Abstract
An Echinococcus granulosus cDNA sequence coding for EpC1, a proven serodiagnostic marker for cystic echinococcosis (CE, hydatid disease), has high amino acid sequence identity to a paralogue from Taenia solium, the cause of neurocysticercosis (NCC). To determine diagnostic antibody-binding regions on EpC1 recognized specifically by CE sera, 10 truncated regions (P1–10) of the immunogenic protein were expressed in Escherichia coli and subjected to immunoblotting. One peptide, designated peptide 5 [P5, fused with glutathione-S-transferase (GST)] was positively recognized by sera from mice experimentally infected with oncospheres of E. granulosus and sera from surgically confirmed CE patients. Sera from NCC patients did not react with any of the peptides used. There are four amino acid substitutions in P5 compared with the T. solium sequence and these may form part of the epitope inducing CE-specific antibody. Ninety-seven per cent (58 of 60) of sera from confirmed CE patients recognized P5-GST, which was higher than the parent EpC1 fused with GST which reacted with 92% (55 of 60) of the sera. A population screening survey showed that 424 human sera collected from communities in Xinjiang, an area in China endemic for CE, exhibited 4·5% and 3·3% positivity in immunoblotting analysis to EpC1 and P5, respectively; 19·8% of these sera reacted positively against hydatid cyst fluid (HCF) antigen B. Low numbers of surgical CE cases have been reported from this population, suggesting that HCF-based serology lacks specificity and that EpC1 or its contained P5 peptide may prove more accurate for seroepidemiological surveys of CE.
Keywords: antibody-binding region, Echinococcus granulosus, immunoblotting, immunogenic protein EpC1, serodiagnosis
Introduction
Cystic echinococcosis (CE), a near-cosmopolitan zoonosis caused by the metacestode larval stage of Echinococcus granulosus, is characterized by long-term growth of hydatid cysts in human and other intermediate hosts [1]. Early diagnosis of CE can provide significant improvements in the quality of management and treatment of the disease. The definitive diagnosis for most human CE cases is by physical imaging methods, such as roentgenography, ultrasonography, computed axial tomography (CT scanning) and magnetic resonance imaging, although such procedures are often not readily available in isolated communities. In most cases, the early stages of infection are asymptomatic and not detectable by the imaging techniques [2]. Therefore, immunodiagnostic tests, which are relatively easy to use and inexpensive, would be advantageous for large-scale screening of populations at high risk [3]. However, serodiagnosis of CE remains a challenge. The major problem is that extensive serological cross-reactivity has been reported for E. granulosus antigens with sera from patients with cysticercosis [4].
We recently cloned a cDNA (EpC1) from an E. granulosus protoscolex cDNA library; expression of the cDNA and purification of the resulting recombinant protein (EpC1) yielded a highly immunogenic molecule that was recognized by 92% of sera from patients with CE [5]. The predicted protein sequence for EpC1, however, displays high sequence similarity to a molecule from Taenia solium [6], the causative pathogen responsible for neurocysticercosis (NCC). Nevertheless, the serological cross-reactivity was limited (about 9%), indicating that EpC1 has B cell epitopes not cross-reactive with antibodies in NCC sera. In this report, we mapped antibody-binding epitopic regions on EpC1 and identified two peptide regions (P1 and P5) that probably form epitopes recognized only by antibodies in sera from patients with CE, thus accounting for the high level of serodiagnostic specificity recorded for EpC1.
Materials and methods
Preparation of recombinant proteins
The expression and purification of recombinant EpC1 fused with glutathione-S-transferase (EpC1-GST) have been described previously [5]. Double-affinity purification involving TALON™ Metal Affinity Resins (Clontech, Palo Alto, CA, USA) and GST-tag Affinity Resins (Novagene, Madison, WI, USA) was employed in order to obtain highly purified recombinant protein.
Ten truncated cDNA fragments designated as P1–P10 (Fig. 1) were amplified by polymerase chain reaction (PCR) from the parent EpC1 cDNA using primers listed in Table 1. The amplified cDNA fragments for the 10 peptides were ligated to the pET-41b(+) [the exception being P7, which incorporated pET-41a(+)] vector (Novagene Madison, WI, USA) and transformed into Escherichia coli BL21(DE3) (Novagene) by heat shock transformation [7]. The open reading frames of all peptide constructs were confirmed by DNA sequencing. Procedures for expression and purification of the recombinant peptide fusion proteins were identical to those used for the parent EpC1-GST.
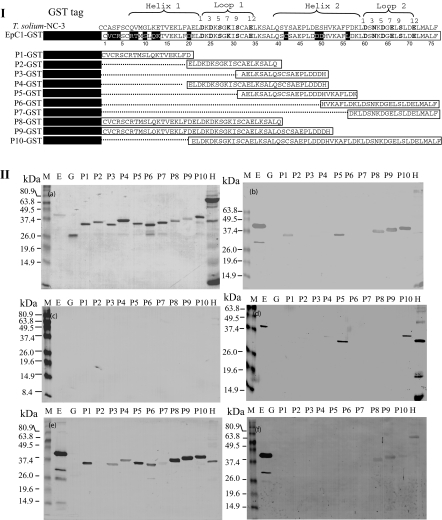
Fig. 1.
Panel I. Schematic representation and positioning of truncated peptides against the Echinococcus granulosus EpC1 amino acid sequence (Accession number AF481884) aligned with the homologue of Taenia solium (NC-3, Accession number AJ012669). Identical residues against NC-3 are blocked in black, similarities are shown with a lightly shaded background, while different residues are not blocked. Amino acid numbers for EpC1 are scaled at the bottom of the sequence. The glutathione-S-transferase (GST) fusion partner is shown as a black solid box and expressed sequences are shown in open boxes. Dotted lines indicate deleted regions. The predicated secondary structure of the EpC1 protein is shown with two helices and two loops according to protein sequence analysis using the MacVector version 6·5.3 software package (Oxford Molecular Group, Oxford, UK) and blastp (http://www.ncbi.nlm.nih.gov/BLAST/). Panel II. Immunoblot analysis showing different sera recognizing recombinant EpC1-GST and truncated fragments of EpC1 fused with GST. Lane E, EpC1-GST; lane G, GST; lane P1–10, peptides 1–10 fused with GST; lane H, hydatid cyst fluid. Proteins and peptides were first separated on a 12% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel (a, stained by Coomassie blue), transferred onto nitrocellulose membrane and probed with a mouse infection serum pool (b); a serum pool from neurocysticercosis patients (c); a serum pool from cystic echinococcosis patients; (d), a rabbit hyperimmune serum (e); and a pool of mouse hyperimmune sera (f). Markers (M) are shown on the left-hand side of each panel in kilodaltons (kDa).
Table 1.
Primers used for construction of truncated regions of the EpC1 parent protein.
| Name | Sequence (5′→ 3′) | Use |
|---|---|---|
| EPC3-1 | 5′-GGCTCGAGTCAATCGAAAAGCTTCTCAACAGTT-3′ | DSP for P1 |
| EPC3-2 | 5′-GGCTCGAGTCAATGGTCGTCATCGAGAGGCTC-3′ | DSP for P3, P4 and P8 |
| EPC3-4 | 5′-GGCTCGAGTCACTGTAGTGCGGATTTAAGCTCG-3′ | DSP for P2 and P8 |
| EPC3-10 | 5′-GGCTCGAGTCACTTATCTAAGAAAGCCTTCACATG-3′ | DSP for P5 |
| EPC5-1 | 5′-GGGATCCTGAGTTGGACAAGGACAAGTCG-3′ | USP for P2, P4 and P10 |
| EPC5-2 | 5′-GGGATCCACATGTGAAGGCTTTCTTAGAT-3′ | USP for P6 |
| EPC5-8 | 5′-GGAATTCGCCGAGCTTAAATCCGCACTA-3′ | USP for P3 and P5 |
| EPC5-10 | 5′-GGGATCCGATAAGCTCGACTCCAACAAG-3′ | USP for P7 |
| C1R | 5′ GCCCGAGCAACTTAATCTCGA 3′ | DSP for P6, P7 and P10 |
| S-TAG | 5′ TCGAACGCCAGCACATGGACAG 3′ | USP for P1, P8 and P9 |
Sequences in italics are restriction enzyme cut sites. TCA is a stop codon. DSP, downstream primer; USP, upstream primer.
Hyperimmune anti-EpC1 anti-sera (HIS)
Purified EpC1-GST was digested with enterokinase using a recombinant enterokinase kit (Novagen) to remove the GST tag. EpC1 was further purified on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The EpC1 band was cut from the gel and recovered using an elution apparatus (Bio-Rad, Hercules, CA, USA).
New Zealand White hybrid rabbits were obtained from the Central Breeding House, University of Queensland, Australia and BALB/c mice were purchased from the Animal Resource Centre, Perth, Western Australia. The animals were maintained in the animal house at the Queensland Institute of Medical Research. Two rabbits and five BALB/c mice were, respectively, immunized subcutaneously (s.c.) with 200 µg and 20 µg of recombinant EpC1-GST emulsified in an equal volume of Freund's complete adjuvant (CFA) (Sigma, St Louis, MO, USA). The animals were boosted s.c., then intraperitoneally (i.p.) with the same amount of EpC1 emulsified in Freund's incomplete adjuvant (IFA) (Sigma) 21, 42, and 56 days later, respectively; the animals received a final boost i.p. with the same amount of EpC1 after 63 days. Additionally, two rabbits and five BALB/c mice were immunized with recombinant GST protein using the same amount and schedule of injections as for the EpC1 protein. Blood samples for serum analysis were collected on day 70. Both anti-EpC1 and GST sera used for immunoblotting were absorbed against bacterial cell lysates to remove non-specific antibodies, following a published protocol [5].
Infection sera
Sera were also obtained from outbred Chinese Kunming mice challenged with oncospheres (eggs) of E. granulosus [5, 8], 26 weeks post-infection. Human sera were collected from patients with surgically confirmed CE infection from Australia (n = 7) and China (n = 53) and sera from patients (n = 6) infected with T. solium NCC from China were used to evaluate the diagnostic performance of the individual recombinant protein fragments. A further 424 sera were collected from subjects in communities in or near Urumqi, Xinjiang, an area endemic for CE in China [9], to test the usefulness of the recombinant proteins in community surveys for CE infection. The participants had an average age of 32 years (range 19–59 years). Considering that sex and ethnicity are major risk factors influencing echinococcosis infection rates in Xinjiang [10, 11], the subjects were divided into males and females, and Muslim Chinese and Han Chinese ethnic groups (Table 2). All sera were stored at −20°C until use.
Table 2.
Population screening for cystic echinococcosis in and around Urumqi, Xinjiang, China using immunoblotting with recombinant and native proteins.
| No. positive (%) | ||||
|---|---|---|---|---|
| Subjects | Ethnicity | EpC1-GST | P5-GST | HCF (antigen B) |
| Females | Muslim Chinese (n = 67) | 2 (2·9) | 1 (1·5) | 11 (16·4) |
| Han Chinese (n = 210) | 10 (4·8) | 7 (3·3) | 46 (21·9) | |
| Subtotal (n = 277) | 12 (4·3) | 8 (2·9) | 57 (20·6) | |
| Males | Muslim Chinese (n = 60) | 4 (6·7) | 3 (5·0) | 8 (13·3) |
| Han Chinese (n = 87) | 3 (3·4) | 3 (3·4) | 19 (21·8) | |
| Subtotal (n = 147) | 7 (4·8) | 6 (4·1) | 27 (18·4) | |
| Total | 424 | 19 (4·5*) | 14 (3·3*) | 84 (19·8*) |
Significant difference, P < 0·01 between hydatid cyst fluid (HCF) (antigen B) and EpC1-glutathione-S-transferase (GST) or P5-GST.
Immunoblot analysis
Affinity purified EpC1 and peptides fused with GST were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. Native hydatid cyst fluid as control antigens was prepared as described previously [5]. Sera from patients, mice and rabbits were diluted 1 in 100–500 with phosphate-buffered saline (PBS) containing 0·05% (v/v) Tween-20 (PBST) and incubated with the transferred nitrocellulose membranes after blocking with 5% (w/v) skimmed milk in PBS. The second antibody conjugates, anti-mouse IgG, anti-rabbit IgG horseradish peroxidase (HRP) (Bio-Rad) or anti-human IgG HRP (Sigma), diluted 1 in 1000 in PBST, were used to probe primary antibodies precipitated on the membranes. Blots were developed using H2O2 and 4-chloro-1-naphthol (Sigma) in PBS.
Statistical analysis
The χ2 test (Fisher's exact method) with spss (version 11) was used to compare percentage of positive values of groups with different ethic backgrounds and antigens used for the tests. P < 0·01 was taken to indicate a statistically significant difference.
Results
Two specific CE antibody binding regions are located in the helical structure of EpC1
In order to identify specific antibody binding regions on EpC1, we truncated the parent EpC1 fragment into 10 peptides (P1–10), based on the predicated secondary structure and amino acid substitutions of the protein against a homologous sequence termed NC-3 from T. solium (Fig. 1, I) [6]. Sequencing analysis showed that all open reading frames for P1–P10 were identical to those designed (data not shown). To analyse specific antibody binding ability of the designed peptides, we used immunoblotting to probe pooled sera from patients and mice infected with E. granulosus, and NCC patients. Recombinant GST was not recognized by any of the pooled sera regardless of source (lane G in Fig. 1, Ia–f), suggesting that GST does not contain B cell epitopes recognized by antibodies in sera from individuals or mice infected with E. granulosus or patients with T. solium. These negative reactions with GST were confirmation that all sera reactivity was specific to EpC1 or the EpC1-derived peptides.
Sequence analysis using blast showed that EpC1 is a calcium-binding protein containing two EF-hand motifs (data not shown). A typical EF-hand motif contains a loop (functioning as the calcium-binding site) flanking with two helical structures [12]. Two peptides, P2 and P7, which overlap predicted loops in the EpC1 sequence with 100% sequence identity to the T. solium NC-3 sequence [6] (Fig. 1, I), showed no reactivity with any of the test sera (Fig. 1, II), including the NCC sera. Two peptides, P1 and P5, which overlap two alpha-helices in the protein, were both recognized by pooled sera from mice infected with oncospheres of E. granulosus. P1 covers the first helical structure with nine substitute residues compared with the T. solium sequence (Fig. 1, I). There was no recognition of P1 by sera from patients with CE or NCC, indicating that P1 does not contain epitopes stimulating human B cell responses, but can induce CE-specific antibody responses in mice.
To determine precise antibody-binding epitope regions in EpC1, we used an overlapping and spanning strategy to map the peptides. P8, which spans the regions covered by P1 and P2, showed reactivity with sera from the E. granulosus-infected mice, indicating that the binding was due exclusively to P1 as P2 was not recognized by any of the sera. P9, a fragment that is 11 residues longer than P8 at the C-terminus, overlaps P1, P2, P3 and P4. Only P1 was recognized by CE infection sera from mice; P2, P3 and P4 did not bind antibodies in sera from E. granulosus-infected mice (Fig. 1, IIb), indicating that P1 contains the epitope region in P9 which covers more than two-thirds of the EpC1 sequence at the N-terminus. There are nine amino acid substitutions in P1 compared to the homologous T. solium NC-3 sequence (Fig. 1, I), indicating that the replacements may format the specific binding sites to antibodies in the sera from mice infected with E. granulosus. However, P1 was not recognized by sera from CE patients and there was no reactivity to its related peptides, P2, P3, P4, P8 and P9.
Only P5 and its related peptide 10 (P10) were recognized by pooled sera from patients with CE (Fig. 1, IId). P10 overlaps P4, P5, P6 and P7, of which only P5 was recognized by the CE sera from humans and mice; P5 did not react with sera from the NCC patients. These serological analyses indicate that P5 contains specific antibody-binding epitope(s) to CE infection recognized by multiple mammalian host species, but is not cross-reactive with sera from subjects infected with T. solium (Fig. 1, IIc).
P3 differs from the T. solium NC-3 sequence by three residues (Fig. 1, I). The peptide is identical to P5 except that it lacks seven residues (VKAFLDK) at the C terminal end. Among the seven residues, only leucine positioned at number 56 (L56) in EpC1 is replaced by phenylalanine in NC-3 (Fig. 1, I). The P3 predicted sequence encompasses about 60% of the second alpha-helical structure of EpC1, but despite this P3 did not react with sera from mice (Fig. 1, IIb) or patients (Fig. 1, IId) infected with E. granulosus. Furthermore, P6 contains these same seven residues near its N terminus, but it was also not recognized by any of the sera. Combining these results, L56 appears to play a crucial role in forming specific epitope(s) with three other substitute residues, possibly a conformational epitope on EpC1 recognized by CE sera.
Hyperimmune anti-EpC1 anti-sera (HIS) from mice and rabbits showed different patterns of peptide recognition. Mouse HIS recognized the parent EpC1 molecule strongly, but recognized the peptides only weakly (Fig. 1, IId). In contrast, rabbit HIS reacted strongly to all peptides except P2 and P7 (which contains a loop structure) (Fig. 1, IId), suggesting that the secondary structure of the protein affects antibody reactivity strongly. The helical structure appeared to be more antigenic than the loop structure.
Recombinant peptide 5 has similar diagnostic capacity for detection of specific antibodies in sera of mice and patients infected with E. granulosus
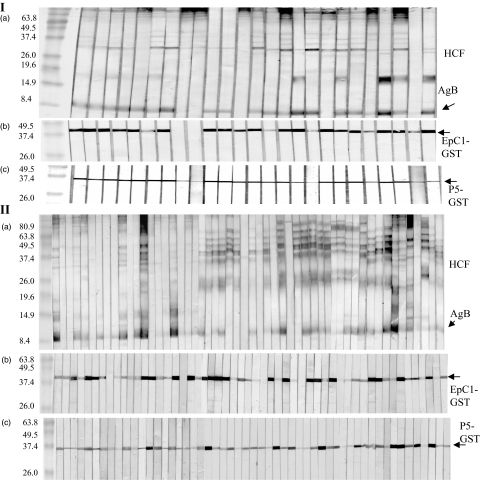
We subjected P5 to further testing of its serodiagnostic sensitivity. We initially tested 25 sera from mice infected experimentally with oncospheres of E. granulosus. These mice harboured different numbers of cysts at autopsy, as described in a previous study [5]. Both EpC1-GST and P5-GST bound antibodies in the sera, with P5-GST exhibiting 100% sensitivity (25 of 25, Fig. 2, Ic) and EpC1-GST showing 92% sensitivity (23 of 25, Fig. 2, Ib). Twenty-one of 25 (84% sensitivity) sera bound native hydatid cyst fluid (HCF) antigen B (Fig. 2, Ia), which were similar to results we obtained previously [5].
Fig. 2.
Immunoblot analysis of 25 sera obtained from outbred Chinese Kunming mice challenged with oncospheres (eggs) of Echinococcus granulosus [5, 8] 26 weeks post-infection (panel I) and 53 human sera from confirmed cystic echinococcosis (CE) cases from China (panel II) recognizing hydatid cyst fluid antigen B (a), EpC1-glutathione-S-transferase (GST) (b) and P5-GST (c).
We then tested these antigens against 60 sera from patients with surgically confirmed CE. The results showed that 97% (58 of 60) of the sera produced visible reactions using P5-GST (Fig. 2, IIc). In comparison, 92% (55 of 60) of the sera showed positive responses with EpC1-GST (Fig. 2, IIb). These reactions were much clearer than when using native HCF antigen B, which was recognized by 78% (47 of 60) of the sera (Fig. 2, IIa).
Community screening with EpC1-GST and P5-GST
To assess the performance of EpC1-GST and P5-GST in community screening, we tested 424 human sera collected in Xinjiang, China, by immunoblotting and compared the results with those obtained with HCF antigen B. The results showed that native HCF antigen B recognized 19·8% of the sera, whereas P5-GST and EpC1-GST reacted with 3·3% and 4·5% of the sera, respectively (Table 2). There was significant difference between EpC1-GST or P5-GST and native antigen B (P < 0·01). There were no differences between male and females, or between Muslim and Han-Chinese with the same antigens (P > 0·05).
Discussion
We have identified previously a novel recombinant protein, EpC1, from E. granulosus that yielded an overall sensitivity of 92·2% and specificity of 95·6% for diagnosis of cystic echinococcosis with its GST fusion protein [5]. EpC1, comprised of 76 amino acids, has 86% amino acid sequence similarity to a protein (NC-3, Accession number AJ012669) from T. solium [6]. Despite this sequence similarity, only 9% (12 of 172) of sera from confirmed T. solium NCC cases cross-reacted with EpC1 by immunoblotting [5]. It was shown earlier that NC-3 exhibited 96·3% sensitivity and 91·5% specificity in an enzyme-linked immunosorbent assay with 27 confirmed NCC patient serum samples [6]. Hence, both EpC1 and NC-3 exhibited very high specificity and sensitivity in serodiagnosis of CE and NCC, respectively, indicating that the two proteins contain specific B cell epitopes for E. granulosus and T. solium infection. We used immunoblotting to test 20 sera from patients infected with Schistosoma japonicum and 20 sera from mice infected with Plasmodium yoelii; none of these sera reacted with EpC1-GST [5] or P5-GST (data not shown). In the present study, we have confirmed that the high specificity of EpC1 is due to specific B cell epitopes on the protein, which result in its highly specific recognition by human and mouse CE infection sera. These epitopes are located on peptides P1 and P5 of EpC1, which have several amino acid residues that are different from the NC-3 sequence; these substitutions may comprise the specific binding sites for antibodies generated from E. granulosus infection. Our data also show that only P5 contains antibody binding site(s) for human CE infection.
It is noteworthy that there are four substituted residues in the P5 sequence compared with NC-3 from T. solium (Fig. 1) with tyrosine (Y), glutamic acid (E), serine (S) and phenylalanine (F) being replaced by cysteine (C42), aspartic acid (D49), aspartic acid (D50) and leucine (L56), respectively, in EpC1 (Fig. 1, I). These substitutions may form specific epitope(s) on EpC1 that stimulate B cell-producing antibodies during CE infection. The F/L substitution appears important in the composition of the B-cell epitope, as although peptides P3, P4 and P9 contain the other three substitutions, they were not recognized by human CE infection sera (Fig. 1, IId). However, the sequence containing leucine without the other three residues, represented by P6, was not recognized by the CE sera. Protein secondary structure analysis showed that the P5 region of EpC1 is highly helical (Fig. 1). The leucine residue may form a conformational epitope with the other substitutions to form a specific epitope specifically recognized by antibodies in human and mouse CE infection sera.
Both P1 and P5, which are located in the helix sequences (Fig. 1, I) were recognized by murine infection sera (Fig. 1, IIb), indicating that there is an additional antibody-binding epitope on this region, which resulted also in P8 and P9 being recognized (Fig. 1, IIb). Compared with the T. solium NC-3 sequence, there are nine different residues in the 20 amino acid P1 peptide. These differences may form another specific epitope on EpC1 recognized only by antibodies in CE infection sera. However, the peptide was not recognized by sera from confirmed CE patients. Further testing of P1 with sera from different CE patient age groups, especially younger individuals, will indicate whether it can be used for diagnosis of early CE infection.
The different patterns of recognition by infection/hyperimmune sera from the different mammalian hosts suggest that B cells in the different animals can be activated by different epitopes on the same protein. Concerning hyperimmune sera against recombinant protein, B cells from rabbits seemed much more sensitive to EpC1, suggesting that they recognized more EpC1 epitopes than did those from mice. The reasons for this are unclear and represent an interesting area for future study.
The population screening showed that P5-GST (3·3% positivity) and EpC1-GST (4·5% positivity) had significantly lower reactivity than native protein antigen B, which was recognized by 19·8% of the 424 human sera tested by immunoblotting. We found no difference between sex and ethnic (Han or Muslim Chinese) groups, due probably to the fact these subjects came from an urban location. An earlier report showed that 26·6% of inhabitants from villages in the same area in Xinjiang were seropositive to HCF antigens in an enzyme-linked immunosorbent assay (ELISA) in a population survey [13]. However, in this area, the annual surgical case rate was only 43·8 of 100 000 (0·04%) of the population [14], suggesting that the HCF/ELISA lacks specificity. Overall, therefore, serological assays incorporating P5 or EpC1 may be more accurate than native HCF antigen B for CE population screening, given the high specificity we have reported recorded previously for EpC1 [5]. We are continuing our serological studies aimed at confirming the practical specificity of EpC1 and P5 in community surveys of CE in China and in other endemic areas.
In summary, our data, which describe the mapping of antibody binding regions on the diagnostic antigen EpC1, may prove important in future research aimed at further defining specific immune responses to E. granulosus in different mammalian hosts. Future development of a diagnostic assay for CE based on the P5 peptide is worthy of serious consideration.
Acknowledgments
We thank Gu Zhu (Queensland Institute of Medical Research) for help with statistical analysis. The study was supported financially by the Wellcome Trust (GR057212) and the National Health and Medical Research Council of Australia.
References
- 1.Eckert J, Schantz PM, Gasser PR, et al. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: World Organisation for Animal Health and World Health Organisation; 2001. pp. 100–42. [Google Scholar]
- 2.Pawlowski ZS, Eckert J, Vuitton D, et al. Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS, editors. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: World Organisation for Animal Health and World Health Organisation; 2001. pp. 20–71. [Google Scholar]
- 3.McManus DP, Zhang W, Li J, et al. Echinococcosis. Lancet. 2003;362:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 4.Schantz PM. Progress in diagnosis, treatment and elimination of echinococcosis and cysticercosis. Parasitol Int. 2006;55(Suppl. 1):S7–13. doi: 10.1016/j.parint.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zhang WB, Wilson M, et al. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J Infect Dis. 2003;188:1952–61. doi: 10.1086/379976. [DOI] [PubMed] [Google Scholar]
- 6.Hubert K, Andriantsimahavandy A, Michault A, et al. Serological diagnosis of human cysticercosis by use of recombinant antigens from Taenia solium cysticerci. Clin Diagn Lab Immunol. 1999;6:479–82. doi: 10.1128/cdli.6.4.479-482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambrook J, Russell DW. A laboratory manual. 3. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning. [Google Scholar]
- 8.Zhang W, You H, Li J, et al. Immunoglobulin profiles in a murine intermediate host model of resistance for Echinococcus granulosus infection. Parasite Immunol. 2003;25:161–8. doi: 10.1046/j.1365-3024.2003.00622.x. [DOI] [PubMed] [Google Scholar]
- 9.Chi PS, Fan YL, Zhang WB, et al. The epidemic situations of cystic echinococcosis in China. Xinjiang Agric Sci. 1989:35–8. [in Chinese] [Google Scholar]
- 10.Chai J. Sero-epidemiological surveys from cystic echinococcosis in the Xinjiang Uygur Autonomous Region, PRC. In: Andersen FL, Chai J, Liu F, editors. Compendium on cystic echinococcosis − with special reference to the Xinjiang Uyger Autonomous Region, the People's Republic of China. Provo, Utah: Brigham Young University Print Services; 1993. pp. 153–61. [Google Scholar]
- 11.Menghebat L, Jiang L, Chai J. A retrospective survey from surgical cases of cystic echinococcosis in The Xinjiang Uyger Autonomous Region, PRC (1951–90) In: Andersen FL, Chai J, Liu F, editors. Compendium on cystic echinococcosis − with special reference to the Xinjiang Uyger Autonomous Region, the People's Republic of China. Provo, Utah: Brigham Young University Print Services; 1993. pp. 135–45. [Google Scholar]
- 12.Michiels J, Xi C, Verhaert J, et al. The functions of Ca(2+) in bacteria: a role for EF-hand proteins? Trends Microbiol. 2002;10:87–93. doi: 10.1016/s0966-842x(01)02284-3. [DOI] [PubMed] [Google Scholar]
- 13.Chai J, Sultan Y, Wei M. An investigation on the epidemiologic baseline of hydatid disease in Xinjiang, China. I. A sero-epidemiological survey of human hydatidosis. Endemic Dis Bull. 1989;4:1–8. [in Chinese] [Google Scholar]
- 14.Zhang WB, Zhang ZZ, Alili H, et al. A pilot control program for hydatid disease in Hutubi county, Xinjiang. China Endemic Dis Bull. 1994;9:70–1. [in Chinese] [Google Scholar]