFN-γ production was achieved when the highest doses of cytokines were combined with BCG, i.e. 27-fold increase at 9000 pg/ml for rIL-2, 36-fold increase at 500 pg/ml for rIL-12 and 34-fold increase at 25 000 pg/ml for rIFN-α, respectively. Because no saturation of IFN-γ production was observed, a BCG-induced Th1 immune response could be enhanced further by addition of even higher doses of these cytokines. Among the three Th1-stimulating cytokines tested, rIL-12 appeared to be the most potent cytokine in augmenting BCG for IFN-γ production by PBMCs.
Fig. 2.
The effects of exogenous recombinant interleukin (rIL)-2, rIL-12 and recombinant interferon (rIFN)-α, alone or in combination with bacille Calmette–Guérin (BCG), on IFN-γ production by human peripheral blood mononuclear cells (PBMCs). PBMCs were incubated with the indicated doses of rIL-2 (a), rIL-12 (b) or rIFN-α (c), in the presence or absence of BCG [2·5 × 105 colony-forming units (CFU)/ml], for 3 days and then assayed for IFN-γ production in the conditioned media.
Combination of exogenous rIL-2, rIL-12 and rIFN-α enhances IFN-γ production and synergizes with BCG for IFN-γ production by PBMCs
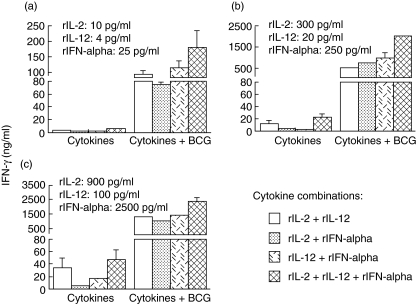
Although all three Th1-stimulating cytokines tested induced no or a negligible amount of IFN-γ in PBMC culture when they were used alone (Fig. 2), a combination of either two or three cytokines increased IFN-γ induction in all doses tested including a minimal dose (Fig. 3a), a moderate dose (Fig. 3b) and a high dose (Fig. 3c). Triple cytokine combination induced higher IFN-γ than dual cytokine combination at the same doses. A dose-dependent induction of IFN-γ was observed for all dual and triple cytokine combinations (Fig. 3cversus3bversus3a). When the dual and triple cytokines were further combined with BCG (2·5 × 105 CFU/ml), a synergistic induction of IFN-γ was observed (Fig. 3a–c). Again, this increased induction of IFN-γ showed a clear dose-dependent manner (Fig. 3cversus3bversus3a) and was not saturated at the highest doses tested (Fig. 3c). This phenomenon suggests further that a BCG-induced Th1 immune response could be enhanced by addition of multiple, high doses of Th1-stimulating cytokines.
Fig. 3.
The effects of exogenous dual or triple cytokines [recombinant interleukin (rIL)-2, rIL-12 and recombinant interferon (rIFN)-α], alone or in combination with bacille Calmette–Guérin (BCG), on IFN-γ production by human peripheral blood mononuclear cells (PBMCs). PBMCs were incubated with the indicated dual or triple cytokines at a minimal dose (a), a moderate dose (b) or a high dose (c) in the presence or absence of BCG [2·5 × 105 colony-forming units (CFU)/ml], for 3 days and then assayed for IFN-γ production in the conditioned media. Refer to Fig. 2 for PBMC IFN-γ production in response to BCG alone, single cytokines alone and BCG plus single cytokines.
Low doses of BCG induce robust IFN-γ production by PBMCs when combined with exogenous triple cytokines rIL-2, rIL-12 and rIFN-α
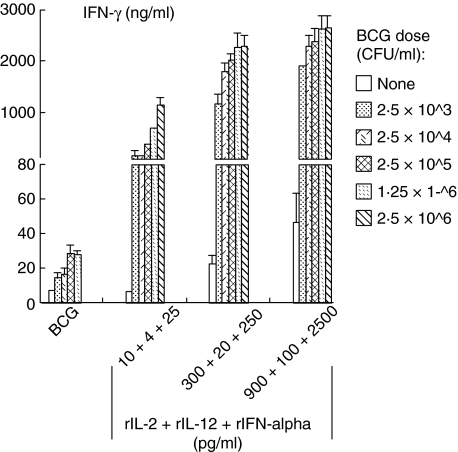
To determine the capacity of triple cytokines in enhancing BCG for IFN-γ production, we stimulated PBMCs with five different doses of BCG ranging from 2·5 × 103 to 2·5 × 106 CFU/ml or BCG combined with the three different doses (i.e. minimal, moderate and high) of triple cytokines used in Fig. 3. The addition of triple cytokines at a minimal dose (10 pg/ml for rIL-2, 4 pg/ml for rIL-12 and 25 pg/ml for rIFN-α) showed a clear synergistic, BCG dose-dependent IFN-γ induction (Fig. 4). This synergistic effect was readily observable even at the minimal BCG dose used (2·5 × 103 CFU/ml). This effect was also apparent at the BCG doses that induced a plateau level of IFN-γ seen in Fig. 1a (i.e. 1·25–2·5 × 106 CFU/ml). The latter observation indicated that addition of low-dose triple cytokines could increase the BCG-dose limitations for IFN-γ induction. The moderate- and high-dose triple cytokines also enhanced BCG-induced IFN-γ at all BCG doses tested including the minimal BCG dose (2·5 × 103 CFU/ml). These results suggest that addition of multiple Th1-stimulating cytokines could compensate a low dose of BCG for induction of a potent Th1 immune response.
Fig. 4.
Bacille Calmette–Guérin (BCG) dose-dependent interferon (IFN)-γ production by human peripheral blood mononuclear cells (PBMCs) in response to combination of BCG plus exogenous triple cytokines recombinant interleukin (rIL)-2, rIL-12 and rIFN-α. PBMCs were incubated with five different doses of BCG ranging from 2·5 × 103 to 2·5 × 106 colony-forming units (CFU)/ml, in the presence or absence of triple cytokines at a minimal dose, a moderate dose or a high dose for 3 days and then assayed for IFN-γ production in the conditioned media.
IL-2- and IFN-α-expressing rBCGs are potent stimulators for PBMC IFN-γ production
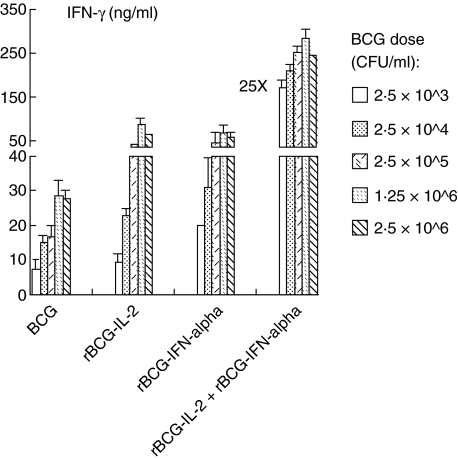
As Th1-stimulating cytokines showed their abilities to enhance BCG-induced IFN-γ production by PBMCs, we investigated whether our previously developed human IL-2- and IFN-α-expressing rBCGs could also induce high IFN-γ production. Indeed, both rBCG–IL-2 and rBCG–IFN-α induced 1·5–3-fold higher levels of IFN-γ than control BCG at all five doses tested (Fig. 5). Both rBCGs-induced IFN-γ showed a BCG dose-dependent manner similar to that of control BCG, however, with an early occurrence of a plateau at a dose of 2·5 × 105 CFU/ml. Also, similar to the combination of BCG with exogenous Th1-stimulating cytokines, a combination of both rBCGs showed a synergistic effect on IFN-γ production. Such rBCG combination was so potent that even at a minimal dose (2·5 × 103 CFU/ml) combined rBCGs increased IFN-γ by 25-fold compared to control BCG at the same dose. These results suggest that IL-2- and IFN-α-expressing rBCGs are superior to BCG for induction of a Th1 immune response.
Fig. 5.
Recombinant bacille Calmette–Guérin (rBCGs) expressing interleukin (IL)-2 or interferon (IFN)-α enhanced IFN-γ production by human peripheral blood mononuclear cells (PBMCs). PBMCs were incubated with five different doses of BCG, recombinant (r)BCG–IL-2, rBCG–IFN-α or both rBCGs, ranging from 2·5 × 103 to 2·5 × 106 colony-forming units (CFU)/ml for 3 days and then assayed for IFN-γ production in the conditioned media. PBMCs without BCG stimulation expressed a basal level of IFN-γ (0·041 ± 0·004 ng/ml).
Discussion
Intravesical BCG immunotherapy has been used for treating bladder TCC for three decades [1–3]. However, the current BCG therapy is empirical and needs to be improved. Because a proper induction of Th1 immune response appears necessary for BCG-mediated anti-tumour immunity, we have evaluated the regulatory cytokines that favour the production of the Th1 cytokine IFN-γ [29, 30, 34]. In this study, we focused on the dose–response of BCG/Th1-stimulating cytokines in IFN-γ production by human PBMCs. We demonstrated that rIL-2, rIL-12 and rIFN-α were potent enhancers for BCG-induced IFN-γ. We also demonstrated that a minimal dose of BCG was sufficient for induction of a high level of IFN-γ when it was combined with triple cytokines rIL-2, rIL-12 and rIFN-α. In addition, we demonstrated that rBCGs expressing IL-2 or IFN-α were superior to BCG for IFN-γ production. These observations provide an immunological basis for the rational use of Th1-stimulating cytokine with BCG in bladder cancer treatment.
BCG is a potent inducer for IFN-γ production by human PBMCs [23, 29, 30, 34]. This IFN-γ production was found to depend on BCG-induced, endogenously produced Th1-stimulating cytokines, including IL-2, IL-12 and IFN-α (Fig. 1b). IL-10, as an antagonist of the Th1 immune response, exhibited an inhibitory effect on BCG-induced IFN-γ production. These observations indicate the complexity of the cytokine network that determines the output of IFN-γ. Based on current understanding, macrophages serve as a first line of defence in anti-mycobacterial infection and produce IL-12, IFN-α and other proinflammatory cytokines after activation [37–39]. These macrophage-derived cytokines act as primary initiators of Th1 immune response and cause T cells and NK cells to produce IFN-γ [38, 40–42]. T cells can also be stimulated by endogenously produced IL-2 through autocrine- and pericrine-feedback processes, leading to a more profound production of IFN-γ [34, 43]. Thus, BCG-induced PBMC IFN-γ production is probably associated with activation of multiple immune cellular components, including at least macrophages, T cells and NK cells.
Although BCG is shown to be a potent inducer for PBMC IFN-γ production, this IFN-γ production was BCG dose-limited as a plateau level of IFN-γ emerged at a BCG dose of ≥1·25 × 106 CFU/ml (Fig. 1a). However, we observed that a subsaturated dose of BCG (2·5 × 105 CFU/ml) could be synergized for IFN-γ induction when it was combined with mono, dual or triple cytokines rIL-2, rIL-12 and/or rIFN-α (Figs 2 and 3). No saturation of IFN-γ increment was observed, even at the highest cytokine doses used. Thus, it is likely that a combination of rIL-2, rIL-12 or/and rIFN-α with an appropriate dose of BCG is capable of inducing a markedly enhanced Th1 immune response in the treatment of bladder cancer. Indeed, we have observed previously that addition of rIL-12 or rIFN-α could reverse BCG non-responsive patients for IFN-γ induction by BCG and clinical response to BCG [29, 30].
The current intravesical BCG immunotherapy of bladder cancer includes the weekly instillation of BCG at approximately 1–8 × 108 CFU/dose for at least 6 weeks. This repeated application of high doses of BCG is believed to be a major cause for the commonly seen BCG-related side effects. Previously, we observed that instillation of one-third of the standard BCG dose plus rIFN-α or rIL-12 induced high urinary IFN-γ [29, 30]. We even observed that instillation of one-tenth the standard BCG dose plus rIFN-α induced a comparably high level of urinary IFN-γ [30]. In this study, we observed further that supplementation of triple cytokines rIL-2, rIL-12 and rIFN-α not only synergized with BCG for PMBC IFN-γ production but also compensated for low BCG doses (Fig. 4). This observation suggests that a reduced BCG dose could be used, when combined with certain Th1-stimulating cytokines, to minimize BCG side effects while retaining its therapeutic effects in bladder cancer treatment.
Because a combination of BCG with Th1-stimulating cytokines showed a synergistic effect on PBMC IFN-γ production, we next tested whether rBCGs expressing IL-2 or IFN-α could also induce a high level of IFN-γ. Indeed, both rBCGs induced higher IFN-γ than control BCG when they were used alone and in combination at the minimal doses (Fig. 5). Thus, these rBCGs are probably superior to BCG for Th1 immune induction and may replace BCG to achieve both improved therapeutic and adverse effects in bladder cancer treatment.
It is generally accepted that successful intravesical BCG immunotherapy of bladder cancer depends on the proper induction of a localized Th1 immune response. The results of the present study suggest that supplementation of Th1-stimulating cytokines could enhance the effect of BCG on Th1 immune induction, which allows the use of lower and safer doses of BCG in bladder cancer treatment. Although the level of IFN-γ reflects the Th1 immune intensity, induction of very high IFN-γ may have detrimental effects in vivo. Elevated expression of IFN-γ has been observed to be associated with tissue destruction in inflammation [44, 45]. Thus, the level of IFN-γ induction should be controlled carefully in bladder cancer treatment.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (RO1DK66079) and Department of Defense (W81XWH-04-1-0070). We would like to thank Mitchell L. Rotman for helping edit the manuscript.
References
- 1.Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette–Guérin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MA, DeWolf DC. Bacillus Calmette–Guérin immunotherapy for superficial bladder cancer. New prospects for an old warhorse. Surg Oncol Clin N Am. 1995;4:189–202. [PubMed] [Google Scholar]
- 3.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573–80. [PubMed] [Google Scholar]
- 5.Dejager R, Guinan P, Lamm D. Long-term complete remission in bladder carcinoma in situ with intravesical Tice bacillus Calmette–Guérin. Urology. 1991;38:507–13. doi: 10.1016/0090-4295(91)80166-5. [DOI] [PubMed] [Google Scholar]
- 6.Shahin O, Thalmann GN, Rentsch C, Mazzucchelli L, Studer UE. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette–Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J Urol. 2003;169:96–100. doi: 10.1016/S0022-5347(05)64044-X. [DOI] [PubMed] [Google Scholar]
- 7.Steg A, Adjiman S, Debre B. BCG therapy in superficial bladder tumours − complications and precautions. Eur Urol. 1992;21(Suppl. 2):35–40. doi: 10.1159/000474920. [DOI] [PubMed] [Google Scholar]
- 8.Lamm DL, Van Der Meijden ADPM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette–Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette–Guérin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–8. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 10.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–42. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- 11.Peuchmaur M, Benoit G, Vieillefond A, et al. Analysis of mucosal bladder leucocyte subpopulations in patients treated with intravesical bacillus Calmette–Guérin. Urol Res. 1989;17:299–303. doi: 10.1007/BF00262987. [DOI] [PubMed] [Google Scholar]
- 12.Saint F, Patard JJ, Groux Muscatelli B, et al. Evaluation of cellular tumor rejection mechanisms in the peritumoral bladder wall after bacillus Calmette–Guérin treatment. BJU Int. 2001;88:602–10. doi: 10.1046/j.1464-410x.2001.02394.x. [DOI] [PubMed] [Google Scholar]
- 13.De Boer EC, De Jong WH, Van Der Meijden AP, et al. Presence of activated lymphocytes in the urine of patients with superficial bladder cancer after intravesical immunotherapy with bacillus Calmette–Guérin. Cancer Immunol Immunother. 1991;33:411–6. doi: 10.1007/BF01741603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Boer EC, de Jong WH, van der Meijden AP, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 15.De Boer EC, De Jong WH, Steerenberg PA, et al. Leukocytes and cytokines in the urine of superficial bladder cancer patients after intravesical immunotherapy with bacillus Calmette–Guérin. In Vivo. 1991;5:671–7. [PubMed] [Google Scholar]
- 16.Bohle A, Nowc C, Ulmer AJ, et al. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette–Guérin immunotherapy. J Urol. 1990;144:59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- 17.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette–Guérin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 18.Saint F, Patard JJ, Maille P, et al. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guérin treatment for superficial bladder cancer. J Urol. 2002;167:364–7. [PubMed] [Google Scholar]
- 19.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette–Guérin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 20.Poppas DP, Pavlovich CP, Folkman J, et al. Intravesical bacille Calmette–Guerin induces the antiangiogenic chemokine interferon-inducible protein 10. Urology. 1998;52:268–75. [PubMed] [Google Scholar]
- 21.Pavlovich CP, Kraling BM, Stewart RJ, et al. BCG-induced urinary cytokines inhibit microvascular endothelial cell proliferation. J Urol. 2000;163:2014–21. [PubMed] [Google Scholar]
- 22.Sanchez-Carbayo M, Urrutia M, Romani R, et al. Serial urinary IL-2, IL-6, IL-8, TNF-alpha, UBC, CYFRA 21-1 and NMP22 during follow-up of patients with bladder cancer receiving intravesical BCG. Anticancer Res. 2001;21:3041–7. [PubMed] [Google Scholar]
- 23.Luo Y, Chen X, O'Donnell MA. Mycobacterium bovis bacillus Calmette–Guerin (BCG) induces human CC- and CXC-chemokines in vitro and in vivo. Clin Exp Immunol. 2007;147:370–8. doi: 10.1111/j.1365-2249.2006.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saint F, Patard JJ, Maille P, et al. T helper 1/2 lymphocyte urinary cytokine profiles in responding and nonresponding patients after 1 and 2 courses of bacillus Calmette–Guerin for superficial bladder cancer. J Urol. 2001;166:2142–7. [PubMed] [Google Scholar]
- 25.Nadler R, Luo Y, Zhao W, et al. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette–Guerin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003;131:206–16. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bercovich E, Deriu M, Manferrari F, Irianni G. BCG vs. BCG plus recombinant alpha-interferon 2b in superficial tumors of the bladder. Arch Ital Urol Androl. 1995;67:257–60. [PubMed] [Google Scholar]
- 27.Stricker P, Pryor K, Nicholson T, et al. Bacillus Calmette–Guérin plus intravesical interferon alpha-2b in patients with superficial bladder cancer. Urol. 1996;48:957–61. doi: 10.1016/s0090-4295(96)00375-5. [DOI] [PubMed] [Google Scholar]
- 28.Clinton SK, Canto E, O'Donnell MA. Interleukin-12. opportunities for the treatment of bladder cancer. Urol Clin North Am. 2000;27:147–55. doi: 10.1016/s0094-0143(05)70242-1. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-γ in response to bacillus Calmette–Guérin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 30.Luo Y, Chen X, Downs TM, DeWolf WC, O'Donnell MA. IFN-α 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette–Guérin immunotherapy. J Immunol. 1999;162:2399–405. [PubMed] [Google Scholar]
- 31.Joudi FN, Smith BJ, O'Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette–Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344–8. doi: 10.1016/j.urolonc.2005.11.026. National BCG-Interferon Phase 2 Investigator Group. [DOI] [PubMed] [Google Scholar]
- 32.Joudi FN, O'Donnell MA. Second-line intravesical therapy versus cystectomy for bacille Calmette–Guerin (BCG) failures. Curr Opin Urol. 2004;14:271–5. doi: 10.1097/00042307-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell MA, Krohn J, DeWolf WC. Salvage intravesical therapy with interferon-alpha 2b plus low dose bacillus Calmette–Guerin is effective in patients with superficial bladder cancer in whom bacillus Calmette–Guerin alone previously failed. J Urol. 2001;166:1300–4. [PubMed] [Google Scholar]
- 34.Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21:17–26. doi: 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell MA, Aldovini A, Duda RB, et al. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, Chen X, Han R, O'Donnell MA. Recombinant bacille Calmette–Guerin (BCG) expressing human interferon-alpha 2B demonstrates enhanced immunogenicity. Clin Exp Immunol. 2001;123:264–70. doi: 10.1046/j.1365-2249.2001.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson S, Valadas E, Smith SM, Lukey PT, Dockrell HM. Monocyte-derived macrophage cytokine responses induced by M. bovis BCG. Tuber Lung Dis. 2000;80:197–207. doi: 10.1054/tuld.2000.0247. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto H, Suzuki K, Tsuyuguchi K, et al. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG. cytokine regulation and effect of NK cells. Infect Immun. 1997;65:4405–10. doi: 10.1128/iai.65.11.4405-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol. 2004;173:6890–8. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 40.Esin S, Batoni G, Pardini M, et al. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette–Guerin. Immunology. 2004;112:143–52. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon-γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theze J, Alzari PM, Bertoglio J. Interleukin 2 and its receptors. recent advances and new immunological functions. Immunol Today. 1996;17:481–6. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- 44.Willemsen LE, Hoetjes JP, van Deventer SJ, van Tol EA. Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol. 2005;142:275–84. doi: 10.1111/j.1365-2249.2005.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi T, Morimoto M, Iwata H, Onodera T. Interferon-gamma plays a role in pancreatic islet-cell destruction of reovirus type 2-induced diabetes-like syndrome in DBA/1 suckling mice. Int J Exp Pathol. 1998;79:313–20. doi: 10.1046/j.1365-2613.1998.670398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]