Abstract
The immune tolerance induced by the liver as an allograft is difficult to reconcile with the evidence that the liver selectively accumulates activated T cells from the circulation. However, much of this information is based on murine liver lymphocytes that were isolated using enzymatic digestion. In the present study we made use of a novel resource, the lymphocytes isolated during the perfusion of living donor liver lobe prior to transplantation. These healthy human liver lymphocytes displayed surface markers indicating a high degree of activation of natural killer cells, CD56+ T cells, CD4+ T cells and CD8+ T cells. These properties were independent of enzymatic treatment or the details of cell isolation. We conclude that the healthy human liver is a site of intense immunological activity.
Keywords: activation, human, liver lymphocytes, tolerance
Introduction
The human liver plays a sentinel role, confronting exogenous antigen absorbed from the gut as well as the pathogen challenge resulting from breaches of the intestine's mucosal barrier. Because of the diversity of the antigens to which it must respond, the liver displays both innate and adaptive immune functions; however, many of the food-derived antigens do not represent a threat, and the liver's immune system must make a nuanced response which correctly selects either an inflammatory, a tolerogenic or an anergic outcome. Furthermore, the liver has been proposed to function as an important regulator of immune cells activated by events at remote locations within the host [1]. Examples of the above include the lack of reaction to the harmless antigens in food or from commensal bacteria, the inflammatory response to gut-derived pathogens and transformed cells, and the well-documented ability to participate in the generation of tolerance to certain oral antigens and to vascularized allografts [2–5]. The diverse pathology that results from maladaptive immune responses within the liver has stimulated the investigation of hepatic immune function, in the hope of developing better therapies for viral, microbial, neoplastic and autoimmune diseases.
Lymphocytes extracted from human liver tissue have been characterized [6, 7]. In comparison to peripheral blood, the most noteworthy differences are the preponderance of natural killer (NK) and CD56+ T cells in the liver, and the diminished frequency of T lymphocytes with a skewing of the CD4+/CD8+ ratio towards CD8+ lymphocytes in the liver. Lymphocytes within healthy liver express activation markers, supporting the concept that they are not quiescent, but continually mediate immune responses [7, 8]. It has been proposed that the liver of mouse and humans supports the local development and maturation of T cells, although much of the evidence is indirect [9–11]. Some studies suggest that a distinctive cytokine milieu within the liver shapes the development of the specialized immune cells represented therein [5], while other studies indicate that, as part of normal surveillance, naive T cells can circulate freely between the peripheral blood, secondary lymphoid tissue and liver [12]. Activated T cells, however, have their migratory pathways determined by changes in their cell surface expression of adhesion receptors [13], and home to specific tissue sites based upon the endothelial expression of appropriate tissue specific ligands. The healthy liver contains activated terminally differentiated T cells, particularly CD8+ T cells, some of which are destined to undergo apoptosis, yet the liver also contains cells that defend the host against tumour cells and pathogens as part of immune surveillance [14, 15]. Furthermore, the liver can suppress the expansion of activated T helper 1 (Th1) type CD4+ cells and promote the outgrowth of Th2 type CD4+ cells, contributing to ‘immune deviation’ [16]. Thus the lymphocytes found in the liver are a mixture of activated and naive cells; some are likely to participate in local immune response, while others are either trapped and die or are modulated according to demands of an integrated systemic immune response of the host.
Our understanding of immune function within the liver has been derived largely from mouse studies, in part because of the limited numbers of lymphocytes that can be obtained from healthy human livers. Lymphocytes can be isolated from small samples (needle or wedge biopsies) of healthy human livers, but an alternative has been explored: the perfusate of liver transplant donors [6, 17]. The majority of studies dealing with human intrahepatic lymphocytes (IHL) have used cells obtained by the dissociation of small liver biopsies [18] or cells isolated from cadaveric liver donors [6, 17]. The latter technique yields large numbers of IHL, but the effects of donor death and prolonged cold ischaemia in situ complicates the interpretation of these cells. Here we compared derived lymphocytes with the IHL obtained by perfusion of living donor livers; these cells were harvested from healthy donors under general anaesthesia, avoiding both the effects of brain death and of extended storage. The results show that human IHL may be obtained in large numbers from the living donor liver lobes prior to transplant. These cells displayed markers characteristic of IHL obtained using other approaches, suggesting that contamination with blood-borne cells was minimal. We anticipate that this source of human IHL will be valuable in studies of basic immunobiology, and will form the most appropriate control tissue in experiments to evaluate pathological changes in IHL obtained from diseased explants.
Materials and methods
Patients and IHL samples
All tissues were obtained under appropriate consent using a study protocol approved by the Institutional Review Board at the University of Rochester Medical Center. Cells eluted from the liver were retrieved from the Viaspan® preservation solution (‘Belzer UW’, Barr Laboratories, Woodcliff Lake, NJ, USA) in which the liver was stored. Peripheral blood (20 ml) was collected into collection tubes containing heparin from living liver donors at the moment of liver graft removal following complete isolation of the right lobe. Liver-associated lymphocytes were eluted from living donor grafts following removal from the donor. Briefly, the grafts were flushed via the portal vein with 5 litres of cold (4°C) preservation solution, either Viaspan or Custodial® (Custodiol® HTK solution; Odyssey Pharmaceuticals, East Hanover, NJ, USA containing heparin (5000 units/l). The effluent was collected in 1·25-litre sequential aliquots. These aliquots were then processed individually and labelled as: flush 1, flush 2, flush 3 and flush 4. As the first aliquot of liver cells retained features of peripheral blood leucocytes, while subsequent aliquots were typical of liver leucocytes isolated by other methods, we used the fourth aliquot for these phenotypic analyses.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density-gradient centrifugation and resuspended in RPMI-1640 medium with supplements (including fetal calf serum 10%, penicillin, streptomycin, and glutamine). Liver perfusates were centrifuged and resuspended in RPMI-1640 complete medium, filtered and centrifuged to obtain IHL. The cell pellets underwent Ficoll-Hypaque density-gradient centrifugation prior to staining and fluorescence activated cell sorter (FACS) analysis. Cells were either stained immediately or frozen in liquid nitrogen for later analysis.
Flow-cytometric analysis
Phenotypic analysis of the matched specimens of peripheral blood lymphocytes, lymph node cells, spleen cells and intrahepatic lymphocytes was performed using flow cytometry. The following antibodies were used in this study: fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), allophycocyanin-conjugated anti-CD3; FITC, R-PE, PE-Cy5-conjugated anti-CD56; FITC, R-PE-conjugated anti-CD4 and anti-CD8: FITC-conjugated anti-CD27, CD28, CD45RA, CD45RO, CD38, CD69, CD95 (Fas), CD62L (l-selectin), CD16, CD25, T cell receptor (TCR)-alpha/beta, TCR-gamma/delta and IgG isotype control (BD PharMingen, San Diego, CA, USA). All data were acquired using a FACSCalibur instrument (BD Biosciences) and were analysed by using FlowJo (Treestar software). All samples were incubated with an Fc blocking antibody before staining.
Statistical analysis
Statistical significance was determined using two-tailed Student's t-tests. Correlation was calculated using the Excel statistical package. A P-value of less than 0·05 was considered to be statistically significant.
Results
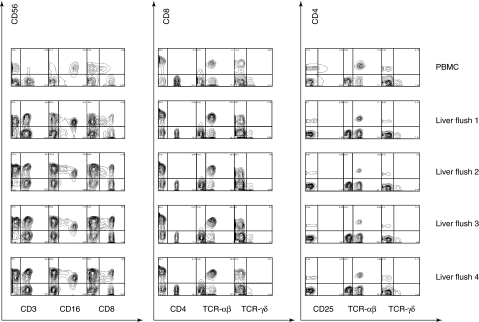
Cells isolated by perfusion of five adult living donor right lobe grafts yielded a mean of 7·8 × 108 cells (range: 2·3–11·8 × 108) IHL after Ficoll separation. This analysis demonstrated a significant phenotypic representation of CD4+ and CD8+ αβΤ cells, CD56+ NK cells, CD56+ CD3+ CD56+ T cells by surface specific staining, and in addition a population of large cells based on forward scatter, which consists of myeloid cells including antigen-presenting cells and Kupffer cells. For comparison of IHL phenotypes to peripheral blood, it was necessary to ensure that peripheral blood contamination from the liver graft was minimal. We therefore examined the lymphocytes from four sequential portal vein flushes of 1200 ml each from living donor graft segments. Aliquots from sequential flushes of a living donor right liver lobe were compared to the donor's PBMC acquired at the same time (Fig. 1). Only the first aliquot of portal vein flush continued to express some features of peripheral blood lymphocytes; such peripheral blood contamination disappeared thereafter with cells displaying the characteristics of IHL in all three subsequent aliquots. This was manifest as changes in the expression of all of the surface markers evaluated. Thus, CD3+ CD56+ cells were rare in the blood, much more abundant in liver flush 1, and stable thereafter. Similarly, CD16+, CD56+ cells and CD8+, CD56+ cells were rare in the blood sample, present in liver flush 1, increased in flush 2 and unchanged thereafter. Among the T cells, the CD8+ T cells were increased in all liver flushes and the CD4+ T cells were decreased; co-staining with CD8 and TCR-αβ confirmed that the increase was due, at least in part, to classical CD8+ T cells. The abundance of TCR-γδ cells among the IHL has been subject to debate, but in our analysis we found that these cells were CD4– and CD8– in the liver, and were detectable but less abundant than among the PBMC.
Fig. 1.
Analysis of four sequential aliquots (1·2 litres each) of fluid flushed from the living donor right liver lobe, immediately prior to transplantation. The samples were subjected to a lymphocyte gate based on light scatter, and analysed by flow cytometry. The data show the change-over from cells that retain some features of blood cells (peripheral blood mononuclear cells) in flush 1 to the typical intrahepatic lymphocytes (IHL) subset distribution of CD56-rich natural killer and CD8+ rather than CD4+ T cells by flush 2.
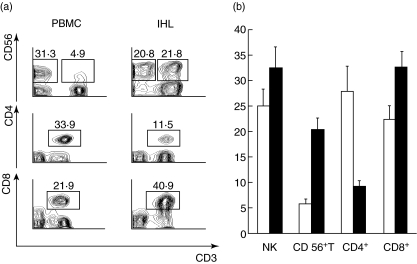
Figure 2 shows the stark contrast between blood lymphocytes and IHL isolated from the living donor liver lobes. Panel A shows that the IHL were enriched in total CD56+ cells, and in CD3+, CD56+ cells in particular (i.e. CD56+ T cells). Within the CD3+ T cells there was a lower percentage of CD3+, CD4+ cells in the IHL, but more CD3+, CD8+ T cells. Panel B shows the results from matched blood versus IHL from five living donors. As in the individual example shown in Fig. 2a, the IHL (solid bars) were enriched for NK, CD56+ T and CD8+ T cells, and depleted of CD4+ T cells. The proportion of NK cells in peripheral blood among these living donors appears somewhat higher than expected. We attribute this to a stress effect of surgery. Peripheral blood was collected precisely at the time of liver graft removal, 4–6 h after initiation of surgery. We chose to use cells collected at this time-point, as they are the valid control in making comparisons of blood versus intrahepatic lymphocytes. We did not think it valid to compare liver lymphocytes collected during major surgery with blood cells collected when this stress was absent.
Fig. 2.
(a) Individual samples of blood lymphocytes versus intrahepatic lymphocytes (IHL), showing staining for CD3 versus CD56, CD4 and CD8. These stains reveal the abundance of CD56+ T cells among the IHL. (b) The frequency of lymphocyte subsets, gated as in Fig. 2a, derived from peripheral blood (open bars) versus liver perfusion (solid black) of five living donors.
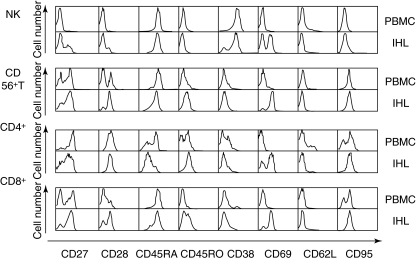
There are many conflicting reports concerning the activation state of resident liver lymphocytes. Because human IHL obtained from living donor-derived tissue is a cell population that has been explored very little, we evaluated the expression of diverse cell surface markers on the four major subsets of IHL. Individual examples of staining of human liver NK cells, CD56+ T cells, CD4+ T cells and CD8+ T cells for cell surface markers are shown in Fig. 3. The liver NK cells differed from NK cells among the PBMC in several ways: a subset expressed CD27, which was negative on the PBMC NK cells; a subset expressed CD28; a subset was CD38-negative; and approximately half the cells expressed CD69 (Fig. 3, top set of panels). The data from a set of 10 matched samples of PBMC and IHL are shown in the upper half of Table 1. These data reveal that there was some individual variation between the samples; we were unable to find a single ‘representative’ set of data that exemplified the overall results in every respect. Thus, across the 10 sets of samples, the IHL showed statistically significant (P < 0·05) increases in the expression of CD27, CD28 and CD69, but in addition the IHL showed significantly lower expression of CD62L, which was not obvious in the single sample shown in Fig. 3. Thus, we find that human liver NK cells are ‘more activated’ than peripheral blood NK cells, and also that they display the lymph node homing receptor, CD62L, on fewer cells.
Fig. 3.
All four major intrahepatic lymphocytes (IHL) subsets from the living donor liver displayed increased activation markers, compared to blood cells (peripheral blood mononuclear cells). The fluorescence activated cell sorter histograms show a back-to-back comparison of blood versus liver-derived natural killer, CD56+ T, CD4+ T and CD8+ T cells for eight activation and differentiation markers. See also Tables 1 and 2 for a summary of the data from 10 donors.
Table 1.
Markers on liver-derived natural killer and CD56+ T cells.
| PBMC (n = 10) | IHL (n = 10) | P-value | |
|---|---|---|---|
| NK | |||
| CD27+ | 3·741 ± 0·953 | 33·861 ± 9·121 | 0·0041* |
| CD28+ | 1·026 ± 0·435 | 8·771 ± 2·757 | 0·0125* |
| CD45RA+ | 97·7 ± 0·935 | 98·41 ± 0·454 | 0·5035 |
| CD45RO+ | 4·246 ± 1·294 | 2·809 ± 0·962 | 0·3848 |
| CD38+ | 94·97 ± 1·243 | 94·7 ± 1·629 | 0·8966 |
| CD69+ | 6·63 ± 1·046 | 57·98 ± 6·443 | 3·11E-07* |
| CD62L+ | 16·985 ± 4·073 | 5·583 ± 1·414 | 0·0164* |
| CD95+ | 29·651 ± 6·449 | 35·128 ± 7·237 | 0·579 |
| CD 56+T | |||
| CD27+ | 33·221 ± 6·226 | 51·677 ± 7·92 | 0·0835 |
| CD28+ | 31·745 ± 6·451 | 61·53 ± 9·029 | 0·0151* |
| CD45RA+ | 78·08 ± 7·001 | 53·912 ± 11·152 | 0·083 |
| CD45RO+ | 40·34 ± 4·76 | 60·88 ± 7·885 | 0·0387* |
| CD38+ | 19·651 ± 4·25 | 42·42 ± 7·613 | 0·0176* |
| CD69+ | 17·957 ± 2·386 | 87·77 ± 2·824 | 2·6E-13* |
| CD62L+ | 14·745 ± 3·097 | 1·734 ± 0·656 | 0·00066* |
| CD95+ | 86·01 ± 3·98 | 88·44 ± 3·288 | 0·6435 |
IHL: intrahepatic lymphocytes (IHL). NK: natural killer; PBMC: peripheral blood mononuclear cells.
The liver's abundant population of CD56+ T cells was compared with the much smaller population of CD56+ T cells in the blood. An example of such a comparison is shown in Fig. 3, the second set of panels. The liver CD56+ T cells (marked IHL) contained a higher frequency of CD27+, CD28+ and CD69+ cells than the blood-derived cells (marked PBMC). Compared to the NK cells, the CD56+ T cells expressed a higher frequency of all three activation markers. Across the 10 paired sets of PBMC and IHL samples data were consistent with the increased expression of CD27 shown in Fig. 3, but the difference was on the limits of statistical significance (P = 0·0835). However, the increased frequency of CD28+ and CD69+ cells was confirmed (Table 1, lower half). In addition, the IHL samples showed an overall, statistically significant increase in the expression of CD38 (P = 0·0176). These data show that, as with NK cells, the human liver CD56+ T cells displayed a more activated phenotype than CD56+ T cells in the blood.
In the mouse, the resident liver lymphocytes show both abundance of NK, CD56+ T and CD8+ subsets and an increase in the frequency of activated and apoptotic T cells [14]. However, these cells cannot be harvested without mechanical dissociation of the tissue, which in many studies was accompanied by perfusion and/or incubation in collagenase. The direct harvest of human IHL by elution from living donor liver lobes allowed us to obtain a high-quality sample of human liver lymphocytes without tissue dissociation or enzyme incubation; therefore this was an ideal source of cells with which to test the notion that liver T cells are constitutively activated. Figure 3 (third set of panels from the top) shows an example of the direct comparison of CD4+ T cells (identified by gating on the CD3+, CD4+ cells) from blood (PBMC) versus liver (IHL). Strikingly, the liver CD4+ T cells contained an increased subpopulation of CD45RAlow and CD45ROhigh cells, consistent with either an activated or a memory phenotype. Among these cells, many were also CD69+, identifying them as recently activated T cells. The aggregate results of the group of 10 paired samples of PBMC and IHL confirm this impression (Table 2, top half). While the decrease in CD45RA+ cells was not significant (P = 0·1674) due to individual variation, the increase in the frequency of CD45RO+ cells was highly significant (P = 0·00787), as was the increase in the expression of CD69 (P = 2·94 × 10−9). The aggregate data revealed two more features of CD4+ T cells among the IHL; they expressed CD62L on a lower percentage of cells (P = 0·0176), and they expressed more CD95 (P = 0·000636). Overall, these data confirm that impression that human liver CD4+ T cells, like those obtained from dissociated mouse livers, are enriched for activated T cells. The increased expression of CD95 also revealed that these cells were susceptible to death receptor signalling.
Table 2.
Markers on liver-derived classical T cells.
| PBMC (n = 10) | IHL (n = 10) | P-value | |
|---|---|---|---|
| CD4+ | |||
| CD27+ | 70·98 ± 6·403 | 56·62 ± 6·311 | 0·1276 |
| CD28+ | 89·71 ± 2·602 | 89·31 ± 3·465 | 0·9274 |
| CD45RA+ | 43·19 ± 8·031 | 26·548 ± 8·325 | 0·1674 |
| CD45RO+ | 53·8 ± 4·257 | 77·01 ± 3·527 | 0·00054* |
| CD38+ | 37·16 ± 5·163 | 35·264 ± 5·666 | 0·8074 |
| CD69+ | 2·627 ± 0·554 | 43·97 ± 6·290 | 3·75E-06* |
| CD62L+ | 33·981 ± 6·333 | 15·505 ± 3·143 | 0·0176* |
| CD95+ | 62·09 ± 4·234 | 83·1 ± 2·830 | 0·000636* |
| CD8+ | |||
| CD27+ | 48·43 ± 6·774 | 57·963 ± 7·519 | 0·3587 |
| CD28+ | 39·45 ± 5·417 | 55·25 ± 7·073 | 0·0931 |
| CD45RA+ | 67·62 ± 7·341 | 46·501 ± 10·04 | 0·1067 |
| CD45RO+ | 28·954 ± 4·984 | 52·96 ± 6·297 | 0·00787* |
| CD38+ | 19·471 ± 3·617 | 38·38 ± 5·821 | 0·0129* |
| CD69+ | 5·088 ± 1·243 | 71·05 ± 6·014 | 2·94E-09* |
| CD62L+ | 18·397 ± 5·163 | 2·266 ± 0·715 | 0·00625* |
| CD95+ | 62·73 ± 5·263 | 86·36 ± 3·385 | 0·00138* |
IHL: intrahepatic lymphocytes (IHL); PBMC: peripheral blood mononuclear cells.
The CD8+ subset of CD3+ T cells is enriched among IHL and these cells are of particular interest to us, due to studies in the mouse showing that the liver sequesters preferentially activated CD8+ T cells through mechanisms that involve intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and Toll-like receptor (TLR)-4. A direct back-to-back comparison of human PBMC-derived versus IHL-derived CD8+ T cells in shown in Fig. 3 (bottom set of panels). In the examples shown, the IHL CD8+ T cells (identified as CD3+, CD8+ cells) differed from their counterparts in blood in that they contained an increased frequency of cells that expressed the markers CD27, CD28, CD45RO, CD69 and CD95. In the analysis of 10 sets of matched PBMC and IHL (Table 2, bottom half), these differences were broadly sustained. Thus, there were statistically significant increases in the expression of CD45RO, CD69 and CD95. The differences in the frequency of cells expressing CD27 and CD28 were consistent with the data in Fig. 3, but not statistically significant. In addition, the overall data showed that the IHL expressed CD38 on a higher frequency of the cells, but CD62L in a lower frequency of the cells.
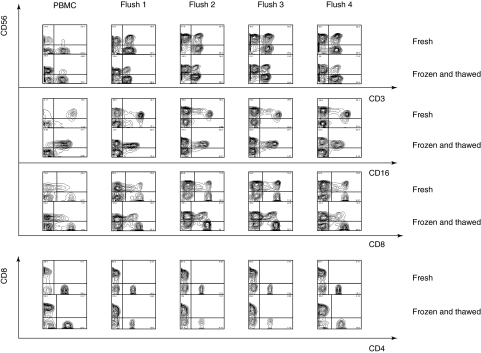
Liver transplantation surgery often occurs at unpredictable times, creating a logistical problem in the collection of human IHL for translational research. We therefore tested the effects of one cycle of cryogenic preservation on the phenotypes of human IHL. Using cells obtained from a living donor, a simple freeze–thaw experiment was performed whereby an aliquot of each 1·2-litre flush of freshly isolated cells was compared to the same donor cells, after cryopreservation and storage for 24 h. The comparison is shown in Fig. 4. There was good preservation of the major IHL phenotypes after cryopreservation, and the characteristic changes in cell subsets through the four sequential flushes were retained. Specifically, the frozen and thawed cells broadly retained the increase in CD56+ cells that was seen in fresh samples, although there was some loss of abundance of CD3+, CD56+ cells, suggesting that these were differentially susceptible to damage. Freezing and thawing appeared to cause a loss of intensity of CD16 expression, but it was still possible to identify CD16+, CD56+ NK cells in the frozen and thawed samples, and to observe their increase in the liver flush specimens relative to the PBMC specimen. The expression of CD8 and CD4 were extremely well preserved in the frozen and thawed cell specimens, and it was possible to observe both the increase in CD8+ cells, and the depletion of CD4+ cells in the IHL samples. These data argue that it will be possible to use frozen and thawed human IHL isolated from living donor lobes in subsequent analysis, but that some caution is in order because of the loss of the CD16 marker, and the selective loss of CD3+, CD56+ CD56+ T cells.
Fig. 4.
Preservation of some natural killer (CD56) and all major T (CD3, CD4, CD8) cell markers in frozen and thawed intrahepatic lymphocytes (IHL). CD16 staining was somewhat impaired by the freezing and thawing procedure but was still apparent. The only cell population that was under-represented after freezing and thawing was the CD56+ T cells, identified as CD56+, CD3+ cells, and as CD56+, CD8+ cells.
Discussion
These data provide insight into the composition of human IHL and supplement the more extensive knowledge of murine systems [14]. Examination of lymphocytes from the livers of living donors confirmed previous findings of increased NK, CD56+ T and CD8+ T cells upon comparison to peripheral blood [6, 8]. Furthermore, these cells displayed a range of activation markers that differ from the same cell subsets isolated from peripheral blood.
Previous work in the mouse suggested that activated T cells were sequestered in the liver, often undergoing apoptosis as a mechanism whereby immune responses were terminated [1, 15]. This re-examination in humans is consistent with the concept that activated T cells, like NK and CD56+ T cells, are sequestered out of the circulation by the liver. One feature of our data was the differential expression of cell surface markers on several or all of the four major subsets of lymphocytes when IHL were compared to PBMC. Thus, the expression of CD28 was increased consistently on both NK and CD56+ T cells among the IHL, but not on classical T cells. CD28 is a co-stimulatory receptor that participates in the classical immunological synapse, which is formed between T cells and antigen-presenting cells. Its presence on a subset of NK cells is interesting, as these signal though a distinct set of receptors. However, NK cells respond to the CD28 ligands, CD80 and CD86 [19]. The CD28 on NK cells is functionally important, participating in the polarizing the microtubule organizing centre and of cytotoxic granules towards the NK cell synapse [20]. Therefore increased CD28 on liver NK and CD56+ T cells may imply an increase in cytotoxic potential.
Classical CD4+ T cells all expressed CD28 in both blood and liver, consistent with its central role in T cell activation (Fig. 3; Table 2). However, it was noteworthy that a subset of CD8+ T cells from the blood was lacking CD28 expression (Fig. 3). The presence of such cells was increased in the liver CD8+ T cells, but with considerable individual variation, rendering the difference non-significant (Table 2). The lack of CD28 on CD8+ T cells has been reported on T cells in elderly humans [21], give rise to non-specific suppressor T cells [22] and share patterns of gene repression with canonical CD4+, CD25+, glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related gene (GITR+), forkhead box P3 (FoxP3)+ regulatory T cells [23]. Therefore, the abundance of these cells in the liver could be linked to ‘liver tolerance’, a local form of immune privilege [24].
CD45RO expression was not evident on NK cells from either source, but was increased on CD56+ T, CD4+ and CD8+ T cells among the IHL. The shift in CD45 isoform expression that occurs following human T cell activation is well documented [25], but it has not been clear that this change also accompanies CD56+ T cell activation. However, based on the co-expression of other markers that suggest liver CD56+ T cells are in a higher activation state, we think it is likely that CD56+ T cells also change their CD45 isoform pattern after TCR engagement. The significance of expression of CD45RO rather than CD45RA on memory T cells is debated, but some data suggest that the CD45RO isoform contributes to a lower activation threshold on memory T cells. The extracellular domains of CD45 associate with CD4 on T cells transfected with modified CD45 molecules lacking a cytoplasmic domain, and the lower molecular weight isoforms of CD45 associate more strongly with both CD4 and the TCR, contributing to enhanced signalling [26]. The strong expression of CD45RA, but lack of expression of CD45RO on NK cells, is therefore consistent with their use of a distinct set of activation and inhibitory receptors that do require the CD4 coreceptor.
Perhaps the most striking difference between PBMC-derived and IHL-derived lymphocytes was in the expression of CD69. This is an acute activation marker that is present transiently on recently activated lymphocytes. The expression of CD69 was increased, and the increase was highly statistically significant, on all four subset of human liver lymphocytes (Fig. 3, Table 1, Table 2). This observation helps to distinguish between two possibilities: that the expression of diverse activation markers on IHL implies that these cells are memory cells, or that they are acutely activated. While both naive and memory cells may exist among the IHL, the high expression of CD69 on all subsets implies that these cells are being continuously subjected to activating signals. These might arise because (1) the liver is exposed to a continuous flux of non-self antigens from the intestine in the form of digestion products, or (2) that the liver is exposed to endotoxin, which is present constitutively in the portal vein [27]. This issue is difficult to approach in the human, where endotoxin-free and antigen-free conditions do not occur; however, the specific issue of the contribution of endotoxin to CD69 expression could be addressed in the mouse, where natural mutant and gene-targeted animals exist lacking the endotoxin receptor.
Recent experiments in normal mice suggest recruitment of lymphocyte subsets mirrors the resident population [28]. Our studies in the human differ from those in the mice in that CD62L was low in all four subsets of IHL; CD62L engages with an addressin on high endothelial venules and facilitates the extraction of lymphocytes form the circulation; among previously activated T cells its expression is the hallmark of ‘central memory’ cells, while tissue or peripheral memory cells express a lower level of this homing receptor. CD62L was essentially absent from IHL-derived NK, CD56+ T and CD8+ T cells, indicating a strong selection bias. It has been postulated the population of intrahepatic lymphocytes is shaped by selective recruitment [12], and the expression of CD62L supports this idea. The tethering interactions that exist in blood vessels with rapid flow may be irrelevant in sinusoidal beds, where direct integrin interactions may have a larger role in cell trafficking. The specialized anatomy of the sinusoid in the liver [14, 29, 30] and spleen, where blood flow is inherently slow, may make the initial tethering interactions of selectins–addressins less important. Tissue specificity of lymphocyte subsets within the liver appears to be influenced both by adhesion molecules and by ligands expressed on the lymphocytes and the vascular epithelium, and by the chemokines expressed within the organ [30]. This is most probably true of all tissues, both lymphoid and non-lymphoid. The observations made here point to an overall preferential representation of activated over naive lymphocytes within liver as well as peripheral lymphoid organs, while the peripheral blood carries predominantly naive lymphocytes.
This study establishes that simple flushing of the excised right lobe of the donor liver provides a high yield of up to a billion (average 7·81 × 108) viable, normal IHL. This has clear-cut advantages over the widely used mechanical homogenization of tissue obtained from small biopsies. First, the yield is high due to the enormous mass of liver from which the cells are extracted, and this occurs using cells that become available during the preparation of the graft; there is no negative impact on either donor or recipient. Using this source of cells avoids the inherent risk of biopsy, and there is less risk of altering cell surface proteins than when enzymatic digestion is employed to assist the liberation of cells. Probably the most important advantage of simple flushing is the high yield of cells that have undergone minimal manipulation. These cells can be purified readily, placed in short-term cell cultures and generate cytokine responses to co-cultured stimulatory or regulatory cells. These properties of the human IHL will be documented in a subsequent publication. These cells will be useful in the analysis of the biology of human IHL subsets, and in prospective studies of the influence of donor liver immunological status on the outcome of transplants.
References
- 1.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 2.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:1969. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Nakafusa Y, Flye MW. Portal vein administration of donor cells promotes peripheral allospecific hyporesponsiveness and graft tolerance. Surgery. 1994;116:229–34. discussion 234–5. [PubMed] [Google Scholar]
- 4.Nakafusa Y, Goss JA, Roland CR, Flye MW. The effect of portal venous tolerance on the survival of small bowel allografts in the rat. Transplantation. 1993;56:1279–82. [PubMed] [Google Scholar]
- 5.Hashimoto W, Takeda K, Anzai R, et al. Cytotoxic NK1.1 ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–40. [PubMed] [Google Scholar]
- 6.Jonsson JR, Hogan PG, Balderson GA, et al. Human liver transplant perfusate: an abundant source of donor liver-associated leukocytes. Hepatology. 1997;26:1111–4. doi: 10.1002/hep.510260504. [DOI] [PubMed] [Google Scholar]
- 7.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 8.Norris S, Doherty DG, Collins C, et al. Natural T cells in the human liver. cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 9.Collins C, Norris S, McEntee G, et al. RAG1, RAG2 and pre-T cell receptor alpha chain expression by adult human hepatic T cells: evidence for extrathymic T cell maturation. Eur J Immunol. 1996;26:3114–18. doi: 10.1002/eji.1830261243. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H, Miyaji C, Seki S, Abo T. C-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosbie OM, Reynolds M, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. In vitro evidence for the presence of hematopoietic stem cells in the adult human liver. Hepatology. 1999;29:1193–8. doi: 10.1002/hep.510290402. [DOI] [PubMed] [Google Scholar]
- 12.Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–4. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 14.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 15.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–10. [PubMed] [Google Scholar]
- 16.Knolle PA, Schmitt E, Jin S, et al. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–40. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 17.Curry MP, Norris S, Golden-Mason L, et al. Isolation of lymphocytes from normal adult human liver suitable for phenotypic and functional characterization. J Immunol Meth. 2000;242:21–31. doi: 10.1016/s0022-1759(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 18.Norris S, Collins C, Doherty DG, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JL, Charo J, Martin-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163:4207–12. [PubMed] [Google Scholar]
- 20.Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA. 2006;103:10346–51. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 22.Filaci G, Fravega M, Negrini S, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142–56. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Scotto L, Naiyer AJ, Galluzzo S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28– T suppressor cells. Hum Immunol. 2004;65:1297–306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–18. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 26.Leitenberg D, Novak TJ, Farber D, Smith BR, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–59. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268–70. [PubMed] [Google Scholar]
- 28.Klugewitz K, Blumenthal-Barby F, Eulenburg K, Emoto M, Hamann A. The spectrum of lymphoid subsets preferentially recruited into the liver reflects that of resident populations. Immunol Lett. 2004;93:159–62. doi: 10.1016/j.imlet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–92. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- 30.Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]